Abstract
Nanogel (NG) drug delivery systems have emerged as promising tools for targeted and controlled drug release, revolutionizing treatment approaches across various diseases. Their unique physicochemical properties, such as nano size, high surface area, biocompatibility, stability, and tunable drug release, make them ideal carriers for a wide range of therapeutic agents. Nanogels (NGs), characterized by their 3D network of crosslinked polymers, offer unique edges like high drug loading capacity, controlled release, and targeted delivery. Additionally, the diverse applications of NGs in medical therapeutics highlight their versatility and potential impact on improving patient outcomes. Their application spans cancer treatment, infectious diseases, and chronic conditions, allowing for precise drug delivery to specific tissues or cells, minimizing side effects, and enhancing therapeutic efficacy. Despite their potential, challenges such as scalability, manufacturing reproducibility, and regulatory hurdles must be addressed. Achieving clinical translation requires overcoming these obstacles to ensure therapeutic payloads' safe and efficient delivery. Strategies such as surface modification and incorporating stimuli-responsive elements enhanced NG performance and addressed specific therapeutic challenges. Advances in nanotechnology, biomaterials, and targeted drug design offer opportunities to improve the performance of NGs and address current limitations. Tailoring NGs for exploring combination therapies and integrating diagnostics for real-time monitoring represent promising avenues for future research. In conclusion, NG drug delivery systems have demonstrated tremendous potential in diverse disease applications. Overcoming challenges and leveraging emerging technologies will pave the way for their widespread clinical implementation, ushering in a new era of precision medicine and improved patient care.
Graphical Abstract
Role of NGs in treatment of various diseases.
The figure was created using BioRender (www.biorender.com) (accessed 4 January 2024).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nanoparticle (NP) technology is rapidly advancing, offering innovative and effective treatments for various medical conditions such as cancer, inflammation, cardiovascular diseases, psoriasis, diabetes, bone regeneration, gene therapy etc. These nanoparticles (NPs) are designed to overcome challenges like poor selectivity, known targeting sites, and side effects on various body tissues. They also address the limitations of micron-size particles, including surface area, site specificity, retention at the targeting site, swelling behaviour, drug loading, and release behaviour. Nanogels (NGs) type of NPs have garnered significant attention over the past 20 years due to their bio-compatibility, biodegradability, versatility, and safety from leakage [1,2,3,4,5]. In addition to in-vitro and in-vivo tests, other methods like ex-vivo and in-silico testing are used to validate drug delivery systems [6]. With the wide range of nanosized materials involved in nanotechnology-developed drug delivery systems, each material exhibits unique properties dependent on its nano-size. These properties allow for enhanced intracellular drug delivery, subcellular targeting, and the capability to access previously inaccessible body areas [7].
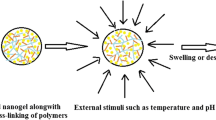
NGs are typically 3-dimensional submicron-sized networks of hydrophilic polymers developed by chemical or physical cross-linking that exhibit the full range of characteristics of both NPs and hydrogels (HGs). Ionic interactions, hydrogen bonds, electrostatic interactions, and hydrophobic interactions are the main types of physical cross-linking [8, 9]. While some studies have shown that NGs as large as 1,000 nm are reasonable, others have found that those as small as 200 nm are ideal for use in the medical field [10]. NGs may absorb a considerable amount of water. NG cross-linking network may be used as a grid to contain the internal fluid system, while the absorbed water is used as a filtration medium for cargo diffusion. NGs with an unsuitable zeta potential help avoid immune phagocytosis and may resist the adsorption of negatively charged proteins [2, 11]. Considering their high-level performance in extending blood circulation and boosting healing effectiveness, several drug carriers, including liposomes, polymer vesicles, and micelles, have drawn broad interest in studying controlled drug delivery [12,13,14].
NGs have been extensively researched for integrating and releasing actions of bioactive substances such as proteins, vitamins, and drugs [15], DNA, antigens, oligonucleotides, genes, as well as inorganic molecules such as quantum dots, silver NPs, magnetic NPs [16]. NGs may be delivered using one of two methods: passive targeting or active targeting. In the case of passive targeting, the size, swelling, surface charge, and various physicochemical attributes of the NGs reveal drug release. Active targets involve conjugating NGs with particular scaffolds that selectively identify and bind with some over-expressed receptors at the target areas, like in tumours. This process causes conjugated NGs to accumulate at the target site [17], attaining more than 98% loading efficiency. Since NGs are HGs, their capacity to resemble tissue is another distinctive quality that distinguishes them because of the significant water content and the bio-compatible ingredients utilized. Such gel topically has a calming effect that is highly helpful in treating conditions like wounds [18].
Drug delivery system
Developing effective treatments and tackling disorders is a significant challenge for formulation nowadays. The existing drugs and active molecules are often effective mechanisms for treating specific disorders, but their efficacy is sometimes severely constrained by challenges in their delivery. To bring drugs and active molecules to the target sites where they will have the most incredible pharmacological effects, a process known as a drug delivery system has been developed [19]. One of these systems' significant benefits is the ability to regulate the rate, timing, and target site of a drug's delivery to a patient [20]. NPs are essential in drug delivery as well-synthesized nanocarriers can meet high drug-loading levels and regulate drug release [21]. Their nanostructures and functions may increase delivery processes such as selectivity, decreasing toxicity and side effects. Polymeric NPs are essential in this context since their structure provides good bio-compatibility and biodegradability and can be readily functionalized [22]. Furthermore, while dealing with suitably functionalized polymer chains, it is reasonable to modify factors such as mechanical qualities, composition, or degradation rate. In the interest of completeness, these formulations are expensive, restricting their use in clinical practice.
Controlled and targeted drug delivery system
Compared to traditional formulations, controlled and specifically designed drug delivery systems are intended to produce significant benefits. They are optimizing the drug release from the delivery system since the rate and duration of the procedure dramatically affect the therapy's efficacy [23]. Controlled drug delivery systems (CDDS) should avoid restrictions on drug concentration within the targeted therapeutic range. Actuality, the drug release profile of traditional drug delivery techniques, is characterized by a rapid decline below the lowest effective concentration, followed by an increase to a peak concentration over the maximum safe concentration. A correctly constructed CDDS ensures the drug's blood concentration profile stays within these limits [24]. However, controlled drug release is not enough if it fails to take place in the tissues that we wish to address; a targeted drug delivery system (TDDS) is a different approach that can preferentially access a specific target area, emphasizing therapeutic benefits and minimizing undesirable effects produced by the drug's interaction with various body tissues [25]. TDDS and CDDS provide the potential to utilize a smaller dose of the drug compared to conventional therapies, and the carrier's structure may keep the drug in-vivo, preventing early deprivation or quick breakdown [26].
NGs
NGs are promising for therapeutics, diagnostics, macromolecules, and other applications. NGs, primarily hydrophilic and with a large capacity for guest molecules, are highly bio-compatible and have significant benefits over other nanomaterials for biomedical applications. Due to their distinctive characteristics, including stimuli-responsive behaviour, softness, and swelling, NGs shield the cargo from deterioration and removal and actively engage in the delivery process to create a regulated, triggered reaction at the target region. [27]. NGs offered merits are as follows:
-
High water content makes materials more bio-compatible, which causes them to behave like actual tissue and elicit favourable immune reactions.
-
Nanocarriers are biodegradable, making them non-toxic.
-
Drug loading capacity is high.
-
Controlling drug release by adjusting crosslinking densities [28].
-
Resist entrapment by the reticuloendothelial system quickly [29].
-
Tiny size makes them better at permeating biological membranes.
-
Drugs and charged solutes that are both hydrophilic and hydrophobic may be included (Fig. 1) [30].
-
Superior transport qualities [31].
Macroscopic and microscopic view of NGs. The figure was developed using BioRender (www.biorender.com) (accessed 5 October 2023)
Limitations of NGs
NGs provide many benefits but have specific vital points that sometimes prevent their application. The limits of NGs are listed below:
-
(a)
The solvent and surfactants must be removed entirely using expensive methods.
-
(b)
There may be residual residues of monomers or surfactants, which might be hazardous.
-
(c)
Variation in manufacturing, whereby the usual qualities of NGs are only attainable within a specific range of dimensions [32].
Types of NGs
A cross-linked HG particle with a polymer basis known as an NG is sub-micron-sized [2]. NGs are highly customizable in size, shape, surface functionalization, and degradation processes and may be natural, synthetic, or a combination [33]. Based on the kind of crosslinking, reactivity to external stimuli (including pH, temperature, light, ionic concentration, etc.), and production techniques, NGs may be classified into several types (Fig. 2).
Polymers used for NGs
Hydrophilic polymers, which typically absorb abundant water within their crosslinked frameworks, are the key components of NGs. These NGs are distinguished by their biological inertness and water sorption capabilities, which cause them to expand up to 1000 times in water, according to the 3D structure of the polymers utilized in their manufacturing (Fig. 3).
Surface functionalization of NGs for delivery of drugs
Drug and biological molecule entrapment is possible using NGs. As a result, they may be used extensively in transporting genes and proteins. Both passive and active drug targeting are possible by adjusting the particle size and surface characteristics to prevent fast clearance by phagocytic cells. Controlled and prolonged drug is delivered at the desired site, increasing therapeutic effectiveness and minimizing side effects [34]. Due to their small volume, NPs can penetrate tissues, even the smallest capillaries, via paracellular or transcellular routes. NGs offer a significant drug-load capacity, a low floating density, and a high degree of stability during dispersion in aqueous mediums (Fig. 4). In comparison to other NPs, particularly in terms of drug loading, NGs show potential as suitable nanomedicine carriers [35].
Coating strategies of surface functionalization of NGs for selective targeting the cells. The figure was developed using BioRender (www.biorender.com) (accessed 14 October 2023)
NGs: Drug incorporation techniques
Due to their incredible ability to target a wide variety of organs, NGs have been widely accepted in nanotechnology, resulting in dual nature: (a) As an HG system, which boosts their ability to load drugs, and (b) a system with nanoparticulate that enables them to reach deeper organs and tissues.
Incorporation of drugs inside the NGs may be achieved by numerous methods (Fig. 5), including-
-
Biological agents can covalently conjugate either during or after the development of NGs. Develop nanosized HGs (Table 2), mutated enzymes can copolymerize using acrylamide for inverse micro-emulsion and diluted water-based solutions. [36, 37].
-
Drug molecules are physically trapped inside NGs. Proteins have been included in cholesterol-modified pullulan NGs, and small interfering RNAs (siRNAs) have been incorporated into hyaluronic acid (HA) NGs using this approach [38, 39].
-
Drug loading through passive/diffusion, for instance, NPs and dexamethasone, can be separately introduced within the dextran lysozyme NGs via diffusion, and the NGs are agitated in excessive drug or NP solutions. Generally, the drug loading produced by these methods is small, often less than 10% by weight [40,41,42].
Potential applications of NGs in the treatment of various diseases
NGs have gained significant attention from research groups worldwide. These structures have been developed to treat various pathologies, including cancer, spinal cord injury, ischemic stroke, cardiovascular diseases, wound healing, bone regeneration, psoriasis, inflammation, etc. They have also been used for delivering anaesthetic drugs. In the following sections, we will analyze the different pathologies in which NGs have been applied and tested. We will provide examples and references to highlight the importance of these formulations in nanomedicine.
NG in CNS-related diseases
Numerous drugs are used to treat CNS-related diseases and other brain disorders. Still, their low bioavailability in such organs, owing to the inadequate permeability of the blood–brain barrier (BBB), has permanently restricted their capacity to enter the brain [43, 44]. A more effective nano-system might lead to better therapy for diseases associated with the brain (Table 1). However, many current methods of brain targeting in nanotechnology involve improving drug access to the brain. Drug bioavailability has constantly been significantly enhanced by polymeric NPs, some of which can cross BBB [45]. The incorporation of NGs is an efficient nanoparticulate technology for effective brain-targeted therapy. Methotrexate (MTX), an anticancer drug, has been developed as a NG and is an extensively used chemotherapeutic drug with a vital role in malignancies and autoimmune disease treatment. MTX was added to the NG system to increase BBB permeability, and polysorbate was used to functionalize the surface [46, 47].
NG significantly boosted the capacity and efficiency of drug loading. The in-vitro experiments confirm the NPs' suitability for brain administration. A study found that even though the drug plasma concentration decreased due to intravenous administration, the drug crossed the BBB and safely entered the brain slowly and controlled. It demonstrates that the drug was absorbed gradually and without risks into the brain. Compared with free drugs, MTX concentrations in the brain were considerably more significant in both kinds of NGs (surface-modified and untreated NGs). Results indicated that using drug-loaded NGs increased MTX concentration in the brain by 10–15 times, presenting a promising future for NGs used for brain delivery. Oligonucleotides (ODN) have also been developed as NG to target CNS against neurodegenerative diseases. Research demonstrated that NG formulations with ODN successfully crossed the BBB. When transferrin or insulin is added to the NG's surface, the effectiveness of the transport is significantly improved to the target. Compared to free ODN, the concentration of phosphorothioate ODN rose onefold inside the brain after 1 h of intravenous NG injection while decreasing twofold in the spleen and liver [48,49,50]. Another researcher developed cisplatin-loaded NGs coupled over monoclonal antibody conjugates to mark exceedingly expressed connexin 43 (Cx43), a tumour-specific membrane protein, and BSAT1, an anion transporter specific to the brain in human glioblastoma, the most aggressive and common brain disorder in the world. The cisplatin-loaded NG formulation for treating gliomas showed higher efficacy, and the survival rate of rats was increased for around 27 days compared to the control group [51].
NG in cancer
Cancer is a chronic illness that includes around 277 forms of cancer pathology [52]. Several treatments have been available, including radiation, surgeries, and targeted therapies [53, 54]. Several teams of researchers are keen on NGs since they offer an opportunity for specific delivery of drugs for cancer therapy (Fig. 6). Specific formulations and surface functionalization using particular ligands may be helpful strategies to selectively target malignant cells within the body and give non-invasive treatments [55]. NGs have the potential to cure diseases such as breast cancer. In this work, the researchers produced dextrin NG encapsulated by Plerixafor and supplied with Dox (Table 1) [56,57,58]. They coupled with the PLG-g-m polyethene glycol (PEG)/combretatatinA4 nano-formulations using azobenzene and cyclodextrin attached to polyglutamic acid (PGA)-graft-PEG methyl ether NG revealed a tumour reduction rate of 68.7%, that was increased to 91.7%. NGs based on cholesterol are a significant and actively researched method for treating cancer. This study created IL-12-loaded pullulan (CHP)-based NGs that include cholesterol [59].
Fate of NGs to act on cancer using endocytosis. The figure was developed using BioRender (www.biorender.com) (accessed 1 November 2023)
In-vivo experiments showed that this technique may slow down fibrosarcoma development. In separate research, vascular endothelial growth factor (VEGF)-specific siRNA was administered via cholesterol-endowed cycloamylose through spermine NG, and the ability to inhibit neo-vascularization and proliferation of renal cell carcinoma [60]. Hyaluronic acid is a significant polymer incorporated to produce NGs to treat cancer. Contesting it, researchers developed a cisplatin-crosslinked hyaluronic acid (HA) NG comprising Dox [61]. Dox and cisplatin had a synergistic effect, increasing the biological activity while decreasing toxicity, resulting in favourable outcomes during in-vivo tests. The identical polymer produced a zein NG, including curcumin, crosslinking using HA, which was efficient towards a CT26 tumour model (Table 1) [62]. As a result, the scenarios provided do not represent the formulae that may be employed. The reduction-responsive polypeptide NG containing Dox demonstrate encouraging in-vivo results due to its exceptional security and cancer-inhibitory properties [63]. Researchers also developed pullulan NGs featuring similar characteristics [64]. In this approach, two independent pullulan NGs for administering Dox were formed with two distinct cross-linking agents, leading to tumour suppression (83.37%) in-vivo studies utilizing an ortho ester-modified Pluronic copolymer (acid-labile) as the crosslinking mediator. Another investigation looked into another NG composition [65]. Researchers developed a lactobionic acid-modified soy-protein NG to deliver Dox. In-vivo investigations demonstrated that this combination facilitated tumor targeting and treatment efficacy. The potential to alter the composition of NGs for selective drug release activities is an attractive feature. In this context, transferrin-modified poly-sulfamide NGs enabling Dox loading were developed [66]. In-vivo studies presented that the formulation had tumour-targeting attributes, which could improve cancer treatment. This investigation used a different approach. In this instance, they synthesize an irinotecan-loaded gelatin NG membrane with platelets embedded. The prepared NGs decrease in-vivo cancer cell growth, reducing side effects [67].
However, hepatic cancer (HCC) is thought to be the third leading cancer-related cause of death worldwide [68,69,70,71,72]. Among the most often used chemotherapeutic drugs for the treatment of liver carcinoma is doxorubicin (Dox), [73]. However, it requires an effective therapy potential because of its decreased effectiveness as a consequence of its severe toxicity. Additionally, Dox has a history of being rapidly metabolized into inactive derivatives, which further reduces its effectiveness. A composite biodegradable NG that are pH-sensitive for local injectable administration was synthesized to counteract the harmful effects of Dox, such as cardiotoxicity and the present issues with HCC [74]. The NG system target tumour tissues due to the enhanced permeability and retention (EPR) effects. It releases drugs through pH-controlled hydrolysis in endosomes and lysosomes through the endocytic route (Table 1). This results in more precise drug delivery with fewer side effects and enhanced effectiveness in cancer treatment. Chitin-poly L-lactic acid composite NGs (CNGs) successfully included Dox, with 86% entrapment efficiency. At an acidic pH, chitin-PLA and Dox-chitin-PLA CNGs resulted in more enlargement and drug release. Additionally, these NGs demonstrated no haemolysis of RBCs, demonstrating the systemic route's safety. In-vitro tests have demonstrated improved cytotoxicity employing pH-sensitive NGs, which deliver the drug with low pH, specifically where the tumour occurs without producing adverse effects via systemic drug distribution [75].
Nano formulations with appropriate surface charge and size offer a vast opportunity for cytotoxic drugs to target specific regions owing to their superior biological membrane permeability. Concerning the topical distribution of drugs through small gels, NGs among contemporary Nano formulations are gaining attention. Chitin NG of 5-fluorouracil (5-FU) is a skin cancer drug thus far produced. The study's findings led to the effective loading of 90% of the drug in the NG, which had a higher capacity for swell and drug release at an acidic pH. Although introducing 5-FU to the NG didn't enhance its penetration for various reasons, it might increase the gel's retention period for the deepest layers of skin (up to 5 times) and is advantageous since the therapy's targets, melanocytes, are found in deeper layers [76].
Moreover, hyperthermia is an abnormal elevation in body temperature or overheating [77]. Whole-body hyperthermia (WBH) and hyperthermic perfusion treatments, such as hyperthermic isolated limb perfusion (HILP) and hyperthermic peritoneal perfusion (HPP), differ from local/interstitial and regional hyperthermia [78]. Clinical investigations on people with locally advanced malignancies supported the application of regional and local hyperthermia [79]. These studies found a remarkable association between administered doses and outcomes. In phase II investigations, regional and local hyperthermia were used in addition to chemotherapy and radio-chemotherapy. The findings suggest that hyperthermia has many therapeutic benefits [80]. The initial heat-activated formulation of a liposomal carrier for drugs to be used in human clinical trials is also thermosensitive liposomal Dox (Table 1) [81]. One of the most popular antineoplastic drugs used in the treatment of human cancer is Dox [82]. The study conducted by the researchers involved the synthesis of a dual pH and temperature-sensitive PNA NG, which was used to deliver drugs. Under normal conditions, the PNA NG was hydrophilic (Table 1).
However, upon heating through its LCST (lower critical solution temperature), the NG undergoes a phase transition influenced by pH value. Because of the pH difference across cancer and normal tissues, tumour cells could preferentially absorb Dox-PNA NGs. Consequently, such NGs might deliver chemotherapeutic drugs directly to tumour cells, increasing cellular internalization during region hyperthermia therapies. Dox was covalently linked to PNA through an acid-labile bond to form NGs. The association was robust at extracellular and physiological pH, but it cleaved to release the drug when it came into contact with moderately acidic conditions in tumour cell endosomes. The approach could lessen the adverse impacts of anti-cancer drugs while boosting their ability to target tumour cells. Dox-PNA NGs may significantly enhance the combination treatment of hyperthermia and chemotherapy [83].
NG in spinal cord injury
A spinal cord injury (SCI) constitutes a devastating CNS disorder that can come from both traumatic and non-traumatic occurrences [84]. The 'primary injury' involves immediate neurological impairment to the spinal cord, and the 'secondary injury' is marked by a sequence of biochemical and inflammatory responses. In the present instance, the most essential aspect of the second stage is inflammation, which is intensively studied to develop an efficient therapeutic candidate for reducing it. The two polymer-based vehicles for drug delivery developed for the treatment of SCI are NGs and NPs. The capacity to penetrate the CNS's intrinsic barrier and selectively address its cells is essential in all conditions. In a recent study, rolipram ( an anti-inflammatory drug) was put into a PEG and PEI-NGs coated with amines (Table 1) [85]. In-vivo studies indicated that formulations might specifically target the astrocytes and restore motor functions in animal models in the initial stages of spinal cord injuries despite lowering the pro-inflammatory events triggered by the activation of astrocytes.
Similarly, a researcher developed poly lactic-co-glycolic acid (PLGA) microspheres carrying paclitaxel and minocycline hydrochloride introduced to alginate HG [86]. In-vivo examinations on rats with dual-drug regimens demonstrated that it successfully decreased inflammatory responses after seven days of treatment, scar tissue development, and neuronal regeneration after four weeks. In a study, the researcher developed a new and significant approach involving polymeric NPs [87]. They employed poly-caprolactone-based NPs loaded with minocycline to target microglia in their investigation. By regulating specific microglial cells, the acute therapy of the NGs in a mouse model with SCI reduced the pro-inflammatory responses while maintaining pro-regenerative surroundings for up to 10 week’s post-injury. SCI was also treated with minocycline. This study developed a sialic acid-PEG-PLGA co-polymer that specifically targets E-selectin and can assemble itself into micelle formulation [88]. In-vivo experiments revealed that these micelles could be assembled in SCI sites in mice, lowering the extent of the lesions and enhancing axon and myelin survival. The scientist also used polymeric NPs carrying IRF5 siRNA, which were delivered into the wounds of SCI mice [89]. Introducing these NPs altered the anti-inflammatory reaction in the wound by minimizing M1 macrophages while boosting the amount of M2 macrophages. Many conditions, other than SCI, can influence the CNS. Thus, NGs can help treat them successfully. Brain tumours, such as glioblastoma, represent an excellent illustration, as they may be highly aggressive and threatening to human life [90]. NGs have the potential to deliver immunotherapy onto glioblastoma cells in an efficient manner. They can be done by consistently developing thermo-reversible PEG-chitosan HGs designed to release T-lymphocytes [91]. These HGs have a solid capability to kill glioblastoma cells and are an essential tool for targeted immunotherapy [92].
NG in ischemic stroke
Ischemic stroke can be treated through thrombolytic treatment, which includes drugs including streptokinase, urokinase, anistreplase, and tissue plasminogen activator [93, 94]. Respondent NGs, such as pH-sensitive NGs, may be beneficial for developing urokinase delivery and are considered a practical approach in stroke management. In one instance, researchers developed pH-sensitive PEG-urokinase NGs (Table 1) [95]. When the pH drops owing to microcirculatory clots, which typically cause an oxygen deficit in this sickness, the NG releases urokinase. In-vivo studies found that urokinase was introduced one hour after cerebral artery congestion, minimizing ischemia damage by preserving the BBB, strengthening ischaemic brain tissues, suppressing apoptosis, and minimizing neurotoxicity. Another investigation [96] discovered that using the same formulation outside the conventional thrombolysis interval produced encouraging outcomes for in-vivo assessment in a second study. In this research, the loaded NGs protected the BBB and decreased stimulate-neurotoxicity among rats with chronic middle cerebral occlusion. Another study developed a hollow NG carrying urokinase (made by combining chitosan glycol and aldehyde-capped PEG) for delivering urokinase under ultrasonic diagnosis conditions [97]. In-vivo investigations revealed that this formulation might prolong urokinase circulation time. The formulation provided urokinase more rapidly, increasing clot thrombolysis, and was responsive to diagnostic ultrasonography. The present study suggested by in-vivo tests that similar urokinase-loaded hollow NGs might mitigate severe ischemic stroke by enhancing urokinase's thrombolysis consequences, maintaining the BBB's integrity, preventing adverse brain haemorrhage and death of animals after one week of administration [98].
NG in inflammation
MTX was initially developed as a folic acid antagonist in the 1940s. This drug primarily inhibits malignant cell proliferation by inhibiting the de novo production of purines and pyrimidines. Because high doses of folinic acid and folic acid may counteract MTX's antiproliferative effects, it is clear that MTX is an antifolate drug. Cells take up MTX via a folate carrier and convert it to polyglutamate [99, 100]. MTX is a potent drug used for cancer [101], anti-inflammatory, and immunosuppressant treatments [102]. It has long-lasting metabolites called MTX polyglutamate that retain the parent compound's antifolate actions while altering the potency of inhibiting specific folate-dependent enzymes [103, 104]. A further investigation examined how sodium carbonate (Na2CO3) affected the transport of MTX within a NG in vitro and the modification of prostaglandin E2 (PGE2) synthesis in skin ex vivo. A NG containing MTX was administered to resected porcine epidermal membranes. The introduction of saturated aqueous Na2CO3 boosted MTX flow while decreasing PGE2 synthesis (Table 1). The findings suggest a unique mechanism in which temperature changes caused de-swelling and ejection of MTX in situ. At the same time, the addition of Na2CO3 resulted in more solubilization and MTX release, lowering PGE2 production [105].
NG in gene therapy
Gene therapy is a legitimate therapeutic option nowadays. However, the initial clinical trials were discontinued due to serious adverse effects [106]. Two out of ten patients who underwent retroviral gene therapy for X-linked severe combination immunodeficiency (X-SCID) developed T-cell leukaemia [107]. Indeed, among the most promising areas of gene therapy is RNA interference (RNAi), which employs siRNAs [108]. Antisense RNA (asRNA) was initially utilized for gene silencing in rats, and this work found that long dsRNA triggered selective mRNA degradation in C. elegans. The sense and antisense strands within dsRNA produced up to tenfold quieter than either strand alone, resulting in Post Transcriptional Gene Silencing (PTGS). After RNAi was discovered in lower eukaryotes, biomedical researchers found it also occurs in mammalian cells [109,110,111]. A new method for delivering siRNA into cells has been developed using a platform called cationic dendritic polyglycerol (dPG-PEI) NG (Table 1). This platform has demonstrated a similar transfection performance to the conventional 25 kDa branched polyethyleneimine (PEI), significantly reducing cytotoxicity. The NG synthesis method employs a thiol-Michael nano-precipitation approach, allowing sensitive contents to be added directly during the NG synthesis. pH-sensitive benzacetal linkages in the NG network help to release the content. The cationic NG platform represents a ready-for-use transfection solution that may be administered directly to cells without requiring complex polyplex production techniques. The new platform ensures that polyplexes and their exact sizes are specified irrespective of the preparation technique [112].
NG in cardiovascular diseases
These are the significant causes of death globally [113]. It covers a variety of diseases, including cerebrovascular, venous thromboembolism, peripheral artery, congenital and coronary heart disease [114]. Various factors, like genetics, hypertension, diabetes, and obesity, can cause cardiovascular diseases. Several researchers are exploring using NGs to deliver drugs to treat these diseases (Fig. 7). Multiple approaches are available to treat hypertension. Still, a novel therapy option has been found that can also treat pneumococcal pneumonia. The technique employs drug nanocarriers to provide an intranasal vaccine (Table 1) [115]. The novel therapy involves cationic-charged cholesteryl-pullulan NG bearing Pneumococcal surface-protein A (PspA) and Angiotensin 1 Receptor (AT1R) from the pneumococcal surface. AT1R antagonists efficiently decrease blood pressure, excluding side effects in rat models. PspA can trigger immunity against Streptococcus pneumoniae. This novel therapy has the potential to alter the treatment of hypertension and pneumococcal pneumonia. In-vivo studies have shown that immunization effectively avoids lethal pneumococcal infections by lowering blood pressure. Another study presents a distinct approach to treating hypertension using NG compositions [116]. The researchers developed NG of amphiphilic Karaya gum with a 3.24 propyl group substitution to distribute Bosentan monohydrate inside the colon selectively. In-vivo studies demonstrated that the NG could effectively lower blood pressure for up to 10 h following delivery, with the most significant decrease occurring after 8 h, reducing by approximately 31%. Besides hypertension, other potential cardiovascular disease treatments use NG-based drug delivery systems. This study developed an N-isopropylacrylamide-methyl methacrylate NGs to administer N, L-rhamnopyranosyl vincosamide and determine cardioprotective attributes. The study revealed that these NGs have intriguing cardioprotective capabilities [117]. In-vivo studies demonstrated that these structures could significantly reduce heart damage in the Dox-induced toxicity model. In further studies, researchers developed temperature-sensitive poly (N-isopropyl amine-co-acrylic acid) NGs containing cardiac stem cells of humans to treat myocardial infarction. These NGs were in-vivo against mouse and pig models, proving their capability to decrease scar size and maintain heart function without causing systemic inflammation [118].
Schematic representation of treatment of cardiovascular disease (myocardial infection) using NGs. The figure was developed using BioRender (www.biorender.com) (accessed 7 November 2023)
NG in wound healing
The wound healing process consists of four phases: inflammation, proliferation, production of the extracellular matrix components, and remodelling [119, 120]. This biological process involves various elements, including keratinocytes, platelets, immunological cells, fibroblasts, and microvascular cells [121]. Historically, various compounds, especially those derived from plants with medicinal properties, have been utilized to accelerate wound healing [122]. Nanocarrier-based techniques, like NGs, have been extensively researched to enhance treatment effectiveness and targeted delivery of active substances. During the process of wound healing, there is a possibility of infection. Researchers have developed chitosan-based NGs packed with an antibacterial drug called silver sulfadiazine to tackle this issue. These NGs were then compared to commercial formulations containing the same chemical (Table 1) [123]. The results of in-vivo experiments have shown that the NGs are an effective treatment for burn wounds. The concentration of silver sulfadiazine necessary to accomplish this effect is lesser than that of commercial formulations [124]. Their research created a lysine-based NG with the antiseptic/disinfectant chemical chlorhexidine diacetate. The loaded NGs were combined with HGs containing methacrylate methoxy PEG and aminoethyl methacrylate HA. In-vivo studies have shown that HGs containing loaded NGs possess strong antibacterial capabilities, as no bacterial biofilm was detected.
Additionally, these HGs were observed to have a quick hemostasis outcome, hastening the healing process. Researchers have also explored alternative methods of controlling inflammation during wound healing. Scientists synthesized gellan-cholesterol NGs carrying baicalin, a flavone often used to treat inflammatory conditions [125]. In-vivo studies demonstrated that these NGs suppressed several inflammatory markers, such as TNF- and myeloperoxidase, more efficiently than marketed formulations and baicalin in phosphate buffer solution. Natural compounds, like curcumin, have demonstrated remarkable wound-healing properties. In another study, researchers created NGs containing fish-scale collagen-HPMC to treat related diseases [126]. In-vivo, studies have shown that combining collagen with curcumin improves wound tightening and reduces irritation symptoms compared to alternative curcumin preparations. IL-2 is a different significant molecule in the healing process because of its positive effect on T-lymphocyte formation. The researchers designed a chitosan-based NG containing IL-2 [127], which provided exciting in-vivo results revealing a reduction in malondialdehyde, a lipid peroxidation biological indicator, and a rise in glutathione concentration. This renowned antioxidant had positive results towards wound recovery applications. In a different study, researchers developed heparin-modified pluronic NGs containing the VEGF195 and BFGF genes, stimulating wound site neovascularization [128]. In-vivo studies revealed that this composition effectively increased endothelial differentiation of cells and neovascularization.
NG in bone regeneration
Bone diseases constitute one of the most prevalent causes of disability globally. Fractures, osteoporosis, and tumours are among the pathologies that cause bone diseases (Table 1) [129]. When bones are healthy, they may reestablish themselves without forming tissue with scars [130]. Fortunately, specialized treatments are frequently needed, like the application of bone alternatives with osteoconductive and osteoinductive properties, as well as the utilization of cells such as mesenchymal stem cells (human), growth factors including bone morphogenic proteins (BMP), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelets-derived growth factor (PDGF) [129]. In this instance, scientists have developed pullulan NGs containing cholesteryl and acryloyl [131]. Recombinant human FGF18, which is used to enhance the activity of low BMP2 doses, and recombinant human BMP2 were produced using these frameworks to generate fast degradable HGs. In in-vivo studies, these formulations induced bone regeneration more efficiently than free BMP2 or a mixture of independent BMP2 and FGF18. The study showed an alternative method for using BMP2 to induce bone healing [132]. To deliver BMP2, the investigators combined polycaprolactone (PCL) with redox-sensitive c-6A PEG-PCL NG (Table 1). This combination yields nanofibers with a core–shell configuration. In-vivo investigations revealed that the NGs controlled distribution of BMP2 might promote bone defect repair.
Similarly, in a different research p-(N-isopropylacrylamide-co-butyl methylacrylate), NGs were designed to develop moieties that function as carriers for delivering mesoporous bioactive surface. The findings (in-vivo) indicate that the bioactive glass-loaded NG-based framework may aid in the repair of femur deformity in osteoporotic animals. The W9-peptide, a TNF-α and receptor activator of kappa beta antagonist, is a potent molecule that has the potential to accelerate bone regeneration. Scientists developed a CHP NG that delivers the W9 peptide [133]. Their research concluded that this framework might prevent the loss of bone in bone resorption models in-vivo. PGE2, a nonpeptide anabolic molecule, may also help with bone rebuilding. The substance's high dosage and short half-life lead to adverse effects. Researchers produced a CHP NG for PGE2 to address these issues [134]. In in-vivo assessments, PGE2 encouraged new bone development when paired with an HG sphere that cross-linked NGs.
NG in psoriasis
Psoriasis is a persistent, inflammatory skin disorder that affects 1–3% of the global population [135]. Because T cells in the epidermis and dermis are activated, it is an immunologically mediated illness [136]. Traditionally, various dosage forms of MTX, retinoids, and cyclosporin are used to treat psoriasis. Various NG preparations have been developed to distribute this type of medication effectively. In this work, scientists proposed a vital method to address this issue [137]. They formulate chitin-based NGs containing clobetasol (CLCNG) for skin application (Table 1). In their study, they compared the effectiveness of prepared NGs to commercial costate cream, and results suggested that CLC NG attained equal anti-psoriatic properties with reduced skin irritation, which made it a suitable choice for skin application.
Moreover, the same team developed an MTX-loaded chitin NG for topical use (Table 1) [138]. They achieved an overall Psoriatic Area and Severity Index (PASI) reduction of 73.11–89.22% (depending on drug dose) using these NG formulations, which was greater than the ideal reduced percentage (73–75%) for taking into account them clinically valuable, demonstrating anti-psoriatic property. They also compared their simplicity for use with a commercial MTX gel, which showed a lower PASI drop and no adverse induction. In a second experiment, they discovered that their MC NG outperformed traditional MTX oral tablets because of their lower toxicity induction [139]. Babchi oil, a naturally existing essential oil with fewer adverse effects than other produced medications, is another helpful constituent in psoriasis treatment. Cyclodextrin-based NGs carrying Babchi oil for application on the skin were developed to test their efficacy for psoriasis therapy by comparing their effectiveness to the native Babchi oil gel [140]. Their in-vivo investigations revealed that these NG were effective against psoriasis without producing visible skin irritation, inflammation, or erythema. Earlier studies indicated that MiRNA-210 plays a crucial role in this type of disease. A high-density lipoprotein NG carrying miR-210 antisense was developed, and its efficiency in reducing inflammation comparable to psoriasis in mice was demonstrated in the study, indicating its potential use in topical treatments [141].
NG in diabetes
MIT and Boston Children's Hospital researchers are developing a self-operating insulin delivery system utilizing a unique nanotech technique comprising just one NG injection stabilizing blood glucose levels for up to 10 days. Due to its glucose sensitivity, the NG can monitor glucose levels and release insulin as necessary. The MIT method uses NG of a combination of oppositely charged dextran NPs, which are attracted to one another electrostatically and help the gel maintain its mechanical consistency. The inner core of the NPs comprises glucose oxidase, modified dextran, and insulin. When exposed to high blood glucose levels, the enzyme transforms glucose into gluconic acid. The dextran spheres are broken down by the gluconic acid that has thus been generated, which also releases insulin, bringing the blood glucose level back to normal. Due to biocompatibility, Dextran and gluconic acid eventually disintegrate in the body [142, 143]. In recent years, a poly (4-vinylphenylboronic acid-co-2-(dimethylamino) ethyl acrylate) [p(VPBADMAEA)] silver NP NG with insulin loading has been developed (Table 1). In the research, the polymer-bound Ag NPs were given the glucose-sensitive p(VPBADMAEA) shell, which caused the Ag NPs to react to glucose. The glucose-responsive polymer, p(VPBADMAEA), detects any variation in the concentration of glucose in the blood throughout a therapeutically meaningful range (0–30 mM) and converts this variation into an optical signal that is recognized by the optically responsive silver core (10 ± 3 nm) [144].
Challenges and future perspective
NG drug delivery systems represent a promising frontier in medical science, addressing challenges in conventional drug administration. NG offers a unique platform for controlled drug release, enhancing therapeutic efficacy while minimizing side effects. However, their widespread application faces several challenges. One primary obstacle is the intricate design required to optimize drug encapsulation and release kinetics. Achieving a balance between stability and responsiveness is critical to ensuring the NGs effectively deliver drugs to target sites.
Moreover, the potential toxicity of nanomaterials and their long-term effects on the body demand a thorough investigation and regulatory scrutiny. Another challenge involves the scalability of NG production. Developing cost-effective manufacturing processes that maintain consistent quality poses a hurdle in translating these innovations from the lab to large-scale clinical applications. Despite these challenges, the future perspective of NG drug delivery is highly promising. The versatility of NGs allows for tailored solutions to treat various diseases. In oncology, for instance, NGs can enhance the specificity of chemotherapy, redutreatcing damage to healthy tissues. Neurological diseases may benefit from targeted drug delivery across the BBB, improving treatment outcomes. Moreover, the advent of smart NGs, responsive to specific physiological cues, further amplifies their therapeutic potential [169, 170].
As research progresses, addressing challenges and refining NG technologies will propel them into mainstream medical practice, revolutionizing drug delivery and significantly improving patient outcomes across a spectrum of diseases. The collaboration between researchers, clinicians, and regulatory bodies will be instrumental in realizing the full potential of NG drug delivery systems in the future of medicine [83, 171,172,173,174,175].
NG: Status of patents for treatment of various diseases and their marketed products
Conclusion
NGs, versatile nanoscale structures, exhibit immense potential in revolutionizing medical treatments across various diseases. Their unique properties, such as high surface area and tunable drug release kinetics, make them promising candidates for targeted drug delivery. In oncology, NGs have shown remarkable efficacy by delivering chemotherapeutic agents directly to cancer cells, minimizing collateral damage to healthy tissues. The application of NGs extends beyond oncology; they have successfully treated inflammatory diseases, tumours, liver disease, SCI, infections, cardiovascular diseases, hyperthermia, diabetes, and neurological diseases, and also in the biomedical field. Their adaptability allows customization to suit specific therapeutic needs, heralding a new era in precision medicine. However, the journey from the laboratory to clinical implementation is fraught with challenges. One significant hurdle is ensuring the biocompatibility and safety of NGs. Ethical concerns surrounding the long-term impact of NGs on the human body necessitate thorough investigation.
Moreover, scalability and cost-effectiveness are addressed to make these innovations accessible on a global scale. The ongoing clinical trials involving NGs are crucial milestones to determining their real-world viability. Researchers are diligently evaluating their performance, side effects, and therapeutic impact. Looking ahead, the future of NGs appears bright. Continued research and advancements in nanotechnology promise to overcome current challenges, fostering widespread adoption of NG-based therapies. NGs may be pivotal in this paradigm shift, offering precise and efficient strategies for many medical conditions.
In conclusion, NGs represent a groundbreaking innovation with far-reaching implications for medical science. While challenges persist, ongoing clinical trials and a commitment to rigorous research pave the way for a future where NGs become indispensable tools in the fight against various diseases, offering hope for more effective, targeted, and controlled treatments.
Data availability statement
Data will be available on request.
Abbreviations
- TDDS:
-
Targeted drug delivery system
- Dox:
-
Doxorubicin
- BBB:
-
Blood-brain barrier
- CDDS:
-
Controlled drug delivery system
- CNS:
-
Central nerovous system
- NG:
-
Nenogel
- NGs:
-
Nenogels
- CNGs:
-
Composite NGs
- HG:
-
Hydrogel
- HGs:
-
Hydrogels
- NP:
-
Nanoparticle
- NPs:
-
Nanoparticles
- MTX:
-
Methotrexate
References
Su H, Wang Y, Liu S, Wang Y, Liu Q, Liu G, et al. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm Sin B. 2019;9:49–58.
Cuggino JC, Blanco ERO, Gugliotta LM, Alvarez Igarzabal CI, Calderón M. Crossing biological barriers with nanogels to improve drug delivery performance. J Control Release. 2019;307:221–46.
Kesharwani P, Gorain B, Low SY, Tan SA, Ling ECS, Lim YK, et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res Clin Pract. 2018;136:52–77.
Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205.
Gupta J, Ahuja A, Gupta R. Green Approaches for Cancers Management: An Effective Tool for Health Care. Anticancer Agents Med Chem. 2022;22:101–14.
Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8:233–78.
Banerjee R. Nanotechnology in drug delivery: Present status and a glimpse into the future. Ther Deliv. 2018;9:231–2.
Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017;24:539–57.
Uthaman S, Maya S, Jayakumar R, Cho CS, Park IK. Carbohydrate-based nanogels as drug and gene delivery systems. J Nanosci Nanotechnol. 2014;14:694–704.
Akiyama E, Morimoto N, Kujawa P, Ozawa Y, Winnik FM, Akiyoshi K. Self-assembled nanogels of cholesteryl-modified polysaccharides: Effect of the polysaccharide structure on their association characteristics in the dilute and semidilute regimes. Biomacromol. 2007;8:2366–73.
Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–46.
Zhu Y, Yang B, Chen S, Du J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog Polym Sci. 2017;64:1–22.
Iqbal S, Blenner M, Alexander-Bryant A, Larsen J. Polymersomes for therapeutic delivery of protein and nucleic acid macromolecules: From design to therapeutic applications. Biomacromol. 2020;21:1327–50.
Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851.
Sekine Y, Moritani Y, Ikeda-Fukazawa T, Sasaki Y, Akiyoshi K. A hybrid hydrogel biomaterial by nanogel engineering: Bottom-up design with nanogel and liposome building blocks to develop a multidrug delivery system. Adv Healthc Mater. 2012;1:722–8.
Jiang L, Zhou Q, Mu K, Xie H, Zhu Y, Zhu W, et al. pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials. 2013;34:7418–28.
Rigogliuso S, Sabatino MA, Adamo G, Grimaldi N, Dispenza C, Ghersi G. Polymeric nanogels: Nanocarriers for drug delivery application. Chem Eng Trans. 2012;27:247–52.
Almoshari YH. Novel hydrogels for topical applications: An updated comprehensive review based on source. Gels (Basel, Switzerland). 2022;8(3):174.
Tiwari G, Tiwari R, Bannerjee S, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2:2–11.
Tibbitt MW, Dahlman JE, Langer R. Emerging Frontiers in Drug Delivery. J Am Chem Soc. 2016;138:704–17.
Ramasamy T, Ruttala HB, Gupta B, Poudel BK, Choi HG, Yong CS, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J Control Release. 2017;258:226–53.
Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J Control Release. 2013;166:182–94.
Kumar B, Jalodia K, Kumar P, Gautam HK. Recent advances in nanoparticle-mediated drug delivery. J Drug Deliv Sci Technol. 2017;41:260–8.
Srivastava VK, Singh N, Gupta T, Mishra U. Sustained and controlled drug delivery system - as a part of modified release dosage form. Int J Res Pharm Nano Sci. 2015;4:347–64.
Wen H, Jung H, Li X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015;17:1327–40.
Gujral S, Khatri S. A review on basic concept of drug targeting and drug carrier system. Int J Adv pharmacy, Biol Chem. 2013;2:130–6.
Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418–29.
Ryu JH, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. Self-cross-linked polymer nanogels: A versatile nanoscopic drug delivery platform. J Am Chem Soc. 2010;132:17227–35.
Yadav KS, Chuttani K, Mishra AK, Sawant KK. Effect of size on the biodistribution and blood clearance of etoposide-loaded PLGA nanoparticles. PDA J Pharm Sci Technol. 2011;65:131–9.
Murphy EA, Majeti BK, Mukthavaram R, Acevedo LM, Barnes LA, Cheresh DA. Targeted nanogels: A versatile platform for drug delivery to tumors. Mol Cancer Ther. 2011;10:972–82.
Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, Craft J, et al. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J Clin Invest. 2013;123:1741–9.
Vinogradov SV. Polymeric nanogel formulations of nucleoside analogs. Expert Opin Drug Deliv. 2007;4:5–17.
Suhail M, Rosenholm JM, Minhas MU, Badshah SF, Naeem A, Khan KU, et al. Nanogels as drug-delivery systems: a comprehensive overview. Ther Deliv. 2019;10:697–717. https://doi.org/10.4155/tde-2019-0010.
Bencherif SA, Siegwart DJ, Srinivasan A, Horkay F, Hollinger JO, Washburn NR, et al. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials. 2009;30:5270–8.
Soni G, Yadav KS. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc. 2016;24:133–9.
Khmelnitsky YL, Neverova IN, Gedrovich AV, Polyakov VA, Levashov AV, Martinek K. Catalysis by α-chymotrypsin entrapped into surface-modified polymeric nanogranules in organic solvent. Eur J Biochem. 1992;210:751–7.
Yan M, Ge J, Liu Z, Ouyang P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J Am Chem Soc. 2006;128:11008–9.
Akiyoshi K, Sasaki Y, Sunamoto J. Molecular chaperone-like activity of hydrogel nanoparticles of hydrophobized pullulan: Thermal stabilization with refolding of carbonic anhydrase B. Bioconjug Chem. 1999;10:321–4.
Lee Sh, Choi Sh, Kim Sh, Tg P. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: Swelling induced physical disruption of endosome by cold shock. J Control Release. 2008;125:25–32.
Coll Ferrer MC, Dastgheyb S, Hickok NJ, Eckmann DM, Composto RJ. Designing nanogel carriers for antibacterial applications. Acta Biomater. 2014;10:2105–11.
Coll Ferrer MC, Shuvaev VV, Zern BJ, Composto RJ, Muzykantov VR, Eckmann DM. ICAM-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS ONE. 2014;9:e102329.
Coll Ferrer MC, Ferrier RC, Eckmann DM, Composto RJ. A facile route to synthesize nanogels doped with silver nanoparticles. J Nanopart Res. 2012;15:1323.
Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6(2):252–273. https://www.academia.edu/3296321/Drug_delivery_to_the_central_nervous_system_a_review (accessed November 21, 2023).
Agnihotri TG, Jadhav GS, Sahu B, Jain A. Recent trends of bioconjugated nanomedicines through nose-to-brain delivery for neurological disorders. Drug Deliv Transl Res. 2022;12:3104–20.
Tao HQ, Meng Q, Li MH, Yu H, Liu MF, Du D, et al. HP-β-CD-PLGA nanoparticles improve the penetration and bioavailability of puerarin and enhance the therapeutic effects on brain ischemia-reperfusion injury in rats. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:61–70.
Azadi A, Hamidi M, Khoshayand MR, Amini M, Rouini MR. Preparation and optimization of surface-treated methotrexate-loaded nanogels intended for brain delivery. Carbohydr Polym. 2012;90:462–71.
Mishra MK, Gupta J, Gupta R. Self-assemble amphiphilic PEO-PPO-PEO Tri-block co-polymeric methotrexate nanomicelles to combat MCF7 cancer cells. Curr Drug Deliv. 2021;18:794–804.
Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2003;15:50–60.
Vinogradov SV, Poluektova LY, Makarov E, Gerson T, Senanayake MT. Nano-NRTIs: Efficient inhibitors of HIV type-1 in macrophages with a reduced mitochondrial toxicity. Antivir Chem Chemother. 2010;21:1–14.
Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J Control Release. 2005;107:143–57.
Baklaushev VP, Nukolova NN, Khalansky AS, Gurina OI, Yusubalieva GM, Grinenko NP, et al. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. Drug Deliv. 2015;22:276–85.
Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Pract. 2017;4:127–9.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:1–35.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–96. https://doi.org/10.1038/s41573-018-0006-z.
Ghavami S, Bardajee GR, Mirshokraie A, Didehban K. A novel pH, thermo, and magnetic responsive hydrogel nanocomposite containing nanogel for anticancer drug delivery. Polym Sci - Ser B. 2019;61:376–86.
Zhang F, Gong S, Wu J, Li H, Oupicky D, Sun M. CXCR4-targeted and redox responsive dextrin nanogel for metastatic breast cancer therapy. Biomacromol. 2017;18:1793–802.
Li D, Xu W, Liu H. Fabrication of chitosan functionalized dual stimuli-responsive injectable nanogel to control delivery of doxorubicin. Colloid Polym Sci. 2023;301:879–91.
Singh S, Maurya P, Rani S, Mishra N, Nisha R, Singh P, et al. Development of doxorubicin hydrochloride–loaded whey protein nanoparticles and its surface modification with N-acetyl cysteine for triple-negative breast cancer. Drug Deliv Transl Res. 2022;12:3047–62.
Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, et al. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367:330–5.
Fujii H, Shin-Ya M, Takeda S, Hashimoto Y, Mukai SA, Sawada SI, et al. Cycloamylose-nanogel drug delivery system-mediated intratumor silencing of the vascular endothelial growth factor regulates neovascularization in tumor microenvironment. Cancer Sci. 2014;105:1616–25.
Zhang Y, Wang F, Li M, Yu Z, Qi R, Ding J, et al. Self-stabilized hyaluronate nanogel for intracellular codelivery of doxorubicin and cisplatin to osteosarcoma. Adv Sci. 2018;5:1700821.
Seok HY, Sanoj Rejinold N, Lekshmi KM, Cherukula K, Park IK, Kim YC. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J Control Release. 2018;280:20–30.
Huang K, Shi B, Xu W, Ding J, Yang Y, Liu H, et al. Reduction-responsive polypeptide nanogel delivers antitumor drug for improved efficacy and safety. Acta Biomater. 2015;27:179–93.
Zheng Y, Lv XD, Xu Y, Cheng X, Wang X, Tang R. pH-sensitive and pluronic-modified pullulan nanogels for greatly improved antitumor in vivo. Int J Biol Macromol. 2019;139:277–89.
Cheng X, Qin J, Wang X, Zha Q, Yao W, Fu S, et al. Acid-degradable lactobionic acid-modified soy protein nanogels crosslinked by ortho ester linkage for efficient antitumor in vivo. Eur J Pharm Biopharm. 2018;128:247–58.
Peng S, Wang H, Zhao W, Xin Y, Liu Y, Yu X, et al. Zwitterionic Polysulfamide Drug Nanogels with Microwave Augmented Tumor Accumulation and On-Demand Drug Release for Enhanced Cancer Therapy. Adv Funct Mater. 2020;30:2001832.
Xu L, Su T, Xu X, Zhu L, Shi L. Platelets membrane camouflaged irinotecan-loaded gelatin nanogels for in vivo colorectal carcinoma therapy. J Drug Deliv Sci Technol. 2019;53:101190.
de Oliveria Andrade LJ, Argemiro D’Oliveira J, Melo RC, De Souza EC, Silva CA, Paraná R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1:33–7.
Liu R, Ye H, Xiong X, Liu H. Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater Chem Phys. 2010;121:432–9.
Tam K. The roles of doxorubicin in hepatocellular carcinoma. ADMET DMPK. 2013;1:29–44.
Gupta R, Kadhim MM, Turki Jalil A, Obayes AM, Aminov Z, Alsaikhan F, et al. Multifaceted role of NF-κB in hepatocellular carcinoma therapy: Molecular landscape, therapeutic compounds and nanomaterial approaches. Environ Res. 2023;228:115767.
Gupta R, Gupta J, Roy S. Exosomes: Key Players for Treatment of Cancer and Their Future Perspectives. Assay Drug Dev Technol. 2024. https://doi.org/10.1089/ADT.2023.026.
Huynh H, Chow PKH, Soo KC. AZD6244 and doxorubicin induce growth suppression and apoptosis in mouse models of hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2468–76.
Shi Y, Moon M, Dawood S, McManus B, Liu PP. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36:296–305.
Arunraj TR, Sanoj Rejinold N, Ashwin Kumar N, Jayakumar R. Bio-responsive chitin-poly(l-lactic acid) composite nanogels for liver cancer. Colloids Surfaces B Biointerfaces. 2014;113:394–402.
Sabitha M, Sanoj Rejinold N, Nair A, Lakshmanan VK, Nair SV, Jayakumar R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr Polym. 2013;91:48–57.
Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: Results of thermoradiotherapy versus radiotherapy. Int J Hyperth. 1990;6:479–86.
Overgaard J, Gonzalez Gonzalez D, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, et al. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia. 1996;12:3–20.
Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163–9.
Hildebrandt B, Wust P, Rau B, Schlag P, Riess H, Van Der Zee J, et al. Regional hyperthermia for rectal cancer [9] (multiple letters). Lancet. 2000;356:771–2.
Needham D, Anyarambhatla G, Kong G, Dewhirst M. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–201.
Carter SK. Adriamycin-a review. J Natl Cancer Inst. 1975;55:1265–74.
Xiong W, Wang W, Wang Y, Zhao Y, Chen H, Xu H, et al. Dual temperature/pH-sensitive drug delivery of poly(N-isopropylacrylamide-co-acrylic acid) nanogels conjugated with doxorubicin for potential application in tumor hyperthermia therapy. Colloids Surf B Biointerfaces. 2011;84:447–53.
Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57.
Vismara I, Papa S, Veneruso V, Mauri E, Mariani A, De Paola M, et al. Selective modulation of A1 astrocytes by drug-loaded nano-structured gel in spinal cord injury. ACS Nano. 2020;14:360–71.
Nazemi Z, Nourbakhsh MS, Kiani S, Heydari Y, Ashtiani MK, Daemi H, et al. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145–58.
Papa S, Caron I, Erba E, Panini N, De Paola M, Mariani A, et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13–24.
Wang XJ, Shu GF, Xu XL, Peng CH, Lu CY, Cheng XY, et al. Combinational protective therapy for spinal cord injury medicated by sialic acid-driven and polyethylene glycol based micelles. Biomaterials. 2019;217:119326.
Li J, Liu Y, Xu H, Fu Q. Nanoparticle-delivered IRF5 siRNA facilitates M1 to M2 transition, reduces demyelination and neurofilament loss, and promotes functional recovery after spinal cord injury in mice. Inflammation. 2016;39:1704–17.
Vashist A, Kaushik A, Vashist A, Bala J, Nikkhah-Moshaie R, Sagar V, et al. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov Today. 2018;23:1436–43.
Tsao CT, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, et al. Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy. Biomacromol. 2014;15:2656–62.
Giacomazza D, Picone P, Ditta L, Antonietta Sabatino M, Militello V, Luigi San Biagio P, et al. Biodistribution of Insulin-nanogels in mouse: A preliminary study for the treatment of alzheimer’s disease. Biophysj. 2017;112:137a.
Rossi UG, Ierardi AM, Cariati M. Pictorial neurological disease acute ischemic stroke. Acta Neurol Taiwan. 2019;28:84–5.
Ali MR, Salim Hossain M, Islam MA, Saiful Islam Arman M, Sarwar Raju G, Dasgupta P, et al. Aspect of thrombolytic therapy: A review. Sci World J. 2014;2014:586510.
Cui W, Liu R, Jin H, Lv P, Sun Y, Men X, et al. pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J Control Release. 2016;225:53–63.
Cui W, Liu R, Jin H, Huang Y, Liu W, He M. The protective effect of polyethylene glycol-conjugated urokinase nanogels in rat models of ischemic stroke when administrated outside the usual time window. Biochem Biophys Res Commun. 2020;523:887–93.
Jin H, Tan H, Zhao L, Sun W, Zhu L, Sun Y, et al. Ultrasound-triggered thrombolysis using urokinase-loaded nanogels. Int J Pharm. 2012;434:384–90.
Teng Y, Jin H, Nan D, Li M, Fan C, Liu Y, et al. In vivo evaluation of urokinase-loaded hollow nanogels for sonothrombolysis on suture embolization-induced acute ischemic stroke rat model. Bioact Mater. 2018;3:102–9.
Chabner BA, Allegra CJ, Curt GA, Clendeninn NJ, Baram J, Koizumi S, et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest. 1985;76(3):907–12. https://doi.org/10.1172/JCI112088.
Saraf S, Jain SK. pH-sensitive liposomes bearing a chemotherapeutic agent and a natural apoptosis modulator for effective intracellular delivery to the solid tumor. Drug Deliv Transl Res. 2023;13(12):2961–81. https://doi.org/10.1007/s13346-023-01364-1.
Farber S, Toch R, Sears EM, Pinkel D. Advances in chemotherapy of cancer in man. Adv Cancer Res. 1956;4:1–71.
Rau R, Schleusser B, Herborn G, Karger T. Long-term treatment of destructive rheumatoid arthritis with methotrexate. J Rheumatol. 1997;24:1881–9.
Baggott JE, Vaughn WH, Hudson BB. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J. 1986;236:193–200.
Allegra CJ, Drake JC, Jolivet J, Chabner BA. Inhibition of phosphoribosyl aminoimidazole carboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci U S A. 1985;82:4881–5.
Singka GSL, Samah NA, Zulfakar MH, Yurdasiper A, Heard CM. Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Eur J Pharm Biopharm. 2010;76:275–81.
Singh P, Muhammad I, Nelson NE, Tran KTM, Vinikoor T, Chorsi MT, et al. Transdermal delivery for gene therapy. Drug Deliv Transl Res. 2022;12:2613–33.
Milhavet O, Gary DS, Mattson MP. RNA interference in biology and medicine. Pharmacol Rev. 2003;55:629–48.
Rutz S, Scheffold A. Towards in vivo application of RNA interference - New toys, old problems. Arthritis Res Ther. 2004;6:78–85.
Grishok A, Mello CC. RNAi (Nematodes: Caenorhabditis elegans). Adv Genet. 2002;46:339–60.
Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404(6775):293–6.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8.
Dimde M, Neumann F, Reisbeck F, Ehrmann S, Cuellar-Camacho JL, Steinhilber D, et al. Defined pH-sensitive nanogels as gene delivery platform for siRNA mediated in vitro gene silencing. Biomater Sci. 2017;5:2328–36.
Dimmeler S. Cardiovascular disease review series. EMBO Mol Med. 2011;3:697.
Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6:2048004016687211.
Azegami T, Yuki Y, Hayashi K, Hishikawa A, Sawada SI, Ishige K, et al. Intranasal vaccination against angiotensin II type 1 receptor and pneumococcal surface protein A attenuates hypertension and pneumococcal infection in rodents. J Hypertens. 2018;36:387–94.
Laha B, Das S, Maiti S, Sen KK. Novel propyl karaya gum nanogels for bosentan: In vitro and in vivo drug delivery performance. Colloids Surfaces B Biointerfaces. 2019;180:263–72.
Cheraghi M, Namdari M, Daraee H, Negahdari B. Cardioprotective effect of magnetic hydrogel nanocomposite loaded N, α-L-rhamnopyranosyl vincosamide isolated from Moringa oleifera leaves against doxorubicin-induced cardiac toxicity in rats: in vitro and in vivo studies. J Microencapsul. 2017;34:335–41.
Tang J, Cui X, Caranasos TG, Hensley MT, Vandergriff AC, Hartanto Y, et al. Heart repair using nanogel-encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano. 2017;11:9738–49.
Moeini A, Pedram P, Makvandi P, Malinconico M, Gomez DG. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr Polym. 2020;233:115839.
Gonzalez ACDO, Andrade ZDA, Costa TF, Medrado ARAP. Wound healing - A literature review. An Bras Dermatol. 2016;91:614–20.
Grimaudo MA, Concheiro A, Alvarez-Lorenzo C. Nanogels for regenerative medicine. J Control Release. 2019;313:148–60.
Hajialyani M, Tewari D, Sobarzo-Sánchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int J Nanomedicine. 2018;13:5023–43.
El-Feky GS, El-Banna ST, El-Bahy GS, Abdelrazek EM, Kamal M. Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydr Polym. 2017;177:194–202.
Zhu J, Li F, Wang X, Yu J, Wu D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl Mater Interfaces. 2018;10:13304–16.
Manconi M, Manca ML, Caddeo C, Cencetti C, di Meo C, Zoratto N, et al. Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur J Pharm Biopharm. 2018;127:244–9.
Pathan IB, Munde SJ, Shelke S, Ambekar W, Mallikarjuna SC. Curcumin loaded fish scale collagen-HPMC nanogel for wound healing application: Ex-vivo and In-vivo evaluation. Int J Polym Mater Polym Biomater. 2019;68:165–74.
Aslan C, Celebi N, Degim IT, Atak A, Ozer C. Development of interleukin-2 loaded chitosan-based nanogels using artificial neural networks and investigating the effects on wound healing in rats. AAPS PharmSciTech. 2017;18:1019–30.
Yang HN, Choi JH, Park JS, Jeon SY, Park KD, Park KH. Differentiation of endothelial progenitor cells into endothelial cells by heparin-modified supramolecular pluronic nanogels encapsulating bFGF and complexed with VEGF165 genes. Biomaterials. 2014;35:4716–28.
Iaquinta MR, Mazzoni E, Manfrini M, D’Agostino A, Trevisiol L, Nocini R, et al. Innovative biomaterials for bone regrowth. Int J Mol Sci. 2019;20:618.
Ansari M. Bone tissue regeneration: Biology, strategies and interface studies. Prog Biomate. 2019;8:223–37.
Fujioka-Kobayashi M, Ota MS, Shimoda A, Nakahama KI, Akiyoshi K, Miyamoto Y, et al. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials. 2012;33:7613–20.
Gong T, Liu T, Zhang L, Ye W, Guo X, Wang L, et al. Design redox-sensitive drug-loaded nanofibers for bone reconstruction. ACS Biomater Sci Eng. 2018;4:240–7.
Alles N, Soysa NS, Hussain MA, Tomomatsu N, Saito H, Baron R, et al. Polysaccharide nanogel delivery of a TNF-α and RANKL antagonist peptide allows systemic prevention of bone loss. Eur J Pharm Sci. 2009;37:83–8.
Kato N, Hasegawa U, Morimoto N, Saita Y, Nakashima K, Ezura Y, et al. Nanogel-based delivery system enhances PGE2 effects on bone formation. J Cell Biochem. 2007;101:1063–70.
Suresh PK, Singh P, Saraf S. Novel topical drug carriers as a tool for treatment of psoriasis: Progress and advances. African J Pharm Pharmacol. 2013;7:138–47.
Masson W, Lobo M, Molinero G. Psoriasis and Cardiovascular Risk: A Comprehensive Review. Adv Ther. 2020;37:2017–33.
Panonnummal R, Jayakumar R, Sabitha M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur J Pharm Sci. 2017;96:193–206.
Panonnummal R, Sabitha M. Anti-psoriatic and toxicity evaluation of methotrexate loaded chitin nanogel in imiquimod induced mice model. Int J Biol Macromol. 2018;110:245–58.
Panonnummal R, Jayakumar R, Anjaneyan G, Sabitha M. In vivo anti-psoriatic activity, biodistribution, sub-acute and sub-chronic toxicity studies of orally administered methotrexate loaded chitin nanogel in comparison with methotrexate tablet. Int J Biol Macromol. 2018;110:259–68.
Kumar S, Singh KK, Rao R. Enhanced anti-psoriatic efficacy and regulation of oxidative stress of a novel topical babchi oil (Psoralea corylifolia) cyclodextrin-based nanogel in a mouse tail model. J Microencapsul. 2019;36:140–55.
Feng H, Wu R, Zhang S, Kong Y, Liu Z, Wu H, et al. Topical administration of nanocarrier miRNA-210 antisense ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J Dermatol. 2020;47:147–54.
Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7:4194–201.
Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, et al. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7:6758–66.
Wu W, Mitra N, Yan ECY, Zhou S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano. 2010;4:4831–9.
Oh NM, Oh KT, Youn YS, Lee DK, Cha KH, Lee DH, et al. Poly(l-aspartic acid) nanogels for lysosome-selective antitumor drug delivery. Colloids Surfaces B Biointerfaces. 2013;101:298–306.
Sahu P, Kashaw SK, Kushwah V, Sau S, Jain S, Iyer AK. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorg Med Chem. 2017;25:4595–613.
Don TM, Lu KY, Lin LJ, Hsu CH, Wu JY, Mi FL. Temperature/pH/enzyme triple-responsive cationic protein/PAA-b-PNIPAAm nanogels for controlled anticancer drug and photosensitizer delivery against multidrug resistant breast cancer cells. Mol Pharm. 2017;14:4648–60.
Curcio M, Diaz-Gomez L, Cirillo G, Concheiro A, Iemma F, Alvarez-Lorenzo C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur J Pharm Biopharm. 2017;117:324–32.
Chang R, Tsai WB. Fabrication of photothermo-responsive drug-loaded nanogel for synergetic cancer therapy. Polym. 2018;10:1098.
Rancan F, Volkmann H, Giulbudagian M, Schumacher F, Stanko JI, Kleuser B, et al. Dermal delivery of the high-molecular-weight drug tacrolimus by means of polyglycerol-based nanogels. Pharm. 2019;11:394.
Guo Q, Zhang X. Synthesized of glucose-responsive nanogels labeled with fluorescence molecule based on phenylboronic acid by RAFT polymerization. J Biomater Sci Polym Ed. 2019;30:815–31.
Wang Y, Zheng J, Tian Y, Yang W. Acid degradable poly(vinylcaprolactam)-based nanogels with ketal linkages for drug delivery. J Mater Chem B. 2015;3:5824–32.
Xin F, Wei M, Jiang S, Gao Y, Nie J, Wu Y, et al. Design of hydrophilic photocleavage o-nitrobenzyl acrylate-modified nanogels with outstanding biocompatibility prepared by RAFT polymerization for drug carrier. Eur Polym J. 2020;122:109364.
Li C, Liu X, Liu Y, Huang F, Wu G, Liu Y, et al. Glucose and H2O2 dual-sensitive nanogels for enhanced glucose-responsive insulin delivery. Nanoscale. 2019;11:9163–75.
Zan M, Li J, Huang M, Lin S, Luo D, Luo S, et al. Near-infrared light-triggered drug release nanogels for combined photothermal-chemotherapy of cancer. Biomater Sci. 2015;3:1147–56.
Ma X, Zhang T, Qiu W, Liang M, Gao Y, Xue P, et al. Bioresponsive prodrug nanogel-based polycondensate strategy deepens tumor penetration and potentiates oxidative stress. Chem Eng J. 2021;420:127657.
Shah PP, Desai PR, Patel AR, Singh MS. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials. 2012;33:1607–17.
Chen J, He H, Deng C, Yin L, Zhong Z. Saporin-loaded CD44 and EGFR dual-targeted nanogels for potent inhibition of metastatic breast cancer in vivo. Int J Pharm. 2019;560:57–64.
Si X, Ma S, Xu Y, Zhang D, Shen N, Yu H, et al. Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic. J Control Release. 2020;320:83–95.
Zhang Q, Chen X, Geng S, Wei L, Miron RJ, Zhao Y, et al. Nanogel-based scaffolds fabricated for bone regeneration with mesoporous bioactive glass and strontium: In vitro and in vivo characterization. J Biomed Mater Res Part A. 2017;105:1175–83.
Yurdasiper A, Ertan G, Heard CM. Enhanced delivery of naproxen to the viable epidermis from an activated poly N-isopropylacrylamide (PNIPAM) Nanogel: Skin penetration, modulation of COX-2 expression and rat paw oedema. Nanomedicine Nanotechnology, Biol Med. 2018;14:2051–9.
Yeo J, Lee J, Yoon S, Kim WJ. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater Sci. 2020;8:1148–59.
Aminu N, Chan SY, Yam MF, Toh SM. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int J Pharm. 2019;570:118659.
Onishi H, Ikeuchi-Takahashi Y, Kawano K, Hattori Y. Preparation of chondroitin sulfate-glycyl-prednisolone conjugate nanogel and its efficacy in rats with ulcerative colitis. Biol Pharm Bull. 2019;42:1155–63.
Hoare T, Young S, Lawlor MW, Kohane DS. Thermoresponsive nanogels for prolonged duration local anesthesia. Acta Biomater. 2012;8:3596–605.
Rodrigues da Silva GH, Geronimo G, Ribeiro LNM, Guilherme VA, de Moura LD, Bombeiro AL, et al. Injectable in situ forming nanogel: A hybrid Alginate-NLC formulation extends bupivacaine anesthetic effect. Mater Sci Eng C. 2020;109:110608.
Liu Z, Qiao J, Nagy T, Xiong MP. ROS-triggered degradable iron-chelating nanogels: Safely improving iron elimination in vivo. J Control Release. 2018;283:84–93.
Wu T, Liao W, Wang W, Zhou J, Tan W, Xiang W, et al. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr Polym. 2018;197:403–13.
Manimaran V, Nivetha RP, Tamilanban T, Narayanan J, Vetriselvan S, Fuloria NK, et al. Nanogels as novel drug nanocarriers for CNS drug delivery. Front Mol Biosci. 2023;10:1232109.
Zhang Y, Zou Z, Liu S, Miao S, Liu H. Nanogels as novel nanocarrier systems for efficient delivery of CNS therapeutics. Front Bioeng Biotechnol. 2022;10:954470.
Attama AA, Nnamani PO, Onokala OB, Ugwu AA, Onugwu AL. Nanogels as target drug delivery systems in cancer therapy: A review of the last decade. Front Pharmacol. 2022;13:874510.
Jiao X, Peng X, Jin X, Liu N, Yu Y, Liu R, et al. Nano-composite system of traditional Chinese medicine for ocular applications: molecular docking and three-dimensional modeling insight for intelligent drug evaluation. Drug Deliv Transl Res. 2023;13(12):3132–44. https://doi.org/10.1007/s13346-023-01376-x.
Aldaais EA. A comprehensive review on the COVID-19 vaccine and drug delivery applications of interpenetrating polymer networks. Drug Deliv Transl Res. 2023;13(3):738–56. https://doi.org/10.1007/s13346-022-01254-y.
Giacalone G, Quaillet M, Huang N, Nicolas V, Boulogne C, Gillet C, et al. An injectable, nanostructured implant for the delivery of adenosine triphosphate: towards long-acting formulations of small, hydrophilic drugs. Drug Deliv Transl Res. 2024. https://doi.org/10.1007/s13346-024-01631-9
Ansari MD, Shafi S, Pandit J, Waheed A, Jahan RN, Khan I, et al. Raloxifene encapsulated spanlastic nanogel for the prevention of bone fracture risk via transdermal administration: Pharmacokinetic and efficacy study in animal model. Drug Deliv Transl Res. 2024;14(6):1635–47. https://doi.org/10.1007/s13346-023-01480-y.
Radosz M, Shen Y, Vankirk EA, Laramie WJ. Degradable nanogel for drug delivery. US 2015/0250899A1. 2015. Accessed 29 Nov 2023.
Radosz M, Shen Y, VanKirk EA, Murdoch WJ. Degradable nanogel for drug delivery. US 2007/0224164. 2007. Accessed 29 Nov 2023.
Vinod Labhasetwar SV, Saunthararajah Y. US Patent Application for AIRCRAFT Patent Application 20230286661, 2023 - Justia Patents Search. https://patents.justia.com/patent/20230286661 (accessed November 29, 2023).
Stansbury JW. Water compatible nanogel compositions. US 10,829,672. 2020. Accessed 29 Nov 2023.
Fahmy TM, Look M, Craft J. Nanolipogel comprising a polymeric matrix and a lipid shell. US 10,709,664. 2020. Accessed 29 Nov 2023.
Dash AK, Trickler WJ. Mucoadhesive nanoparticles for cancer treatment. US 8242165B2, 2012. Accessed 29 Nov 2023.
Li W, Cai X, Kim C, Sun G, Zhang Y, Deng R, et al. Graphene hydrogel and method for using the same. Nanoscale. 2013;3(3):1724–30. https://doi.org/10.1039/C0NR00932F.
Irvine DJ, Zheng Y, Tang L. Cell surface coupling of nanoparticles. US11261226B2, 2022. (accessed November 29, 2023) https://patents.google.com/patent/US11261226B2/en?q=(nanogel+for+cancer)&oq=nanogel+for+cancer+.
Jølck RI, Albrechtsen M, Bjerg LN, Andresen TL. Formulation of solid nano-sized particles in a gel-forming system. US10434192B2, 2019. (accessed November 29, 2023) https://patents.google.com/patent/US10434192B2/en?q=(nanogel+for+cancer)&oq=nanogel+for+cancer+
Yeoman RR, Winchurch RA. Targeted therapeutic nanoparticles. US9694085B2, 2017. (accessed November 29, 2023). https://patents.google.com/patent/US9694085B2/en?q=(nanogel+for+cancer+treatment)&oq=nanogel+for+cancer+treatment&page=5
Harald R, Tobias V, Burkhardt L, Nicola B. Hydrogel prodrugs. EP2906617B1, 2015. (accessed November 29, 2023). https://patents.google.com/patent/EP2906617B1/en?q=(nanogel+drug+incorporation+of+cancer+drug)&oq=nanogel+drug+incorporation+of+cancer+drug&page=3
Sinko PJ, Deshmukh M, Priya Anumolu SN, Menjoge AR, Gordon MK. Hydrogel formulation for dermal and ocular delivery. US9763968B2, 2017. (accessed November 29, 2023). https://patents.google.com/patent/US9763968B2/en?q=(nanogel+drug+incorporation+of+cancer+drug)&oq=nanogel+drug+incorporation+of+cancer+drug&page=6
Zheng Y, lrvine DJ, Tang L. Cell surface coupling of nanoparticles. AU2016305087B2, 2018. (accessed November 29, 2023). https://patents.google.com/patent/AU2016305087B2/en?q=(nanogel+drug+incorporation+diabetes)&oq=nanogel+drug+incorporation+for+diabetes
Cui Z, Dharmika SP, Lansakara P, Sandoval MA. Lipophilic monophosphorylated derivatives and nanoparticles. US2013/0131008A1, 2013. (accessed November 29, 2023). https://patents.google.com/patent/US20130131008A1/en?q=(nanogel+drug+incorporation+for+cancer)&oq=nanogel+drug+incorporation+for+cancer
Jhan HJ, Ho Hsiu-O, Sheu MT, Shen SC, Ho YS, Liu JJ. Thermosensitive injectable hydrogel for drug delivery. US9364545B2, 2016. (accessed November 29, 2023). https://patents.google.com/patent/US9364545B2/en?q=(nanogel+drug+incorporation+for+cancer)&oq=nanogel+drug+incorporation+for+cancer
Labhasetwar V, Vijayaraghavalu S, Nanogel-mediated drug delivery. US10729659B2, 2020. (accessed November 29, 2023). https://patents.google.com/patent/US10729659B2/en?q=(nanogel+drug+incorporation+for+cancer)&oq=nanogel+drug+incorporation+for+cancer&page=4
Chauhan S, Jaggi M, Yallapu MM. Magnetic nanoparticle formulations, methods for making such formulations, and methods for their use. US9642925B2, 2017. (accessed November 29, 2023). https://patents.google.com/patent/US9642925B2/en?q=(nanogel+drug+incorporation+for+cancer)&oq=nanogel+drug+incorporation+for+cancer&page=9
Scales CW. McCabe KP, Healy BM. Polymers and nanogel materials and methods for making and using the same. US11029539B2, 2021. (accessed November 29, 2023). https://patents.google.com/patent/US11029539B2/en?q=(nanogel+latest+patents)&oq=nanogel+latest+patents
Scales CW. McCabe KP, Healy BM. Polymers and nanogel materials and methods for making and using the same. US10502867B2, 2019. (accessed November 30, 2023). https://patents.google.com/patent/US10502867B2/en?q=(nanogel)&oq=nanogel+
Nair DP, Simberg D, Saraswathy M. Targeted nanogels for urinary bladder therapies. US20200030244A1, 2020. (accessed November 30, 2023). https://patents.google.com/patent/US20200030244A1/en?q=(nanogel+disorder)&oq=nanogel+for+disorder&page=3
Oxalgin Nano Gel: Buy tube of 30.0 gm Gel at best price in India | 1mg. https://www.1mg.com/otc/oxalgin-nano-gel-otc159302 (accessed November 30, 2023).
Adalene Nanogel Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/adalene-nanogel-gel-134348 (accessed November 30, 2023).
Zeldinac Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/zeldinac-nano-gel-333685 (accessed November 30, 2023).
Acnesol A Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/acnesol-a-nano-gel-142805 (accessed November 30, 2023).
Zyclin Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/zyclin-nano-gel-162581 (accessed November 30, 2023).
S-Shield Sunscreen Lotion 30: Buy tube of 50.0 gm Lotion at best price in India | 1mg. https://www.1mg.com/otc/s-shield-sunscreen-lotion-30-otc668633 (accessed November 30, 2023).
D F O Nano Gel: Buy tube of 30.0 gm Gel at best price in India | 1mg. https://www.1mg.com/otc/d-f-o-nano-gel-otc323312 (accessed November 30, 2023).
Silvercure Nanogel: Buy packet of 50.0 gm Gel at best price in India | 1mg. https://www.1mg.com/otc/silvercure-nanogel-otc347316?qv=1&iv=1 (accessed November 30, 2023).
Uv-Aid Nano Gel Spf 30+: Buy tube of 50.0 gm Gel at best price in India | 1mg. https://www.1mg.com/otc/uv-aid-nano-gel-spf-30-otc438841 (accessed November 30, 2023).
Warflam Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/warflam-nano-gel-807215 (accessed November 30, 2023).
Turnup Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/turnup-nano-gel-680740 (accessed November 30, 2023).
Fasiclo 4X Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/fasiclo-4x-nano-gel-726227 (accessed November 30, 2023).
Acgel Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/acgel-nano-gel-308447 (accessed November 30, 2023).
Skinlite Ever Nano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/skinlite-ever-nano-gel-342960 (accessed November 30, 2023).
Nanomac Gel: Buy tube of 10.0 gm Gel at best price in India | 1mg. https://www.1mg.com/otc/nanomac-gel-otc288339 (accessed November 30, 2023).
Meganano Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/meganano-gel-329848 (accessed November 30, 2023).
Aveil With Heat Shield Gel SPF 50: Buy bottle of 50.0 ml Gel at best price in India | 1mg. https://www.1mg.com/otc/aveil-with-heat-shield-gel-spf-50-otc388969 (accessed November 30, 2023).
Nan Cola Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/nan-cola-gel-740703 (accessed November 30, 2023).
Instaplus Gel: View Uses, Side Effects, Price and Substitutes | 1mg. https://www.1mg.com/drugs/instaplus-gel-329667 (accessed November 30, 2023).
S Gel: Buy tube of 25.0 gm Gel at best price in India | 1mg. https://www.1mg.com/otc/s-gel-otc266541 (accessed November 30, 2023).
Acknowledgements
The authors acknowledge the efforts of management, Institute of Pharmaceutical Research, GLA University, Mathura, U.P., for their assistance.
Funding
The authors have no source of funding.
Author information
Authors and Affiliations
Contributions
GS: Data curation, Writing, Language, Figures, Tables. JG: Original draft, Figures, Data curation, Software, Reviewing, editing, and conceptualization.
Corresponding author
Ethics declarations
Ethics approval
It’s a review article. So, no ethical approval and no consent from participants is required.
Consent for publication
All the authors read and approved the final version of the manuscript. The authors do not want open access to the manuscript. Therefore, we assure you that this manuscript was not send for publication in any other journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nanogels enable precise drug delivery to specific cells or tissues, minimizing side effects and enhancing therapeutic outcomes.
• A 3-D network of crosslinked polymeric nanogel allows efficient encapsulation of diverse drug types, promoting flexibility in treatment approaches for various diseases.
• Nanogels offer controlled release kinetics, ensuring a sustained and prolonged therapeutic effect, improving patient compliance, and reducing the need for frequent dosing.
• Nanogels are often biocompatible and can be engineered to be biodegradable, reducing potential toxicity concerns and allowing for safe elimination from the body.
• Nanogels enhance drug stability, protecting the encapsulated drugs from degradation, leading to improved shelf life and effectiveness in diverse medical applications.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, J., Sharma, G. Nanogel: A versatile drug delivery system for the treatment of various diseases and their future perspective. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01684-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01684-w