Abstract
Breast cancer due to the unpredictable and complex etiopathology combined with the non-availability of any effective drug treatment has become the major root of concern for oncologists globally. The number of women affected by the said disease state is increasing at an alarming rate attributed to environmental and lifestyle changes indicating at the exploration of a novel treatment strategy that can eradicate this aggressive disease. So far, it is treated by promising nanomedicine monotherapy; however, according to the numerous studies conducted, the inadequacy of these nano monotherapies in terms of elevated toxicity and resistance has been reported. This review, therefore, puts forth a new multimodal strategic approach to lipid-based nanoparticle-mediated combination drug delivery in breast cancer, emphasizing the recent advancements. A basic overview about the combination therapy and its index is firstly given. Then, the various nano-based combinations of chemotherapeutics involving the combination delivery of synthetic and herbal agents are discussed along with their examples. Further, the recent exploration of chemotherapeutics co-delivery with small interfering RNA (siRNA) agents has also been explained herein. Finally, a section providing a brief description of the delivery of chemotherapeutic agents with monoclonal antibodies (mAbs) has been presented. From this review, we aim to provide the researchers with deep insight into the novel and much more effective combinational lipid-based nanoparticle-mediated nanomedicines tailored specifically for breast cancer treatment resulting in synergism, enhanced antitumor efficacy, and low toxic effects, subsequently overcoming the hurdles associated with conventional chemotherapy.
Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With becoming the major cause of mortality and morbidity among women [1], breast cancer has become the most commonly diagnosed [2, 3] and prevalent type of cancer in women worldwide [1]. Among 30% of the total cancer cases (878,980 cancer cases), invasive breast cancer constituted about 266,120 cases in 2018 out of which approximately 40,920 cases were considered to be fatal by the American Cancer Society in 2018 [2]. Furthermore, every year nearly 1.5 million breast cancer cases are reported globally [4]. By resulting in 350,000 new breast cancer cases and 130,000 deaths per annum, it has become the most commonly diagnosed type of cancer in European women. Additionally, women over 50 years of age are reported to have the majority of breast cancer cases, developing the risk of breast cancer fatality with every 1 in 77 women by their 85th birthday [5]. Although astounding research and treatment have been carried out for managing this disastrous disease, 30% of the patients diagnosed with primary-stage breast cancer go on to develop metastatic breast cancer that has a 5-year survival rate of less than 30%. Thus, novel therapeutic strategies that lead to unprecedented results in breast cancer management are needed to be explored [6]. Therefore, management and treatment of breast cancer have become an issue of major concern.
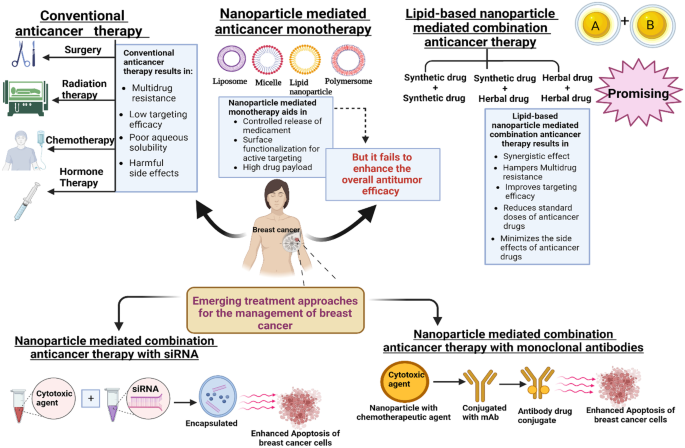
Breast cancer on the molecular level can usually be categorized into luminal A and B, human epidermal growth factor receptor 2 (HER2), triple-negative breast cancer (TNBC), and estrogen (ER) and progesterone (PR) positive receptor subtypes [7], out of which the HER2, ER, and PR positive cancers consist of overexpression of these receptors on the cancer cell membrane whereas TNBC displays an absence of HER2, ER, and PR receptors [2]. Breast cancer similar to other conventional treatment modalities is also treated via radiotherapy, surgery, and chemotherapy with the main aim of exterminating the cancerous cells thereby extending the patient’s survival rate. However, despite the developments, these standard treatments continue to face major hurdles due to the recurrence of the tumor and the development of drug resistance by metastatic as well as advanced tumors. For instance, in case of recurrences and cancer metastases to obscured organs such as the liver, bone, and lung, surgery by conventional treatment modalities is ineffective. On the other hand, conventional cytotoxic agents are being used before or after the surgical treatment so as to disrupt the cell cycle and division of the cancerous cells. Moreover, the use of radiotherapy implies the interference with the division of tumor cells by the introduction of intense energy waves ultimately resulting in the elimination as well as shrinkage of the tumor cell mass. Although the rate of survival of cancer patients could be increased by the dynamic and compelling radiotherapy and chemotherapy techniques but at the same time these lead to the development of severe acute as well as prolonged adverse effects on the crucial organs of the body [8,9,10]. Moreover, the current single chemotherapeutic agents that are used for the treatment of cancer act by targeting the various pathways involved in the deadly disease and often result in the development of multi-drug resistance upon their repetitive administration. A further difficulty in treating the deadly cancer disease by single drug chemotherapy is pinned on various other reasons that include accessibility of the cytotoxic agents in an insubstantial manner to the tumor cell mass thereby necessitating the administration of high drug dose consequently leading to nonspecific targeting and serious toxicity [11]. Over the past few decades, an elevation in the adoption of combination therapies has been witnessed for circumventing the challenges associated with the delivery of single conventional chemotherapeutics [12]. In a much broader sense, combination therapy refers to the simultaneous co-administration of two or more cytotoxic agents or amalgamation of chemotherapy, immunotherapy, radiotherapy, and hormone therapy. But among these two, the approach of delivering two or multiple cytotoxic agents simultaneously is regarded as the most common and effective clinical treatment modality in terms of cancer management [11, 13]. The mutational changes that are required by the cancer cells for their cancer cell adaptation are curbed by the approach of combination therapy. Apart from this, it also promotes target selectivity and therapeutic efficacy by invoking synergism between the combined drugs. Indeed, it is worth mentioning that different chemotherapeutics vary in their biodistribution, pharmacokinetics, and membrane transport properties further making it complicated for the dose optimization of the scheduling of drugs. Moreover, the side effects profile increases due to the simultaneous combination of multiple drugs [14]. However, to date, oncologists prefer administering the multiple anticancer agents freely and not in their encapsulated form often limiting the possible beneficial effects that one can possibly attain from the combinations of various other drugs. This is because the physicochemical, as well as pharmacokinetic aspects of different classes of cytotoxic agents, hinder their co-administration with each other [15]. Therefore, one such promising approach of using multiple cytotoxic agents for the management of cancer is with nanoparticles [16]. With the advent of nanoparticle-mediated combination therapy, multiple cytotoxic agents possessing different physicochemical as well as pharmacological properties were able to be delivered via their successful encapsulation in a single nanoparticle [16,17,18]. This is because the diverse biodistribution, pharmacokinetics, and stability of drugs get normalized upon their encapsulation in nanoparticle due to the controlled drug release offered by them which otherwise possess contrasting pharmacological responses [19]. Additionally, the optimum synergistic ratio of both drugs till their delivery to the target cancerous cells is being maintained by the single nanocarriers [16,17,18]. Apart from this, it offers various other potential advantages that include augmented therapeutic efficacy, reduced development of drug resistance, concurred pharmacokinetic profile, improved patient compliance, decreased cases of side effects, and explicit control of individual doses.
The present review, therefore, focuses on the recent progress in the nanomedicine-mediated combination approaches for breast cancer management with an emphasis on various sections that will provide an overview of the co-delivery of synthetic-synthetic chemotherapeutic agents, co-delivery of herbal-synthetic chemotherapeutic agents, co-delivery of herbal-herbal chemotherapeutic agents, and lastly co-delivery of anticancer agents with siRNAs and co-delivery of chemotherapeutics with monoclonal antibodies (mAbs) is highlighted for managing the heterogeneous breast cancer. Furthermore, the perspective of future scope has also been discussed.
Nanotechnology in breast cancer
Marred by numerous limitations, namely, non-specificity, high systemic toxicity, and poor pharmacokinetics, conventional chemotherapies are inadequate in curbing breast cancer disease effectively [20]. Nanomedicine refers to the fabrication of nanoscale assemblies employed for the detection, prevention, and treatment of ailments via their successful application in the field of medicine [21], which has assisted in reduced toxicity, effective chemotherapy [20], and has extensively revolutionized the paradigms of cancer treatment [22]. In comparison with conventional chemotherapies, nanotechnology results in several promising benefits such as protecting the drug from the harsh internal environments both chemical and biological consequently causing its less degradation during its transportation, along with enhanced targeting and biocompatibility which ultimately results in the generation of less toxic effects, and aids in delivering an enhanced dose of chemotherapeutic drugs to the mass of tumor cells [23, 24]. Nanotechnology is an emerging integrative research field uniting biology, chemistry, medicine, and engineering that addresses the demands of diagnosing and treating cancers [25]. Its implementation for breast cancer treatment has rendered chemotherapy more efficient and successful [26]. Nanotechnology and nanoparticle-mediated drug delivery due to their overarching benefits such as increased loading, low toxicity, increased stability, high specificity and efficacy, and enhanced tolerability of the nanoparticles are considered as promising treatment options for the management of cancer in comparison to conventional chemotherapeutics. Furthermore, these drug-loaded nanoparticles can efficiently be targeted to breast cancer lesions via active and passive targeting [27]. Additionally, the astounding advantages of the nanoparticles do not only lie in their potential to be created in small sizes but also due to their ability to form a diverse range of materials, namely, solid lipid nanoparticles (SLNs), and liposomes from lipids, polymeric nanoparticles from polymers, gold nanoparticles from inorganic materials, and so on. Among the different nanoparticles mentioned here, micelles, liposomes, SLNs, polymeric, and gold nanoparticles are widely used in the treatment of breast cancer [2]. Although the fabrication of nanoparticles is a cumbersome process but it may prove to be of great importance for the successful treatment of various cancers [28].
Nanomedicinal combination therapy: an efficient approach
Amelioration of heterogeneous breast cancer has become an enormous challenge owing to the development of chemoresistance that is a consequence of over-dramatic expression of multidrug resistance (MDR), disturbance in the apoptosis pathway, and cancer stem cells (CSCs) production [29, 30]. But among all, MDR is allocated as the primary culprit causing the unresponsiveness of the aggressive tumor cells thereby leading to resistance and ultimate failure of the whole chemotherapy. The major MDR efflux transporters, namely, multiple drug resistance proteins (MRPs), breast cancer resistance protein (BCRP), and P-glycoprotein (Pgp), lead to the efflux of drugs from the tumor cells and subsequently reduce their internal accumulation in the tumor vasculature [30]. Additionally, as evident by the clinical trials, conventional chemotherapy often leads to the overexpression of MDR transporters worsening the treatment modality even more [31,32,33].
Nanomedicine encompasses the utilization of nanotechnology for purposes such as diagnosis, prevention, and treatment of several pathological conditions through the application of nanosized devices. In addition to this, nanomedicine displays its potential in various other aspects, namely, drug delivery, in vitro and in vivo diagnostics, imaging, drug therapies, and so on [21]. Nevertheless, despite many advancements such as providing controlled release of the medicament, surface modification for active targeting, and resulting in reduced toxicity, still clinically monotherapy nanomedicines have failed to enhance the antitumor activity of the chemotherapeutic agents. Myocet, an FDA-approved non-PEGylated doxorubicin (DOX) liposomal formulation, was not able to enhance the anticancerous activity against metastatic breast cancer, Kaposi’s sarcoma, and multiple myeloma. However, it was successful in lowering the side effects, namely, nausea, alopecia, depression of bone marrow, and cardiomyopathy subsequently increasing a few others like dermatologic toxicity and palmar-plantar erythrodysesthesia [34]. It was also reported that Myocet when tested against free DOX in phase III clinical trials failed in meaningfully enhancing the anti-tumor effects against metastatic breast cancer despite reducing the congestive heart failure and cardiac arrest incidences. Apart from this, there exist several other monotherapy nanomedicines that fail in generating an enhanced response rate in clinical trials, despite being demonstrating efficacious results in animal models in preclinical trials over the conventional chemotherapy [34]. Whereas it has been reported that co-delivery of drugs aids in augmenting the overall treatment efficacy in comparison to single delivery in patients of cancer [35,36,37], the improved therapeutic efficacy of the multiple drugs at ideal synergistic ratio [38, 39], additionally combination therapy reduces the severe adverse effects associated with single drugs, minimizes the standard doses of chemotherapeutic agents [40], results in synergistic action that further helps in hampering the development of resistance in tumor cells [41]. Also, combinational chemotherapy has been regarded as the most common approach in the management of advanced breast cancer [42]. Moreover, nanoengineering allows for the simultaneous and efficient delivery of multiple agents safely encapsulated and separated from each other without getting exposed to external factors [14]. In accordance with this, delivering multiple agents via nanotechnology offers several unique advantages such as providing controlled drug release, improved serum stability, increased systemic circulation, enhanced carrier capacity, and encapsulation of multiple agents for combinatorial delivery [43]. Moreover, the co-delivery of drugs via nanoparticles aids in accomplishing the “3R approach” of drug delivery that stresses the delivery of drugs at the right place in the right dose at the right time which can be ascribed to the successful accumulation of nanoparticles in the tumor cells that further facilitates the achievement of similar bio-fate along with simultaneous execution of the therapeutic efficacies of both the drugs at an optimum combined ratio [40]. Till now, scientists have stressed on active and passive targeting of cancer nanomedicines that fail to deliver the drugs at the right place, additionally, unsuccessful clinical trials and R&D research have further prompted the researchers to reconsider the fact that tumor-targeted drug delivery has always been the focal point of nanomedicine [44] and thus their focus has now been shifting from targeted tumor therapy to combinational nanomedicine that exhibits the attractive advantage of multiple co-delivery of drugs. Nanocarriers help in the prediction of the ideal combinatory dose ratio that is a critical parameter for obtaining strong therapeutic action, the co-encapsulated agents at the right combination ratio, when delivered to cancerous cells, aids in the delivery of therapeutics at the right place thereby providing synergistic action [40].
Combination index in combinatorial nanomedicine
Combination of multiple drugs in nanocarriers can be achieved by various strategies that include free drug + nano form, nano form + nano form, and co-encapsulation of both the drugs in a single nanocarrier for a combination of two drugs. The first two strategies result in the sequential release of the drugs whereas the third one delivers the drugs simultaneously. It further aids in increased concentration of the multiple administered drug in the intracellular milieu due to the temporal and spatial controlled release of the drugs. Subsequently, providing a potent and coordinated synergistic effect against the cancerous cells and finally resulting in the most effective coordinated drug delivery at cellular levels among the three [39]. Various in vitro as well as in vivo studies conducted in this regard have shown the potential benefits of co-delivery of drugs in the same nanocarrier. An early proof of concept study by Shuhendler et al. depicted the significant enhanced anticancer action on human breast cancer cells, MDA-MB-435/LCC6 by the anticancer agents’ DOX and mitomycin both co-loaded in polymer lipid hybrid nanoparticles rather than the individual nano-drug combinations indicating at the improved drug synergy and amplified antitumor efficacy [45]. Although mostly combinatorial nanomedicine studies are performed for yielding synergistic actions, but this may not be the case always and they sometimes result in additive or antagonistic effects depending upon their therapeutic regimen and the design of the study. Numerous studies have suggested that the drug-to-drug ratio and schedule of administration of the drug are the governing factors behind the synergism effect generation [45,46,47,48,49,50]. The combination theorem given by Chou Talay gives a quantitative definition for attributing any effect as antagonistic, additive, or synergistic based on the values of the combination index (CI), according to which CI > 1, CI < 1, and CI = 1 depict antagonism, synergism, and additive effects, respectively [51, 52]. Nevertheless, co-loading two different drugs with diverse properties are cumbersome and challenging procedure attributing to their solubility profiles, polarity, and stability, henceforth one should be careful in choosing the combination of drugs for their probable co-encapsulation in nanosystems since any change leads to reformulating them for preventing any undesirable effects [39].
Combination with synthetic breast cancer agent
Several preclinical studies have been conducted for assessing the potential of various chemotherapeutic agents belonging to different classes encapsulated in multi-drug nanoparticle formulations for breast cancer management [53]. In a recently conducted study by Guo et al. [54], the efficacy of co-delivered docetaxel (DTX) and verapamil (VRP) against MDR in breast cancer MCF-7 and MCF-7/ADR cells was evaluated by designing mPEG-PLGA-SS (PP-SS-DTX/VRP) nano-micelles utilizing the film dispersion-probe ultrasonic method. Here, reduction sensitive mPEG-PLGA-SS-DTX conjugate was used for loading VRP. The fabricated mPEG-PLGA-SS-DTX/VRP micelles exhibited high drug loading capacity and DTX and VRP resulted in a sustained reduction sensitive release process while the in vitro cytotoxicity assay conducted in MCF-7 as well as in MCF-7/ADR cells revealed dose and time-dependent cytotoxicity, wherein higher cytotoxic action was displayed by both the micelles, i.e., PP-SS-DTX and PP-SS-DTX/VRP in comparison to DTX free solution. Nonetheless, the highest cytotoxic action was exhibited by PP-SS-DTX/VRP micelles. Moreover, the in vitro cellular uptake studies performed in MCF-7 and MCF-7/ADR cells confirmed the higher internalization of both the micelles in the cells in comparison to Rhodamine123 (RH123) fluorescent molecule which complied with the results obtained from the flow cytometry. Furthermore, an increase in apoptosis in both cell lines was exhibited by the PP-SS-DTX/VRP micelles in contrast to free DTX solution and PP-SS-DTX micelles.
Apart from this, the oral bioavailability of DTX was found to be augmented when administered in PP-SS-DTX micelles as confirmed by its 20.96-fold higher AUC in comparison with the DTX solution in male Wistar rats which indicated at the enhanced antitumor efficacy due to higher accumulation of drug and residence time in vivo. Thus, the study suggested a substantial increase in the antitumor activity of DTX and reversal of MDR with improved pharmacokinetic parameters. However, it still lacks acute toxicity and biodistribution studies, which would have been immensely effective in assessing the safety of the formulating micelles and their action on each vital organ (Fig. 1) [54].
A Cell viability study of PP-SS-DTX-VRP and PP-SS-DTX compared to free DTX in MCF-7 and MCF-7/ADR cells (*, p < 0.01 PP-SS-DTX micelle compared with free DTX; #: p < 0.01 PP-SS-DTX/VRP micelle compared with free DTX; + : p < 0.01 PP-SS-DTX/VRP micelle compared with the PP-SS-DTX micelle; mean ± SD, n = 4). B Images of cellular uptake in MCF-7 and MCF-7/ADR cells and C flow cytometry in MCF-7 and MCF-7/ADR cells of (a) free RH123, (b) PP-SS-DTX/RH123 micelles, (c) PP-SS-DTX/(VRP + RH123) micelles, and (d) blank cells. Reproduced with permission from reference [56]. DTX docetaxel, PP-SS-DTX mPEG-PLGA-SS-docetaxel nanomicelles, PP-SS-DTX-VRP nanomicelles mPEG-PLGA-SS-docetaxel-verapamil nanomicelles, RH123 Rhodamine123 copyright Elsevier 2017
In yet another interesting study performed by Elzoghby et al. multi-reservoir nanoformulation comprising of phospholipid shells entrapping protamine, nanocapsules were constructed loaded with both letrozole and celecoxib that showed enhanced increased anti-cancerous action both in vitro and in vivo [55]. Table 1 summarizes some of the recent combinatorial nanomedicine studies conducted with different synthetic chemotherapeutics. Nevertheless, the severe risks associated with the simultaneous delivery of chemotherapeutics cannot be overlooked and hence scientists are inclining more toward the newly emerging concept of traditional herbs administration that is recently gaining more attention due to their natural origin [56]. Also, the anticipated risk of combining an herb with a chemotherapeutic agent is substantially less [57] in breast cancer patients, since the patients of breast cancer are ardent users of complementary and alternative medicine (CAM) therapy accompanied by prostate and melanoma cancer [58]. These complications may be circumvented by combining these agents with herbal/naturally derived agents instead as described in the next section.
Combination of the synthetic agent with the herbal chemotherapeutic agent
New arenas have been opened since the implication of natural compounds in the anticancer field mainly due to their chemical and structural variability [71,72,73,74]. From the year 1981 to 2010, approximately 50% of the anti-tumor drugs approved by the US FDA were natural agents [75] with more than hundreds of the naturally originated compounds being evaluated in clinical trials or are either employed for clinical application [76, 77]. The different aspects exhibited by cancerous cells such as metastasis, viability, and proliferation are often downregulated and inhibited by the multi-target capability of the natural compounds [56] which are known to act via several mechanisms the most common of which include induction of apoptosis thereby causing the death of cancerous cells [78].
As suggested by a recent survey, around 80% of the women suffering from disastrous breast cancer utilize alternative/complementary medication rather than conventional chemotherapeutic agents. Among them herbal medicines represent one of the most widely used alternative sources of medicine by women around the globe for alleviating the heterogeneous breast cancer [79]. In addition to this, numerous studies conducted in the past have shown unraveling interest in natural agents as being acting as potent chemosensitizers and ultimately as saviors. Chemosensitizing the resilient aggressive tumor cells to traditional anticancer drugs by successfully combining them with non-toxic herbal agents to hinder the development of chemoresistance, limit the side effects and offsite toxicity as well as enhance their cytotoxic efficiency has emerged as an innovative and novel strategic approach [80]. These agents act as chemosensitizers, chemopreventive agents as well as chemotherapeutic agents, thereby displaying their versatile nature in the management of cancer. Phytochemicals, identified till now and further categorized into flavonoids, phenolics, steroids, carotenoids, alkaloids, quinones, and terpenoids, have been reported to act as chemosensitive, chemopreventive, and anticancer agents [80, 81]. To mention but a few, curcumin (CUR) when administered along with the chemotherapeutic drug cisplatin made the tumor cells more sensitive to both the drugs along with downregulating the expression of FEN1 as demonstrated by in vitro and in vivo studies [82]. Potentiation of the antiangiogenic effect of DOX, etoposide, gemcitabine, and 5-fluorouracil by resveratrol in addition to causing chemosensitive activity in breast cancer cells toward DOX by downregulating the expression of HSP27 [80]. Simultaneous delivery of tamoxifen with the metabolite of ginsenosides, namely, 20S-protopanaxadiol, demonstrated effective killing of the MCF-7 cancer cells by inhibiting the gene expression of estrogens [83]. Several agents explored in this regard act by enhancing the time of residence of conventional chemotherapeutics inside the tumor vasculature, upregulating the pro-apoptotic proteins thereby causing cell death, inducing the damage of DNA [80], increasing the sensitivity of drug, and modulating the efflux proteins [84, 85]. Taken together, these mechanisms lead to synergistic actions in normal as well as resistant cells subsequently increasing the cytotoxic effect of the anticancer agents [41], enhancing the bioavailability of any one agent among the two [86], and decreasing the standard dose of chemotherapeutic agents consequently reducing the severe side effects that arise due to extensive use of synthetic anticancer agents [87]. In line with this, Vakilinezhad et al. [88] successfully fabricated MTX and CUR-loaded PLGA nanoparticles (MTX-CUR-NPs) using the co-precipitation method and in the same manner MTX-NPs, CUR-NPs, and blank-NPs were formulated as well. It was found from the in vitro studies that CUR resulted in the sustained release action of MTX from the optimized formulation. Moreover, in vitro cytotoxicity studies conducted on SK-Br3 cell lines in 24 and 48 h revealed the significantly lower cytotoxicity of MTX from MTX-NPs in 24 h in comparison to MTX-solution owing to the incomplete release of MTX from MTX-NPs. However, it did exhibit augmented cytotoxicity in about 48 h, whereas CUR-NPs exhibited lower cytotoxicity at both 24 and 48 h than the CUR solution as well as MTX solution. Lastly, the optimized MTX-CUR-NPs-8 depicted the highest cytotoxicity among MTX-NPs, CUR-NPs, and their plain solutions with 2.5 and 1.7 folds lower IC50 at both 24 as well as 48 h than the MTX-NPs. In vivo studies conducted in female Sprague Dawley rats with chemically induced tumor by MNU revealed that compared to the control group any other group that received MTX formulation displayed an improvement in the incidence as well as the size of the tumor, whereas the treatment group that received MTX-NPs resulted in significantly reduced size of the tumor in contrast with MTX solution. Moreover, CUR solution and CUR-NPs depicted similar improvement in the tumor incidence that was comparatively lower than that in the control group. Considering the size of the tumor, the MTX-NPs revealed slow development of tumor size than the CUR-sol and CUR-NPs. Nonetheless, MTX-NPs-8 depicted marked improvement in the size of the tumor thereby stressing at its suitability in the treatment of breast cancer. Although the current study provided deep insight into the effectiveness of co-delivered cargos in breast cancer management, it still lacks pharmacokinetic studies for the fabricated PLGA nanoparticles, including which may help further establish the effect on bioavailability and various other essential pharmacokinetic parameters (Fig. 2) [88].
A Characterization of nanoparticles by scanning electron microscopy (SEM) (1) MTX-NP, (2) CUR-NP, and (3) MTX-CUR-NP-8. B In vitro cell viability study of MTX-CUR-NP-8 (optimized formulation) in comparison to blank NP, MTX, MTX-NP, CUR, and CUR-NP wherein conc. 1, 2, and 3 signify 6.25, 12.5, and 25 µg/ml conc. for MTX and 2.5, 5, and 10 µg/ml conc. for CUR. Reproduced with permission from reference [88] copyright Elsevier 2019. MTX methotrexate, CUR curcumin, MTX-NP methotrexate nanoparticle, CUR-NP curcumin nanoparticle, blank NP blank nanoparticle, MTX-CUR-NP-8 methotrexate-curcumin-nanoparticle-8
Table 2 summarizes recent combinatorial nanomedicine studies conducted with different herbal chemotherapeutics.
Combination with the herbal chemotherapeutic agent for breast cancer management
Nanomediated co-delivery of curcumin with herbal chemotherapeutic agents
Originated from Curcuma longa L., the family Zingiberaceae CUR is obtained as a hydrophobic yellowish component [109]. According to a plethora of research conducted in the past CUR has depicted its valuable role as being a potent anti-inflammator, immunoregulator, anti-tumoral, anti-bacterial, anti-fungus, and anti-oxidant agent [110, 111] in treating various types of malignant cancers such as prostate, breast, gastric, ovarian, pancreatic, cervical, and colorectal cancers [112]. Considering its mechanism of anticancer activity, it is reported to act via interfering with various signaling pathways including the PI3K/Akt, JAK/STAT, p53, Wnt/β-catenin, NF-ĸB, MAPK, and signaling related to apoptosis. Additionally, by non-coding RNA expression modification, it is capable of inhibiting the proliferation, angiogenesis, invasion, metastasis, and epithelial-mesenchymal transition associated with malignant cells [113,114,115,116]. In light of this, in 2022 Oghaz et al. fabricated the zein nanoparticles coated with chitosan, co-loaded with CUR and berberine (CUR-Z-Ber-Ch) by anti-solvent precipitation technique for investigating their potential in breast cancer. In their research, the in vitro drug release studies revealed augmented drug release of both CUR and Ber at acidic pH. Moreover, increased cellular uptake of CUR-Z-Ber-Ch was revealed by cellular uptake studies (confocal imaging and flow cytometry) wherein CUR and Ber having intrinsic green fluorescence properties resulted in strong intensity in comparison to CUR + Ber in MDA-MB-231 cancer cell lines after a 4 h duration. The findings of apoptosis studies carried out on MDA-MB-231 cells after their successful staining with AO and EB dyes resulted in the appearance of green fluorescence with normal cells and red/orange fluorescence with dead or late apoptotic cells. Results revealed green fluorescence by untreated as well as free dug combination treated cells. Also, the cells that were treated with CUR-Z-Ber-Ch nanoparticles were found to be majorly located in the early and the late apoptotic stages as evident by the results of confocal imaging microscopy with the blank nanoparticles having no influence on the cell death. Further, the flow cytometry studies also found the highest proportion of cells stained with ethidium bromide and treated with CUR-Z-Ber-Ch nanoparticles lying in the lower right quadrant depicting the percentage of apoptotic cells. The researchers also inferred the elevated anti-inflammatory action of CUR-Z-Ber-Ch on IL-8 cytokine secretion in MDA-MB-231 cells in comparison to the combination of free drug solutions thereby proving its efficacy as a potent inflammatory cytokine. Although this study suggested a significant role that “layer by layer nanopolymers” can have in the management of breast cancer, however, it still lacks the in vivo pharmacokinetic and pharmacodynamic studies the inclusion of which may further help in validating the therapeutic efficacy of the developed nanocarriers. Further, the inclusion of acute toxicity studies for knowing the toxic effects on the vital organs is advocated too (Fig. 3) [117].
A Flow cytometry analysis images indicating the intracellular internalization of CUR-Z-Ber-Ch nanoparticles and free drug combination of CUR + Ber after a duration of 4 h. B Fluorescent images of AO/EB dual staining indicating the morphology of apoptosis in MDA-MB-231 cells incubated with (a) untreated cells, (b) blank nanoparticles, (c) free CUR + Ber combination, and (d) CUR-Z-Ber-Ch nanoparticles. Reproduced with permission from reference [119] copyright Elsevier 2022. CUR curcumin, BER berberine, CUR-Z-Ber-Ch nanoparticles curcumin-zein-berberine-chitosan, CUR + BER curcumin and berberine, AO/EB dual acridine orange/ethidium bromide fluorescent staining
Another recently conducted study investigated the co-delivery of CUR and paclitaxel (PTX) in biodegradable polymeric nanoparticles and tested their anticancer efficacy against breast cancer both in vitro and in vivo. Results depicted small particle size and slow release with enhanced cellular uptake, increased cytotoxicity, and a high rate of apoptosis in comparison to the free drug combinations in the MCF-7 cell line. Also, when tested in a BALB/C nude mouse bearing MCF-7 cells by intravenous route substantial suppression of tumor growth, decreased side effects, and prolonged rate of survival than the free drug combinations were observed suggesting the therapeutic efficacy of combinatorial drug delivery packaged in nanoformulation [118].
In the investigation of Pushpalatha et al., CUR and resveratrol were encapsulated in cyclodextrin nanosponge-based hydrogel, and their therapeutic effects against breast cancer cells were assessed. They concluded synergistic action with a 0.29 value of CI on the MCF-7 cells with increased photostability and enhanced in vitro release profile of the nanoformulation [119].
An in vitro investigation showed that CUR and chrysin when co-delivered in a nanofiber result in synergistic activity on T47D breast cancer cells in terms of both antiproliferation as well as cytotoxicity. They also downregulated the levels of BCL-2, cyclin D1, Bax, p53, hTERT, caspase-3, and 7 [120]. The study of Danafar et al. demonstrated the antitumor effect of CUR and sulforaphane by their encapsulation in PEGylated gold-coated FE3O4 magnetic nanoparticles. Results depicted increased cytotoxicity on the MCF-7 cell lines than the free drug combinations, enhanced apoptosis, and inhibition of the cells migration as well as induction of necrosis was also observed [121]. In a nutshell, it can be inferred that these novel nano-combinations of CUR with another herbal chemotherapeutic agent hold a promising future in curbing the resilient breast cancer disease.
Nanomediated co-delivery of quercetin with herbal chemotherapeutic agents
Found in several fruits and vegetables, quercetin (QC) is a potent polyphenolic compound [122] that is reported to exhibit various pharmacological activities including anti-inflammatory, anti-proliferative, anti-oxidant, and also anti-diabetic properties [123,124,125]. Growing evidence has shown a remarkable effect of QC against numerous types of cancers such as breast, colon, cervical, ovarian, prostate, lung, and gastric cancer [126,127,128,129,130,131]. Regarding its anticancer mechanisms, it acts by modulating the STAT signaling pathway, PI3K/Akt/mTOR, altering the intracellular pH, modifying the expression of heat shock protein, and is also involved in the regulation of various cancer-related factors that include vascular endothelial growth factor, apoptosis proteins, and matrix metalloproteinases [132,133,134]. In line with this, Liu et al. highlighted that QC sensitizes the MCF-7/ADR cells to PTX wherein they fabricated QC and PTX-loaded mesoporous silica nanoparticles by sol–gel method, further coated with chondroitin sulfate (ChS) for evaluating their MDR reversal potential as well as for enhancing their anticancer activity. The results of in vitro cell cytotoxicity performed on MCF-7/ADR cells revealed that the QC and PTX-loaded mesoporous silica ChS nanoparticles (MSNs-ChS@PQ) formulation exhibited the lowest IC50 value thus displaying better chemotherapeutic action. Moreover, the results of fluorescence microscopy revealed time-dependent cellular uptake of fluorescein5 (6)-isothiocyanate (FITC), MSNs-COOH-FITC, and MSNs-ChS-FITC in MCF-7/ADR cells. It was further concluded that MSNs-ChS-FITC treated cells exhibited augmented fluorescence intensity than the cells treated with either free FITC or MSNs-COOH-FITC. Additionally, the results of flow cytometry were also consistent with the aforementioned results wherein the higher cell intensity was attributed to the targeting of the CD44 receptor by ChS. MSNs-ChS@PQ exhibited a significant increase in the rate of apoptosis in comparison to other samples which was ascribed to the higher internalization of NPs inside the cell along with CD44 mediated endocytosis pathway and the QC action of efflux reversal. Additionally, the cell cycle analysis performed by flow cytometry revealed, the free drug combination of QC + PTX resulted in G2M phase arrest indicating at the MDR reversal efficacy of QC thereby causing the cell cycle arrest by PTX. Nevertheless, MSNs-ChS@PQ due to their CD44 targeting resulted in the highest G2M phase cell cycle arrest disrupting the procedure of tubulin synthesis thereby augmenting the efficacy of chemotherapy. Significant microtubule polymerization was caused by MSNs-ChS@PQ due to co-delivered drugs and potent MDR reversal. Furthermore, it was also found that QC was capable of downregulating the expression of P-gp since the fluorescence intensity got markedly reduced in the MCF-7/ADR cells. The in vivo imaging performed in mice showed a comparatively higher intensity of fluorescence for MSNs-ChS@DiR while the highest optimal suppression of tumor volume by MSNs-ChS@PQ and MSNs@PQ was ascribed to the active targeting property of ChS. On the other hand, the ex vivo studies performed on vital organs such as the heart, liver, spleen, and lungs revealed the enhanced accumulation of MSNs-ChS@DiR which was ascribed to the EPR effect as well as CD44 targeting efficiency. Furthermore, the hemolysis studies performed on blank NPs showed RBCs with slight damage equivalent to only 5% rate of hemolysis. Lastly, histopathological studies were performed in the organs of the NS and MSNs-ChS@PQ group that depicted no lesion formation and no signs of abnormalities were observed (Fig. 4) [135].
A MCF-7/ADR cells treated with different formulations for investigating apoptosis populations. B MCF-7/ADR cells treated with different formulations for investigating the cell cycle process. Reproduced with permission from reference [137] copyright Elsevier 2022. QC quercetin, PTX paclitaxel, (MSNs-ChS@PQ) quercetin and paclitaxel-loaded mesoporous silica chitosan nanoparticles
Another work assessed the co-delivery of QC and vincristine in matrix metalloproteinase (MMP)-triggered dual-targeting hybrid micelle-in-liposome system and investigated their synergistic action on the MDA-MB-231 cell lines. The co-encapsulated nanoformulation exhibited a CI of 0.113 suggesting significant synergism as per the Chou Talay protocol; also reducing the dose of vincristine with a DRI of 115 thereby signifying the multiple possible positive effects that this co-encapsulated system can have when explored further [136]. In general, QC primarily due to its natural source, its safety profile, and its comparatively less cost than synthetic anticancer agents is considered a promising anticancer agent; however, its co-delivery with herbal agents is still very less reported in the literature and thus researchers should focus on exploring this agent in the nanomediated combination therapy targeting the breast cancer cells for harnessing its potential in addressing the challenges.
Nanomediated co-delivery of epigallocatechin-3-gallate with herbal chemotherapeutic agents
Epigallocatechin gallate (EGCG) is a polyphenolic compound present in green tea that exhibits vital therapeutic potential, particularly against several health-damaging disease conditions, namely, Parkinson’s, stroke, diabetes, Alzheimer’s, and diabetes [137]. Apart from exhibiting the aforementioned pharmacological effects, it is also reported to possess anticancer properties wherein it acts by downregulating the various genes involved in the pathogenesis of tumors as well as modifying the signaling pathways associated with tumor proliferation and development [138,139,140,141]. Furthermore, according to several studies, epigallocatechin is known to suppress cancers at all stages that are from initiation till progression, and is also reported to induce the process of apoptosis, induction of cell cycle arrest, and proliferation inhibition [142]. Additionally, suppression of ERK phosphorylation and AKT by epigallocatechin has been observed in in vitro studies; also the cell cycle arrest and apoptosis induction are brought by the successful activation of the transcription factors such as FOXO [143]. Moreover, a growing body of evidence confirms the potent anticancer potential of epigallocatechin. For instance, inhibition in cell proliferation was observed in treating MDA-MB-231 and MCF-7 cells with EGCG, QC, and tamoxifen as well [144].
Another line of the study conducted by Ramadass et al. investigated the anticancer potential of EGCG by potentiating its co-delivery with PTX and further encapsulated them in liposomes. The cytotoxicity studies performed in the MDA-MB-231 cells depicted 1:5 as the optimum synergistic ratio wherein it was reported that with increasing the EGCG concentrations the antitumor activity of the dual loaded liposomes got enhanced. Also, the elevated anticancerous response was attributed to the synergistic action of both drugs acting via different anticancer mechanisms. This was then followed by the apoptosis assessment that revealed higher fluorescence intensity for the PTX/EGCG treated cancerous cells signifying the augmented apoptosis rate in contrast to individual drug loaded liposomes which were confirmed by the elevated levels of caspase activity in the cells treated with PTX/EGCG liposomes. Additionally, the formulated co-loaded liposomes depicted the highest reduction in the levels of both MMP-2 and MMP-9 by the PTX/EGCGC liposomes whereas the individual ECGC and PTX liposomes also resulted in the reduction of MMPs with negligible difference which was ascribed to the PTX enhanced cytotoxic activity that might have resulted in reduced cell viability thereby reducing the MMPs levels. Also, zymography analysis revealed the ECGC’s ability of forming a chelating complex with ions or attaching with proteins subsequently suppressing MMPs levels. Furthermore, the cell invasion assay was carried out on MDA-MB-231 cells using matrigel or type-I coated transwell membrane wherein the maximum inhibition of the cell invasion was depicted by the dual cargo loaded liposomes. This was followed by EGCG liposomes and PTX liposomes wherein the reason behind the reduced cell invasion brought by PTX in comparison to the control group was ascribed to its capability of decreasing cell viability. However, irrespective of this, EGCG liposomes depicted more pronounced suppression of the invasion than PTX probably due to its better MMPs inhibition ability. The present study although provides enough evidence for nano-based combinatorial delivery for the treatment of breast cancer but lacks pharmacokinetics, pharmacodynamics and biodistribution studies including them would have been better in understanding the influence of the dual loaded liposomes in terms of pharmaceutical aspects, efficacy, and safety (Fig. 5) [145].
A Cell viability evaluation of free PTX and EGCG compared to PTX/EGCG co-loaded liposomes conducted by MTT assay on MDA-MB-231 cells. The treatment was performed in triplicate. PTX/EGCG co-loaded liposomes and free drug combinations depicted significant reduction (*, p < 0.001) in viability of cells than the vehicle and PTX/EGCG co-loaded liposomes only resulted in significant treatment (#, p < 0.001) than the treatment by single drugs. B ELISA method employed for evaluating MMP-2 concentration and C MMP-9 concentration by control, PTX liposomes, EGCG liposomes, and PTX/EGCG co-loaded liposomes. Significant reduction (#, p < 0.01) depicted by PTX/EGCG co-loaded in comparison to PTX and EGCG liposomes. D Gelatin zymography for analyzing MMP-2/-9 activity performed in MDA-MB-231 cells on conditioned media. EGCG liposomes and PTX/EGCG co-loaded depicted significant reduction in MMP-2/-9 activity. E Cell invasion assay incubated with control, single drug loaded liposomes. Maximum inhibition of cell invasion exhibited by PTX/EGCG co-loaded compared to single drug loaded liposomes. Reproduced with permission from reference [147] copyright Elsevier 2022. PTX paclitaxel, EGCG epigallocatechin gallate, PTX liposomes paclitaxel liposomes, EGCG liposomes epigallocatechin gallate liposomes, PTX/EGCG co-loaded liposomes paclitaxel/epigallocatechin gallate co-loaded liposomes
In yet another study, the co-delivery of EGCG with PTX fabricated in PLGA-casein core–shell nanoparticles was explored by Narayan et al. wherein results depicted the sequential release of PTX after EGCG. Further, the increased sensitivity of MDA-MB-231 cells to PTX was also reported along with the apoptosis induction, inhibition of the activation of NF-κB, and suppression of various genes associated with the process of angiogenesis, metastasis of tumor as well as tumor survival. Furthermore, inhibition of P-gp was observed both at gene and protein levels with major cytotoxic action on the breast cancer cell lines [146]. In summary, evidence from the reported literature suggested the efficacious synergistic effect of EGCG in combination with another herbal chemotherapeutic agent in the management of the critical condition of heterogenous breast cancer. However, only a few of the studies have investigated the co-delivery of EGCG with other herbal chemotherapeutics and are thus inconclusive. It is, therefore, appropriate that the investigation of this highly promising herbal anticancer agent with other herbal moieties be done more vigorously to gain maximum therapeutic efficacy from these herbs in the management of breast cancer.
Nano-combination of cytotoxic agents with siRNA
A plethora of several complex factors are known to contribute to the astounding development of tumors which are currently treated by the cancer management approaches utilizing the concept of a single magic bullet that are further painfully insufficient in the eradication of the disease [35, 43].
However, the emergence of the powerful RNA interference (RNAi) technology has offered a paragon shift in the treatment strategies of cancer, wherein it results in the downregulation of the post-transcriptional genes when applied to the cancerous gene expressions specifically [147]. By employing the recent RNAi approach, one can carry out the knockdown of the genes particularly causing resistance in conventional chemotherapy. Furthermore, the use of siRNA has been reported to sensitize the cancerous cells more toward the chemotherapeutic agent [148]. Since chemotherapeutic agents develop MDR and thus lose their potential, these siRNAs on the other hand have been reported to inhibit the proliferation of the resistant breast cancer cells due to their silencing capability thereby overcoming MDR. In this regard, various studies conducted so far have shown that different categories of breast cancer cell lines were made resistant by their exposure to DOX and were then further treated by siRNA along with combinations of drugs that finally led to the induction of apoptosis confirmed by the microarray analysis that depicted the elevated level of apoptosis-associated proteins [149]. Moreover, countless studies conducted using the synthetic siRNAs have established their potential in degrading the messenger RNA in cell cytoplasm thereby inhibiting the particular gene of interest and subsequently suppressing their expression [150,151,152]. For instance, it has been hypothesized that the activation of the Pi3-K/Akt signal pathway leads to the increased expression of Lifeguard/LGF, an anti-apoptotic gene that further plays a vital decisive role in the suppression of programmed cell death [153, 154]. In view of this, Bucan et al. potentiated the combinational delivery of a chemotherapeutic agent for suppressing the Pi3-k/Akt cascade, preceded by siRNA therapy for reducing the levels of LGF in the MCF-7 cells wherein the results demonstrated a robust decrease in the proliferation of cells along and their survival along with cumulative apoptosis [155,156,157,158].
In this regard, a combination of a conventional chemotherapeutic agent with siRNA could serve as an efficient and encouraging novel treatment approach sparking the interest of researchers globally; however, both these agents suffer from several hindrances that further impede their clinical efficacy. Conventional chemotherapeutics for instance results in the development of MDR that damages the normal cells along with the malignant ones due to their non-selective action exhibiting poor permeability followed by their low penetration in the malignant cells, increased dose, and frequency of the cytotoxic agents [159]. siRNAs on the other hand generally present low selectivity, ease of clearance from the renal system, and low cellular uptake including instability issues [160].
The clinical efficacy of both chemotherapeutics as well as the siRNA is limited due to these hurdles and thus warrants newer strategies. These challenges can be reiterated by the efficient nanoparticles in which both hydrophobic and hydrophilic moiety can be loaded simultaneously achieving enhanced encapsulation efficiency and further smoothening its internalization inside the tumor resulting in augmented therapeutic efficacy and reduced adverse effects. A vast array of nanoparticles has been found to be of extreme importance in terms of biocompatibility and safety for siRNA delivery and their encapsulation as well. Additionally, successful loading of the siRNAs in nanoparticles not only protects them from in vivo degradation by the external environment but also aids in their delivery at the required site minimizing the off-target silencing [147]. Moreover, these nanoparticles often result in a synergetic effect that can be attributed to the formation of a chemical and physical bond between the siRNA and the cytotoxic drugs that aid in their delivery to the same tumor cell thus emerging as a potential tool for therapeutic intervention [161, 162]. In accordance with this, Tunc et al. potentiated dual delivery of siRNA (Bcl2) with Dox via the formulation of gold nanoparticles wherein siRNA was thiolated on the surface of AuNPs followed by the intercalation of DOX (DOX-siRNA-AuNPs). Furthermore, scrambled siRNAs (Scr-siRNAs) that were devoid of any particular therapeutic potential were prepared as well, for investigating the effect of the carrier system specifically. The researchers investigated the cytotoxic action of the nanoformulations with and without DOX that revealed a pronounced effect of DOX-loaded AuNPs on the viability of TNBC breast cancer cells (MDA-MB-231) with increasing its concentration than the free solution of DOX. Furthermore, an enhanced rate of apoptosis and cancer cell inhibition was exhibited by the dual drug delivery system. Moreover, suppressed formation of colony and cell migration was achieved too by the BCl-2 gene silencing. In addition, the dual nanoformulation when tested on MCF-7 cell line demonstrated effective results wherein enhanced cytotoxicity and increased cellular internalization were observed.
Although the present research demonstrated the astounding beneficial effects of siRNA and chemotherapeutic agents’ dual delivery for the management of breast cancer, yet it lacks the noteworthy pharmacokinetics, pharmacodynamics, and acute toxicity studies that are extremely essential for ascertaining the pharmaceutical aspects as well as for investigating the toxicity and biocompatibility of the designed multifunctional nanosystem (Fig. 6) [163].
A Flow cytometry analysis conducted at various time points for quantitative evaluation of DOX internalization in MDA-MB-231 cells treated with control, DOX-siRNA-AuNPs, and free DOX. B Analysis of cellular internalization (%) of DOX in MDA-MB-231 cells after their treatment with DOX-siRNA-AuNPs and free DOX. C Flow cytometry analysis conducted at various time points for quantitative evaluation of DOX internalization in MCF-7 cells treated with control, DOX-siRNA-AuNPs, and free DOX. D Analysis of cellular internalization of DOX (%) in MCF-7 cells after their treatment with DOX-siRNA-AuNPs and free DOX. (Points are presented as the mean of three repeats with ± SD. Level of significance: ***p < 0.001) Reproduced with permission from reference [165] DOX doxorubicin, DOX-siRNA-AuNPs doxorubicin-siRNA-gold nanoparticles
A simplified description of recent siRNA and cytotoxic agent combinatorial delivery investigated by various researchers has been summarized in Table 3.
The combinations of chemotherapeutics with monoclonal antibodies
The clones of a unique B cell that are further attached to specific positions of antigens, also known as the epitopes, are referred to as monoclonal antibodies (mAbs). In 1973, Schwaber identified the method for the production of mAbs for the very first time that involved the human mouse hybrid cells too, which was thereafter utilized by Kohler and Milstein for human-derived hybridomas generation that serves as the cornerstone for the large-scale production of antibodies for therapeutic use ever since its generation. The discovery of hybridomas soon led to an upsurge in the research of mAbs for the treatment of cancer [175]. mAbs are known for manifesting multiple essential roles in breast cancer that includes cancer cell targeting, rarely attacking them directly, assisting in the location as well as delivery of the drugs to the target, obstructing the growth of cells, and also suppressing the inhibitors of the immune system. However, the introduction of mAbs is considered a distinct and unparalleled breakthrough in the treatment of HER2 breast cancers which is otherwise less explored for other types of breast cancers [176]. The HER-2 directed mABs exhibit the anticancer property by blocking the pathway of HER2 to broad activation of the immune system which subsequently results in the induction of antibody-dependent cellular cytotoxicity [177]. The first humanized mAb “trastuzumab” was approved in 1998 by Food and Drug Administration (FDA), which got approval after 2 years for the treatment of early stage and metastatic HER2 overexpressing breast cancer by the European Medicines Agency (EMA) [178].
Helmi et al. developed DOX encapsulated PEGylated chitosan coated nanoparticles (CNPs) that were conjugated with two types of mAbs, namely, anti-human mammaglobin (Anti-hMAM) and anti-human epidermal growth factor (Anti-HER2) for their effective delivery in breast cancer. The cytotoxicity results obtained of both Anti-HER2 functionalized PEGylated DOX loaded CSNPs and Anti-hMAM functionalized PEGylated DOX loaded CSNPs demonstrated marked cytotoxic effect against MCF-7 cell line while comparatively less response was observed against mouse fibroblast cell line (L-929) cell line. Additionally, the mAbs conjugated CSNPs showed enhanced cellular uptake and internalization which may be attributed to the attachment of particular receptors of mAbs to the cancer cells [179].
In yet another study, Kolahkaj and team formulated PLGA coated epirubicin (EPI) encapsulated nanoparticles (NPs) conjugated with trastuzumab mAb by an amide linkage. The nanoparticles were prepared by the nanoprecipitation technique and were extensively characterized for various parameters. The results of in vitro cellular toxicity studies performed on positive and negative HER-2 cell lines revealed the higher cytotoxic action and enhanced cellular uptake of EPI-NPs on HER-2 positive cell lines indicating at the superior efficacy of antibodies conjugated NPs in breast cancer treatment [180].
In 2019, Mehata et al. formulated TPGS-g-chitosan nanoparticles conjugated with and without trastuzumab loaded with DTX for targeted breast cancer therapy. The results obtained from the in vitro cytotoxicity studies performed on SK-BR-3 cell lines showed that in comparison to the conventional DTX formulation, both targeted as well as non-targeted formulation exhibited increased cytotoxicity and higher cellular internalization along with possessing significant bioadhesion property. Moreover, the in vivo pharmacokinetic studies revealed enhancement in relative bioavailability along with an increase in the other parameters of pharmacokinetics for both targeted as well as the non-targeted.
Thus, as per the literature, the unprecedented potential of mAbs in breast cancer treatment cannot be underrated and must be explored further for developing clinical formulations with relevant efficiency.
Challenges and future perspective
Although a tremendous groundbreaking clinical improvement has been achieved in the field of oncology, still the alarming rate at which the cases of breast cancer are rising is disturbing. Breast cancer; a heterogenous complex disease involving several molecular pathways and origins of development is usually associated with the development of MDR in an unescapable manner further requiring the emergence of an essential strategic approach for the effective management of breast cancer. Hitherto, delivery of anticancer agents via nanomedicine has been grabbing eyeballs worldwide probably due to their manifold advantages including enhanced bioavailability and effective targeting, particularly improving the MDR effect. However, these nanomedicines still suffer from various pitfalls and major issues such as toxicity, biocompatibility, low rate of patient survival, and so on that must be addressed for their effective translation from the laboratory scale to the clinical level. Recently, nano-based combination delivery of chemotherapeutics has been developing as a promising and novel strategic approach that provides the fabrication of a single drug delivery system with multiple drugs encapsulated in an appropriate nanocarrier so as to aid in improved efficacy at a comparatively low dose, penetration inside the tumors barring the physiological barriers and reduced toxic effects associated with the drugs. Additionally, these nanocombinations display several other distinctive attributes including resulting in a synchronized and optimal drug delivery thereby causing effective antitumor action with synergistic effects. Although the recently emerging novel technique of simultaneous/co-delivery offers us a plethora of advantages, there still persist various obstacles hindering the clinical outcome and are thus needed to be addressed such as enough evidence must be provided regarding their stability, cost-effectiveness, and clinical efficacy as well. Apart from this, there exist challenges related to pharmacokinetics that further limit the site-specific delivery to the targeted cancerous cells, other significant limitations such as identification of the synergistic drug combinations and maintenance of synergism in vivo, optimization of the nanoformulations for aiding in appropriate drug loading and enhanced targeting procedure. Other key issues that persist are the extensive biological studies and in-depth knowledge about the various molecular mechanisms that are required for determining the drug combinations to be used; it is also essential to determine the mass ratio of each component present within the nanoformulation by understanding the effect of the ratios of different components on the biological activity. Moreover, in order to develop clinically pertinent nanocombinations for effective breast cancer therapy, researchers must focus on exploring and fabricating stable in vivo combinations with utmost safety, capable of releasing the drug combinations simultaneously reducing the toxic side effects and enhancing the internalization inside the malignant cells. Lastly, the most critical step in these combinations is their clinical development owing to the complex structures and design that they possess. However, these nanocombinations with integrated efforts of academicians, regulatory bodies, and pharmaceutical industries would soon be highly perceived worldwide for opening new horizons capable of achieving great success in the field of cancer management in comparison to the traditional chemotherapeutics and nanomedicine monotherapy.
From the authors’ point of view, the excellent nanoformulation for the combinatorial delivery of chemotherapeutics could be the lipid-based nanoformulations due to their ability to encapsulate both hydrophobic and hydrophilic agents, better biocompatibility, non-toxicity, and improved therapeutic index when compared to other nanoformulations. Besides these, the other nanoformulations that can be considered as outstanding nanoformulation for combinatorial delivery are the polymeric micelles owing to their advantageous properties such as enhanced stability, controlled and sustained release, and high drug loading capacity for hydrophobic drugs.
Availability of data and materials
NA.
Abbreviations
- PCDA:
-
Amphiphilic poly(curcumin-dithiodipropionic acid)-b-poly(ethylene glycol)-biotin (PCDA-PEG-biotin) copolymer
- Anti-HER2:
-
Anti-human epidermal growth factor
- Anti-hMAM:
-
Anti-human mammaglobin
- BCRP:
-
Breast cancer resistance protein
- CSCs:
-
Cancer stem cells
- CNPs:
-
Chitosan coated nanoparticles
- CLNCs:
-
Chitosan-grafted lipid nanoparticles
- CI:
-
Combination index
- CUR:
-
Curcumin
- CUR-Z-Ber-Ch:
-
Curcumin and berberine zein nanoparticles coated with chitosan
- DTX:
-
Docetaxel
- DRI:
-
Dose reduction index
- DOX:
-
Doxorubicin
- DOX-siRNA-AuNPs:
-
Doxorubicin and siRNAs loaded gold nanoparticles
- EPR:
-
Enhanced permeation and retention
- EGCG:
-
Epigallocatechin
- ER:
-
Estrogen positive receptor
- FITC:
-
Fluorescein5 (6)-isothiocyanate
- FDA:
-
Food and Drug Administration
- AuNPs:
-
Gold nanoparticles
- HER2:
-
Human epidermal growth factor receptor 2
- MTX:
-
Methotrexate
- MTX-CUR-NPs:
-
Methotrexate and curcumin-loaded PLGA nanoparticles
- mAbs:
-
Monoclonal antibodies
- PP:
-
MPEG-PLGA
- mPEG-PLGA-SS (PP-SS-DTX/VRP):
-
MPEG-PLGA-SS-docetaxel-verapamil nanomicelles
- MDR:
-
Multidrug resistance
- MRPs:
-
Multiple drug resistance proteins
- NLCs:
-
Nanostructured lipid carriers
- PTX:
-
Paclitaxel
- PTX/EGCG liposomes:
-
Paclitaxel and epigallocatechin liposomes
- Pgp:
-
P-glycoprotein
- PR:
-
Progesterone positive receptor
- MSNs-ChS@PQ:
-
QC and PTX-loaded mesoporous silica ChS nanoparticles
- QC:
-
Quercetin
- RH123:
-
Rhodamine123
- SEM:
-
Scanning electron microscopy
- siRNAs:
-
Small interfering RNAs
- SLNs:
-
Solid lipid nanoparticles
- TPGS:
-
Tocopheryl polyethylene glycol succinate
- TNBC:
-
Triple-negative breast cancer
- VRP:
-
Verapamil
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551.
Tran P, Lee SE, Kim DH, Pyo YC, Park JS. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J Pharm Investig. 2020;50:261–70. https://doi.org/10.1007/s40005-019-00459-7.
Gupta P, Neupane YR, Parvez S, Kohli K, Sultana Y. Combinatorial chemosensitive nanomedicine approach for the treatment of breast cancer. Curr Mol Med. 2022. https://doi.org/10.2174/1566524023666220819122948.
Sindhu RK, Verma R, Salgotra T, Rahman MH, Shah M, Akter R, Murad W, Mubin S, Bibi P, Qusti S, et al. Impacting the remedial potential of nano delivery-based flavonoids for breast cancer treatment. Mol. 2021;26.
Day CM, Hickey SM, Song Y, Plush SE, Garg S. Novel tamoxifen nanoformulations for improving breast cancer treatment: old wine in new bottles. Mol. 2020;25.
Bahreyni A, Mohamud Y, Luo H. Emerging nanomedicines for effective breast cancer immunotherapy. J Nanobiotechnology. 2020;18:180. https://doi.org/10.1186/s12951-020-00741-z.
Gupta P, Neupane YR, Parvez S, Kohli K. Recent advances in targeted nanotherapeutic approaches for breast cancer management. Nanomedicine. 2021;16:2605–31. https://doi.org/10.2217/nnm-2021-0281.
Cai S, Thati S, Bagby TR, Diab H-M, Davies NM, Cohen MS, Forrest ML. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J Control release Off J Control Release Soc. 2010;146:212–8. https://doi.org/10.1016/j.jconrel.2010.04.006.
Dao K-L, Hanson RN. Targeting the estrogen receptor using steroid-therapeutic drug conjugates (hybrids). Bioconjug Chem. 2012;23:2139–58. https://doi.org/10.1021/bc300378e.
Liyanage PY, Hettiarachchi SD, Zhou Y, Ouhtit A, Seven ES, Oztan CY, Celik E, Leblanc RM. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim Biophys Acta - Rev Cancer. 2019;1871:419–33. https://doi.org/10.1016/j.bbcan.2019.04.006.
Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17:1044–52.
Alhalmi A, Amin S, Khan Z, Beg S, Al kamaly O, Saleh A, Kohli K. Nanostructured lipid carrier-based codelivery of raloxifene and naringin: formulation, optimization, in vitro, ex vivo, in vivo assessment, and acute toxicity studies. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14091771.
Lehár J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short GF, Staunton, J.E., Jin, X., et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27:659–66. https://doi.org/10.1038/nbt.1549.
Shanavas A, Jain NK, Kaur N, Thummuri D, Prasanna M, Prasad R, Ganga V, Naidu M, Bahadur D, Srivastava R. Polymeric core − shell combinatorial nanomedicine for synergistic anticancer therapy. 2019. https://doi.org/10.1021/acsomega.9b02167.
Linton SS, Sherwood SG, Drews KC, Kester M. Targeting cancer cells in the tumor microenvironment: opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:208–22. https://doi.org/10.1002/wnan.1358.
Gurunathan S, Kang M-H, Qasim M, Kim J-H. Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int J Mol. Sci. 2018;19.
Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21:223–32.
Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang X-L, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–37.
Ma L, Kohli M, Smith A. Nanoparticles for combination drug therapy. ACS Nano. 2013;7:9518–25. https://doi.org/10.1021/nn405674m.
Rocha M, Chaves N, Bao S. Nanobiotechnology for breast cancer treatment. Breast Cancer - From Biol Med. 2017. https://doi.org/10.5772/66989.
Hafner A, Lovrić J, Lakoš GP, Pepić I. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–23. https://doi.org/10.2147/IJN.S55359.
Fan J, Liu B, Long Y, Wang Z, Tong C, Wang W, You P, Liu X. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020;113:554–69. https://doi.org/10.1016/j.actbio.2020.06.025.
Adair JH, Parette MP, Altinoğlu EI, Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano. 2010;4:4967–70. https://doi.org/10.1021/nn102324e.
Torchilin VP. Nanoparticulates as drug carriers. London, UK: Imperial college press; 2006. ISBN 186094907X.
Patnala K, Vishwas S, Malla RR. Chapter 17 - Nanotechnology advances in breast cancer. In: Malla RR, Nagaraju GP, editors. A theranostic and precision medicine approach for female-specific cancers. Academic Press; 2021. p. 271–287. ISBN 978–0–12–822009–2.
Grewal IK, Singh S, Arora S, Sharma N. Polymeric nanoparticles for breast cancer therapy: a comprehensive review. Biointerface Res Appl Chem. 2021;11:11151–71. https://doi.org/10.33263/BRIAC114.1115111171.
Singh SK, Singh S, Lillard JWJ, Singh R. Drug delivery approaches for breast cancer. Int J Nanomedicine. 2017;12:6205–18. https://doi.org/10.2147/IJN.S140325.
Mirza Z, Karim S. Nanoparticles-based drug delivery and gene therapy for breast cancer: recent advancements and future challenges. Semin Cancer Biol. 2021;69:226–37. https://doi.org/10.1016/j.semcancer.2019.10.020.
Velasco-Velázquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44:573–7. https://doi.org/10.1016/j.biocel.2011.12.020.
Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int J Breast Cancer 2011;967419. https://doi.org/10.4061/2011/967419.
Clarke R, Leonessa F, Trock B. Multidrug resistance/P-glycoprotein and breast cancer: review and meta-analysis. Semin Oncol. 2005;32:S9-15. https://doi.org/10.1053/j.seminoncol.2005.09.009.
Burger H, Foekens JA, Look MP, Meijer-van Gelder ME.,Klijn JGM, Wiemer EAC, Stoter G, Nooter K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer. Clin Cancer Res. 2003;9:827– 836.
Chintamani Singh JP, Mittal MK, Saxena S, Bansal A, Bhatia A, Kulshreshtha P. Role of p-glycoprotein expression in predicting response to neoadjuvant chemotherapy in breast cancer-a prospective clinical study. World J Surg. 2005;3:61. https://doi.org/10.1186/1477-7819-3-61.
He C, Chan C, Weichselbaum RR, Fleming GF, Yamada SD, Lin W. Nanomedicine for combination therapy of cancer. EBioMedicine. 2015;2:366–7. https://doi.org/10.1016/j.ebiom.2015.05.013.
Hu C-MJ, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83:1104–11. https://doi.org/10.1016/j.bcp.2012.01.008.
Valencia PM, Pridgen EM, Perea B, Gadde S, Sweeney C, Kantoff PW, Bander NH, Lippard SJ, Langer R, Karnik R, et al. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine (Lond). 2013;8:687–98. https://doi.org/10.2217/nnm.12.134.
Jia J, Zhu F, Ma X, Cao Z, Cao ZW, Li Y, Li YX, Chen YZ. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–28.
Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev. 2016;98:19–34. https://doi.org/10.1016/j.addr.2015.10.022.
Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy - strategies and perspectives. J Control Release. 2016;240:489–503. https://doi.org/10.1016/j.jconrel.2016.06.012.
Wang H, Huang Y. Medicine in drug discovery combination therapy based on nano codelivery for overcoming cancer drug resistance. Med Drug Discov. 2020;6:100024. https://doi.org/10.1016/j.medidd.2020.100024.
Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. https://doi.org/10.1124/pr.58.3.10.
Esnaashari SS, Muhammadnejad S, Amanpour S, Amani A. A Combinational approach towards treatment of breast cancer: an analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21:166. https://doi.org/10.1208/s12249-020-01710-3.
Hu C-MJ, Zhang L. Therapeutic nanoparticles to combat cancer drug resistance. Curr Drug Metab. 2009;10:836–41. https://doi.org/10.2174/138920009790274540.
Park K. The beginning of the end of the nanomedicine hype. J Control release Off J Control Release Soc. 2019;305:221–2.
Shuhendler AJ, Cheung RY, Manias J, Connor A, Rauth AM, Wu XY. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res Treat. 2010;119:255–69. https://doi.org/10.1007/s10549-008-0271-3.
Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–39. https://doi.org/10.1016/j.leukres.2008.06.028.
Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res. 2007;16:361–74. https://doi.org/10.3727/000000006783980937.
Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–63. https://doi.org/10.1158/1535-7163.MCT-06-0118.
Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, Bermudes DG, Mayer LD. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8:2266–75. https://doi.org/10.1158/1535-7163.MCT-09-0243.
Shuhendler AJ, Prasad P, Zhang RX, Amini MA, Sun M, Liu PP, Bristow RG, Rauth AM, Wu XY. Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmunocompromised breast tumor model. Mol Pharm. 2014;11:2659–74. https://doi.org/10.1021/mp500093c.
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. https://doi.org/10.1158/0008-5472.CAN-09-1947.
Mangla B, Neupane YR, Singh A, Kohli K. Tamoxifen and sulphoraphane for the breast cancer management: a synergistic nanomedicine approach. Med Hypotheses. 2019;132. https://doi.org/10.1016/j.mehy.2019.109379.
Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharm Nanotechnol. 2019;7:3–23. https://doi.org/10.2174/2211738507666190122111224.
Guo Y, He W, Yang S, Zhao D, Li Z, Luan Y. Co-delivery of docetaxel and verapamil by reduction-sensitive PEG-PLGA-SS-DTX conjugate micelles to reverse the multi-drug resistance of breast cancer. Colloids Surf B Biointerfaces. 2017;151:119–27. https://doi.org/10.1016/j.colsurfb.2016.12.012.
Elzoghby AO, Mostafa SK, Helmy MW, ElDemellawy MA, Sheweita SA. Multi-reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm Res. 2017;34:1956–69. https://doi.org/10.1007/s11095-017-2207-2.
Ashrafizadeh M, Zarrabi A, Hushmandi K, Hashemi F, Rahmani Moghadam E, Raei M, Kalantari M, Tavakol S, Mohammadinejad R, Najafi M, et al. Progress in natural compounds/siRNA co-delivery employing nanovehicles for cancer therapy. ACS Comb Sci. 2020;22:669–700. https://doi.org/10.1021/acscombsci.0c00099.
Cheng Y-Y, Hsieh C-H, Tsai T-H. Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics. J Food Drug Anal. 2018;26:S88–95. https://doi.org/10.1016/j.jfda.2018.01.003.
Alsanad SM, Howard RL, Williamson EM. An assessment of the impact of herb-drug combinations used by cancer patients. BMC Complement Altern Med. 2016;16:1–9.
Mokhtar S, Khattab SN, Elkhodairy KA, Teleb M, Bekhit AA, Elzoghby AO, Sallam MA. Methotrexate-lactoferrin targeted exemestane cubosomes for synergistic breast cancer therapy. Front Chem. 2022;10:847573. https://doi.org/10.3389/fchem.2022.847573.
Li L, Tong R, Li M, Kohane DS. Self-assembled gemcitabine–gadolinium nanoparticles for magnetic resonance imaging and cancer therapy. Acta Biomater. 2016;33:34–9. https://doi.org/10.1016/j.actbio.2016.01.039.
Fan Y, Wang Q, Lin G, Shi Y, Gu Z, Ding T. Combination of using prodrug-modified cationic liposome nanocomplexes and a potentiating strategy via targeted co-delivery of gemcitabine and docetaxel for CD44-overexpressed triple negative breast cancer therapy. Acta Biomater. 2017;62:257–72. https://doi.org/10.1016/j.actbio.2017.08.034.
Kushwah V, Katiyar SS, Dora CP, Kumar Agrawal A, Lamprou DA, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018;73:424–36. https://doi.org/10.1016/j.actbio.2018.03.057.
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine Nanotechnology Biol Med. 2018;14:1629–41. https://doi.org/10.1016/j.nano.2018.04.009.
Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199–216. https://doi.org/10.2147/IJN.S230376.
Nezhad-Mokhtari P, Ghorbani M, Mahmoodzadeh F. Smart co-delivery of 6-mercaptopurine and methotrexate using disulphide-based PEGylated-nanogels for effective treatment of breast cancer. New J Chem. 2019;43:12159–67. https://doi.org/10.1039/C9NJ02470K.
Jose A, Ninave KM, Karnam S, Venuganti VVK. Temperature-sensitive liposomes for co-delivery of tamoxifen and imatinib for synergistic breast cancer treatment. J Liposome Res. 2019;29:153–62. https://doi.org/10.1080/08982104.2018.1502315.
Guo X-L, Kang X-X, Wang Y-Q, Zhang X-J, Li C-J, Liu Y, Du L-B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019;84:367–77. https://doi.org/10.1016/j.actbio.2018.12.007.
Li N, Zhang P, Huang C, Song Y, Garg S, Luan Y. Co-delivery of doxorubicin hydrochloride and verapamil hydrochloride by pH-sensitive polymersomes for the reversal of multidrug resistance. RSC Adv. 2015;5:77986–95. https://doi.org/10.1039/C5RA15313A.
Wu D, Chen Y, Wen S, Wen Y, Wang R, Zhang Q, Qin G, Yi H, Wu M, Lu L, et al. Synergistically enhanced inhibitory effects of pullulan nanoparticle-mediated co-delivery of lovastatin and doxorubicin to triple-negative breast cancer cells. Nanoscale Res Lett. 2019;14:314. https://doi.org/10.1186/s11671-019-3146-0.
Yu X, Sun L, Tan L, Wang M, Ren X, Pi J, Jiang M, Li N. Preparation and characterization of PLGA–PEG–PLGA nanoparticles containing salidroside and tamoxifen for breast cancer therapy. AAPS PharmSciTech. 2020;21:85. https://doi.org/10.1208/s12249-019-1523-8.
Wang L, Li L, Han Q, Wang X, Zhao D, Liu J. Identification and biological evaluation of natural product biochanin A. Bioorg Chem. 2020;97:103674. https://doi.org/10.1016/j.bioorg.2020.103674.
Liskova A, Koklesova L, Samec M, Smejkal K, Samuel SM, Varghese E, Abotaleb M, Biringer K, Kudela E, Danko J, et al. Flavonoids in cancer metastasis. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12061498.
Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10. https://doi.org/10.3390/biom10020221.
Varghese E, Liskova A, Kubatka P, Mathews Samuel S, Büsselberg D. Anti-angiogenic effects of phytochemicals on miRNA regulating breast cancer progression. Biomolecules. 2020;10. https://doi.org/10.3390/biom10020191.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. https://doi.org/10.1021/np200906s.
Butler MS, Robertson AAB, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612–61. https://doi.org/10.1039/c4np00064a.
Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. https://doi.org/10.1039/b514294f.
Salehi M, Movahedpour A, Tayarani A, Shabaninejad Z, Pourhanifeh MH, Mortezapour E, Nickdasti A, Mottaghi R, Davoodabadi A, Khan H, et al. Therapeutic potentials of curcumin in the treatment of non-small-cell lung carcinoma. Phytother Res. 2020;34:2557–76. https://doi.org/10.1002/ptr.6704.
Johnson SB, Park HS, Gross CP, Yu JB. Use of alternative medicine for cancer and its impact on survival. J Natl Cancer Inst. 2018;110. https://doi.org/10.1093/jnci/djx145.
de Oliveira Júnior RG, Christiane Adrielly AF, da Silva Almeida JRG, Grougnet R, Thiéry V, Picot L. Sensitization of tumor cells to chemotherapy by natural products: a systematic review of preclinical data and molecular mechanisms. Fitoterapia. 2018;129:383–400. https://doi.org/10.1016/j.fitote.2018.02.025.
Vinod BS, Maliekal TT, Anto RJ. Phytochemicals as chemosensitizers: from molecular mechanism to clinical significance. Antioxid Redox Signal. 2013;18:1307–48. https://doi.org/10.1089/ars.2012.4573.
Zou J, Zhu L, Jiang X, Wang Y, Wang Y, Wang X, Chen B. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget. 2018;9:11268–78. https://doi.org/10.18632/oncotarget.24109.
Yu Y, Zhou Q, Hang Y, Bu X, Jia W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer. 2007;109:2374–82. https://doi.org/10.1002/cncr.22659.
Erstad DJ, Cusack JCJ. Targeting the NF-κB pathway in cancer therapy. Surg Oncol Clin N Am. 2013;22:705–46. https://doi.org/10.1016/j.soc.2013.06.011.
Eichhorn T, Efferth T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbs. J Ethnopharmacol. 2012;141:557–70. https://doi.org/10.1016/j.jep.2011.08.053.
Efferth T, Saeed MEM, Mirghani E, Alim A. Integration of phytochemicals and phytotherapy into cancer precision medicine. 2017;8:50284–304.
Article R. Natural products drug discovery : accelerating the clinical candidate development using reverse pharmacology approaches. 2010;48:220–7.
Vakilinezhad MA, Amini A, Dara T, Alipour S. Methotrexate and curcumin co-encapsulated PLGA nanoparticles as a potential breast cancer therapeutic system: in vitro and in vivo evaluation. Colloids Surfaces B Biointerfaces 2019;184:110515. https://doi.org/10.1016/j.colsurfb.2019.110515.
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y, Ou J, Zhang T, Zhu H, Wu J, et al. Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J Biomed Nanotechnol. 2016;12:973–85. https://doi.org/10.1166/jbn.2016.2231.
Guo S, Lv L, Shen Y, Hu Z, He Q, Chen X. A nanoparticulate pre-chemosensitizer for efficacious chemotherapy of multidrug resistant breast cancer. Sci Rep. 2016;6:21459. https://doi.org/10.1038/srep21459.
Cui T, Zhang S, Sun H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol Rep. 2017;37:1253–60. https://doi.org/10.3892/or.2017.5345.
Mahmoudi R, Hassandokht F, Ardakani MT, Karimi B, Roustazadeh A, Tarvirdipour S, Barmak MJ, Nikseresht M, Baneshi M, Mousavizadeh A, et al. Intercalation of curcumin into liposomal chemotherapeutic agent augments apoptosis in breast cancer cells. J Biomater Appl. 2021;35:1005–18. https://doi.org/10.1177/0885328220976331.
Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, Javidfar S, Lotfi-Attari J, Sadeghzadeh H, Shafiei-Irannejad V, Zarghami N. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artif. Cells, Nanomedicine, Biotechnol. 2018:46:917–925. https://doi.org/10.1080/21691401.2017.1347879.
Zafar S, Akhter S, Garg N, Selvapandiyan A, Kumar Jain G, Ahmad FJ. Co-encapsulation of docetaxel and thymoquinone in mPEG-DSPE-vitamin E TPGS-lipid nanocapsules for breast cancer therapy: formulation optimization and implications on cellular and in vivo toxicity. Eur J Pharm Biopharm. 2020;148:10–26. https://doi.org/10.1016/j.ejpb.2019.12.016.
Zafar S, Akhter S, Ahmad I, Hafeez Z, Alam Rizvi MM, Jain GK, Ahmad FJ. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of docetaxel and thymoquinone by chitosan grafted lipid nanocapsules: formulation optimization, in vitro and in vivo studies. Colloids Surf. B. Biointerfaces 2020;186:110603. https://doi.org/10.1016/j.colsurfb.2019.110603.
Alkhatib MH, Bawadud RS, Gashlan HM. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci Rep. 2020;10:18124. https://doi.org/10.1038/s41598-020-75017-5.
El-Ashmawy NE, Khedr EG, Ebeid E-ZM, Salem ML, Zidan A-AA, Mosalam EM. Enhanced anticancer effect and reduced toxicity of doxorubicin in combination with thymoquinone released from poly-N-acetyl glucosamine nanomatrix in mice bearing solid Ehrlish carcinoma. Eur J Pharm Sci. 2017;109:525–32. https://doi.org/10.1016/j.ejps.2017.09.012.
Kommineni N, Saka R, Bulbake U, Khan W. Cabazitaxel and thymoquinone co-loaded lipospheres as a synergistic combination for breast cancer. Chem Phys Lipids. 2019;224:104707. https://doi.org/10.1016/j.chemphyslip.2018.11.009.
Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W. Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016;23:2608–16. https://doi.org/10.3109/10717544.2015.1039667.
Sabra SA, Elzoghby AO, Sheweita SA, Haroun M, Helmy MW, Eldemellawy MA, Xia Y, Goodale D, Allan AL, Rohani S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharm. 2018;128:156–69. https://doi.org/10.1016/j.ejpb.2018.04.023.
Elzoghby AO, El-Lakany SA, Helmy MW, Abu-Serie MM, Elgindy NA. Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine. 2017;12:2785–805. https://doi.org/10.2217/nnm-2017-0247.
Shrivastava N, Parikh A, Dewangan RP, Biswas L, Verma AK, Mittal S, Ali J, Garg S, Baboota S. Solid self-nano emulsifying nanoplatform loaded with tamoxifen and resveratrol for treatment of breast cancer. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14071486.
Liu Q, Li J, Pu G, Zhang F, Liu H, Zhang Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2016;23:1364–8. https://doi.org/10.3109/10717544.2015.1031295.
Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022. https://doi.org/10.1007/s12010-022-04000-9.
Sandhu PS, Kumar R, Beg S, Jain S, Kushwah V, Katare OP, Singh B. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: systematic approach for improved breast cancer therapeutics. Nanomedicine, Nanotechnology Biol Med. 2017;13:1703–13. https://doi.org/10.1016/j.nano.2017.03.003.
Dong X, Lang T, Yin Q, Zhang P, Li Y. Co-delivery of docetaxel and silibinin using pH-sensitive micelles improves therapy of metastatic breast cancer. Acta Pharmacol Sin. 2017;38:1655–62. https://doi.org/10.1038/aps.2017.74.
Rahmani A, Rahimi F, Iranshahi M, Kahroba H, Zarebkohan A, Talebi M, Salehi R, Mousavi HZ. Co-delivery of doxorubicin and conferone by novel pH-responsive β-cyclodextrin grafted micelles triggers apoptosis of metastatic human breast cancer cells. Sci Rep. 2021;11:21425. https://doi.org/10.1038/s41598-021-00954-8.
Barkat A, Rahman M, Alharbi KS, Altowayan WM, Alrobaian M, Afzal O, Altamimi ASA, Alhodieb FS, Almalki WH, Choudhry H, et al. Biocompatible polymeric nanoparticles for effective codelivery of tamoxifen with ganoderic acid A: systematic approach for improved breast cancer therapeutics. J Clust Sci. 2022. https://doi.org/10.1007/s10876-022-02332-4.
Alagawany M, Farag MR, Abdelnour SA, Dawood MAO, Elnesr SS, Dhama K. Curcumin and its different forms: a review on fish nutrition. Aquaculture. 2021;532:736030.
Forouzanfar F, Majeed M, Jamialahmadi T, Sahebkar A. Curcumin: a review of its effects on epilepsy. Stud Biomarkers New Targets Aging Res. Iran 2021;363–373.
Tang W, Du M, Zhang S, Jiang H. Therapeutic effect of curcumin on oral diseases: a literature review. Phyther Res. 2021;35:2287–95.
Rezaei-Tazangi F, Roghani-Shahraki H, Khorsand Ghaffari M, Abolhasani Zadeh F, Boostan A, ArefNezhad R, Motedayyen H. The therapeutic potential of common herbal and nano-based herbal formulations against ovarian cancer: new insight into the current evidence. Pharmaceuticals. 2021;14. https://doi.org/10.3390/ph14121315.
Li Y, Sun W, Han N, Zou Y, Yin D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer. 2018;18:1–9.
Ghasemi F, Shafiee M, Banikazemi Z, Pourhanifeh MH, Khanbabaei H, Shamshirian A, Moghadam SA, ArefNezhad R, Sahebkar A, Avan A. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol Pract. 2019;215.
Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phyther Res. 2014;28:1553–60.
Wang H, Zhang K, Liu J, Yang J, Tian Y, Yang C, Li Y, Shao M, Su W, Song N. Curcumin regulates cancer progression: focus on ncRNAs and molecular signaling pathways. Front Oncol. 2021;11: 660712.
Ghobadi-Oghaz N, Asoodeh A, Mohammadi M. Fabrication, characterization and in vitro cell exposure study of zein-chitosan nanoparticles for co-delivery of curcumin and berberine. Int J Biol Macromol. 2022;204:576–86. https://doi.org/10.1016/j.ijbiomac.2022.02.041.
Xiong K, Zhang Y, Wen Q, Luo J, Lu Y, Wu Z, Wu Z, Wang B, Chen Y, Zhao L, Fu S. Co-delivery of paclitaxel and curcumin by biodegradable polymeric nanoparticles for breast cancer chemotherapy. Int J Pharm. 2020;589:119875. https://doi.org/10.1016/j.ijpharm.2020.119875.
Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: development, optimization, in vitro and ex vivo evaluation. J Drug Deliv Sci Technol. 2019;52:55–64. https://doi.org/10.1016/j.jddst.2019.04.025.
Rasouli S, Montazeri M, Mashayekhi S, Sadeghi-Soureh S, Dadashpour M, Mousazadeh H, Nobakht A, Zarghami N, Pilehvar-Soltanahmadi Y. Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of curcumin and chrysin: possible application in prevention of breast cancer local recurrence. J Drug Deliv Sci Technol. 2020;55:101402. https://doi.org/10.1016/j.jddst.2019.101402.
Danafar H, Sharafi A, Kheiri S, Kheiri Manjili H. Co-delivery of sulforaphane and curcumin with PEGylated iron oxide-gold core shell nanoparticles for delivery to breast cancer cell line. Iran J Pharm Res IJPR. 2018;17:480–94.
Tavana E, Mollazadeh H, Mohtashami E, Modaresi SMS, Hosseini A, Sabri H, Soltani A, Javid H, Afshari AR, Sahebkar A. Quercetin: a promising phytochemical for the treatment of glioblastoma multiforme. BioFactors. 2020;46:356–66.
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Cancer Agents Med Chem (Formerly Curr Med Chem Agents). 2009;9:138–161.
Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37.
Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A, Hajighadimi S, Moradizarmehri S. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10:1–17.
Shafabakhsh R, Asemi Z. Quercetin: a natural compound for ovarian cancer treatment. J Ovarian Res. 2019;12:1–9.
Liu Y, Tang Z-G, Lin Y, Qu X-G, Lv W, Wang G-B, Li C-L. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed Pharmacother. 2017;92:33–8.
Lei C-S, Hou Y-C, Pai M-H, Lin M-T, Yeh S-L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018;51:105–13.
Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J Surg Oncol. 2018;16:1–12.
Sadhukhan P, Kundu M, Chatterjee S, Ghosh N, Manna P, Das J, Sil PC. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater Sci Eng C. 2019;100:129–40.
Srivastava NS, Srivastava RAK. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine. 2019;52:117–28.
Rauf A, Imran M, Khan IA, ur‐Rehman M, Gilani SA, Mehmood Z, Mubarak MS. Anticancer potential of quercetin: a comprehensive review. Phyther Res. 2018;32:2109–30.
Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8:529.
Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20:3177.
Liu M, Fu M, Yang X, Jia G, Shi X, Ji J, Liu X, Zhai G. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids Surfaces B Biointerfaces. 2020;196:111284.
Wong M-Y, Chiu GNC. Simultaneous liposomal delivery of quercetin and vincristine for enhanced estrogen-receptor-negative breast cancer treatment. Anticancer Drugs. 2010;21:401–10. https://doi.org/10.1097/CAD.0b013e328336e940.
Wang W, Xiong X, Li X, Zhang Q, Yang W, Du L. In silico investigation of the anti-tumor mechanisms of epigallocatechin-3-gallate. Molecules. 2019;24. https://doi.org/10.3390/molecules24071445.
Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–21.
Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013;93:307–12.
Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–5.
Saeki K, Hayakawa S, Nakano S, Ito S, Oishi Y, Suzuki Y, Isemura M. In vitro and in silico studies of the molecular interactions of epigallocatechin-3-O-gallate (EGCG) with proteins that explain the health benefits of green tea. Molecules. 2018;23:1295.
Ahuja R, Panwar N, Meena J, Singh M, Sarkar DP, Panda AK. Natural products and polymeric nanocarriers for cancer treatment: a review. Environ Chem Lett. 2020;18:2021–30. https://doi.org/10.1007/s10311-020-01056-z.
Bartholome A, Kampkötter A, Tanner S, Sies H, Klotz L-O. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys. 2010;501:58–64.
Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA, Harbison RA, Linseman DA. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal. 2009;11:469–80.
Ramadass SK, Anantharaman NV, Subramanian S, Sivasubramanian S, Madhan B. Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surfaces B Biointerfaces. 2015;125:65–72. https://doi.org/10.1016/j.colsurfb.2014.11.005.
Narayanan S, Mony U, Vijaykumar DK, Koyakutty M, Paul-Prasanth B, Menon D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomedicine Nanotechnology, Biol Med. 2015;11:1399–1406. https://doi.org/10.1016/j.nano.2015.03.015.
Xiao B, Ma L, Merlin D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv. 2017;14:65–73. https://doi.org/10.1080/17425247.2016.1205583.
Tavallaei O, Marzbany M, Rasekhian M. Combinational treatments for breast cancer. J Reports Pharm Sci. 2020;9:279.
Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano. 2013;7:9571–84. https://doi.org/10.1021/nn4047925.
Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–17.
Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, Liang X-J. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:1–25.
Saw PE, Song E-W. siRNA therapeutics: a clinical reality. Sci China Life Sci. 2020;63:485–500. https://doi.org/10.1007/s11427-018-9438-y.
Bucan V, Adili MY, Choi CYU, Eddy M-T, Vogt PM, Reimers K. Transactivation of lifeguard (LFG) by Akt-/LEF-1 pathway in MCF-7 and MDA-MB 231 human breast cancer cells. Apoptosis. 2010;15:814–21.
Fernández M, Segura MF, Solé C, Colino A, Comella JX, Ceña V. Lifeguard/neuronal membrane protein 35 regulates Fas ligand-mediated apoptosis in neurons via microdomain recruitment. J Neurochem. 2007;103:190–203.
Gratzke AL, Reimers K, Vogt PM, Bucan V. Sensitising breast cancer cells to chemotherapy by downregulation of lifeguard. Cancer Sci Ther. 2014;6:411–6.
Bucan V, Reimers K, Choi CY, Eddy M-T, Vogt PM. The anti-apoptotic protein lifeguard is expressed in breast cancer cells and tissues. Cell Mol Biol Lett. 2010;15:296–310.
Bucan V, Choi CYU, Lazaridis A, Vogt PM, Reimers K. Silencing of anti-apoptotic transmembrane protein lifeguard sensitizes solid tumor cell lines MCF-7 and SW872 to perifosine-induced cell death activation. Oncol Lett. 2011;2:419–22. https://doi.org/10.3892/ol.2011.285.
Maurer V, Reimers K, Lück HJ, Vogt PM, Bucan V. Anti-apoptotic protein lifeguard does not act as a tumor marker in breast cancer. Oncol Lett. 2017;13:1518–24.
Kumar K, Rani V, Mishra M, Chawla R. New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr Res Pharmacol Drug Discov. 2022;3:100103. https://doi.org/10.1016/j.crphar.2022.100103.
Yalamarty SSK, Filipczak N, Li X, Pathrikar TV, Cotter C, Torchilin VP. Co-delivery of siRNA and chemotherapeutic drug using 2C5 antibody-targeted dendrimer-based mixed micelles for multidrug resistant cancers. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14071470.
Sun T-M, Du J-Z, Yao Y-D, Mao C-Q, Dou S, Huang S-Y, Zhang P-Z, Leong KW, Song E-W, Wang J. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5:1483–94. https://doi.org/10.1021/nn103349h.
Xiong X-B, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5:5202–13. https://doi.org/10.1021/nn2013707.
Tunç CÜ, Aydin O. Co-delivery of Bcl-2 siRNA and doxorubicin through gold nanoparticle-based delivery system for a combined cancer therapy approach. J Drug Deliv Sci Technol. 2022;103603. https://doi.org/10.1016/j.jddst.2022.103603.
Hu Q, Yao J, Wang X, Wang Y, Fu X, Ma J, Lin H, Xu J, Shen L, Yu X. Combinational chemoimmunotherapy for breast cancer by codelivery of doxorubicin and PD-L1 siRNA using a PAMAM-incorporated liposomal nanoplatform. ACS Appl Mater Interfaces. 2022;14:8782–92. https://doi.org/10.1021/acsami.1c21775.
Liu H, Ma D, Chen J, Ye L, Li Y, Xie Y, Zhao X, Zou H, Chen X, Pu J, et al. A targeted nanoplatform co-delivery of pooled siRNA and doxorubicin for reversing of multidrug resistance in breast cancer. Nano Res. 2022;15:6306–14. https://doi.org/10.1007/s12274-022-4254-1.
Yuan Y, Liu J, Yu X, Liu X, Cheng Y, Zhou C, Li M, Shi L, Deng Y, Liu H, et al. Tumor-targeting pH/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and GCN5 siRNA. Acta Biomater. 2021;135:556–66. https://doi.org/10.1016/j.actbio.2021.09.002.
Wang S, Liu X, Chen S, Liu Z, Zhang X, Liang X-J, Li L. Regulation of Ca2+ signaling for drug-resistant breast cancer therapy with mesoporous silica nanocapsule encapsulated doxorubicin/siRNA cocktail. ACS Nano. 2019;13:274–83. https://doi.org/10.1021/acsnano.8b05639.
Norouzi P, Motasadizadeh H, Atyabi F, Dinarvand R, Gholami M, Farokhi M, Shokrgozar MA, Mottaghitalab F. Combination therapy of breast cancer by codelivery of doxorubicin and survivin siRNA using polyethylenimine modified silk fibroin nanoparticles. ACS Biomater. Sci. & Eng. 2021;7:1074–1087. https://doi.org/10.1021/acsbiomaterials.0c01511.
Peng H, Qiao L, Shan G, Gao M, Zhang R, Yi X, He X. Stepwise responsive carboxymethyl chitosan-based nanoplatform for effective drug-resistant breast cancer suppression. Carbohydr Polym. 2022;291:119554. https://doi.org/10.1016/j.carbpol.2022.119554.
Jafari R, Majidi Zolbanin N, Majidi J, Atyabi F, Yousefi M, Jadidi-Niaragh F, Aghebati-Maleki L, Shanehbandi D, Soltani Zangbar M-S, Rafatpanah H. Anti-mucin1 aptamer-conjugated chitosan nanoparticles for targeted co-delivery of docetaxel and IGF-1R siRNA to SKBR3 metastatic breast cancer cells. Iran Biomed J. 2019;23:21–33. https://doi.org/10.29252/.23.1.21.
Jin M, Zeng B, Liu Y, Jin L, Hou Y, Liu C, Liu W, Wu H, Chen L, Gao Z, et al. Co-delivery of repurposing itraconazole and VEGF siRNA by composite nanoparticulate system for collaborative anti-angiogenesis and anti-tumor efficacy against breast cancer. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14071369.
Zhao Z, Li Y, Liu H, Jain A, Patel PV, Cheng K. Co-delivery of IKBKE siRNA and cabazitaxel by hybrid nanocomplex inhibits invasiveness and growth of triple-negative breast cancer. Sci Adv. 2020;6:eabb0616. https://doi.org/10.1126/sciadv.abb0616.
Shakeran Z, Varshosaz J, Keyhanfar M, Mohammad-Beigi H, Rahimi K, Sutherland DS. Co-delivery of STAT3 siRNA and methotrexate in breast cancer cells. Artif cells, nanomedicine, Biotechnol. 2022;50:29–39. https://doi.org/10.1080/21691401.2022.2030746.
Abedi Gaballu F, Cho WC-S, Dehghan G, Zarebkohan A, Baradaran B, Mansoori B, Abbaspour-Ravasjani S, Mohammadi A, Sheibani N, Aghanejad A, et al. Silencing of HMGA2 by siRNA loaded methotrexate functionalized polyamidoamine dendrimer for human breast cancer cell therapy. Genes (Basel). 2021;12. https://doi.org/10.3390/genes12071102.
Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9. https://doi.org/10.3390/antib9030034.
Behl A, Wani ZA, Das NN, Parmar VS, Len C, Malhotra S, Chhillar AK. Monoclonal antibodies in breast cancer: a critical appraisal. Crit Rev Oncol Hematol. 2023;183:103915. https://doi.org/10.1016/j.critrevonc.2023.103915.
Costa RLB, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. npj Breast Cancer. 2020;6:10. https://doi.org/10.1038/s41523-020-0153-3.
Altunay B, Morgenroth A, Beheshti M, Vogg A, Wong NCL, Ting HH, Biersack H-J, Stickeler E, Mottaghy FM. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 2021;48:1371–89. https://doi.org/10.1007/s00259-020-05094-1.
Helmi O, Elshishiny F, Mamdouh W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int J Biol Macromol. 2021;184:325–38. https://doi.org/10.1016/j.ijbiomac.2021.06.014.
Fathian kolahkaj F, Derakhshandeh K, Khaleseh F, Azandaryani AH, Mansouri K, Khazaei M. Active targeting carrier for breast cancer treatment: monoclonal antibody conjugated epirubicin loaded nanoparticle. J Drug Deliv Sci Technol. 2019;53:101136. https://doi.org/10.1016/j.jddst.2019.101136.
Acknowledgements
The graphical abstract was created using BioRender (biorender.com). All other figures were reproduced with permission.
Funding
The authors are thankful to Jamia Hamdard, New Delhi 110062, India, for providing DST-PURSE Fellowship to Priya Gupta. The authors would also like to thank DST-FIST for providing departmental and instrumental facilities to Jamia Hamdard, New Delhi 110062, India.
Author information
Authors and Affiliations
Contributions
Writing original draft preparation, conceptualization: Priya Gupta; writing—review and editing and conceptualization: Yub Raj Neupane; writing—review and editing: Mohd. Aqil; supervision: Kanchan Kohli; supervision: Yasmin Sultana.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
NA.
Consent for publication
NA.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, P., Neupane, Y.R., Aqil, M. et al. Lipid-based nanoparticle-mediated combination therapy for breast cancer management: a comprehensive review. Drug Deliv. and Transl. Res. 13, 2739–2766 (2023). https://doi.org/10.1007/s13346-023-01366-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01366-z