Abstract
Microemulsions are interesting formulations for cosmeceutical applications due to their good appearance, high solubilization power, thermodynamic stability, and enhancement of skin penetration. In addition, they can spontaneously form when suitable types and amounts of components are simply mixed. In this study, the phase behavior of the nonionic systems with various parameters was studied by construction of phase diagrams using titration method. Natural oils, i.e., coconut oil (CO), rice bran oil (RBO), and palm oil (PO), were analyzed for their fatty acid compositions and then mixed with blends of nonionic surfactants (Tween80: Span80) and water or mixtures of water and a cosolvent, propylene glycol (PG), to find the microemulsion regions. Subsequently, some microemulsions were selected for physical characterization. The largest microemulsion regions which were obtained from CO, RBO, and PO covered the sizes of 11.65, 9.84, and 9.24 %, respectively. The surfactant mixture at weight ratio of 1:1 was the most suitable for CO and PO, but for RBO, it was 2:1. PG could increase the microemulsion regions of PO from 9.24 to 15.33 %, depending on PG concentrations. Hence, the sizes of the microemulsion regions were related to oil types, surfactant mixtures, and ratios between water and PG. The studied microemulsions were water-in-oil (w/o) type, and their internal droplets were in the nanosize range. They exhibited Newtonian flow behavior and their mean viscosity values were from 247.53 to 690.35 cP which were correlated with the types and concentrations of the components in the formulations. In conclusion, natural oils could form w/o microemulsions with nonionic surfactants. The microemulsion formation and characteristics were related to many parameters of the components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microemulsions are visually transparent systems of two immiscible liquids, i.e., oil and water, stabilized by an interfacial film of a surfactant. The advantages of microemulsions are spontaneous formation, ease of manufacture, thermodynamic stability, high soluble capacity for many compounds with different solubility properties, and potential for skin penetration enhancement. Thus, they have been useful carriers for many cosmeceuticals such as skin-lightening, anti-wrinkle, and sunscreen agents [1, 2]. Recently, microemulsions have also been reported for application as cleansing products to remove oily makeup cosmetics [3]. In addition to oil, water, and surfactant phases, a cosurfactant or a cosolvent may be necessary in some systems. Another surfactant, a short-chain alcohol, or a polyhydroxy compound can be used as a cosurfactant or a cosolvent in the microemulsion system. Microemulsion formation and properties are also dependent on the chemical types and amounts of the components in the systems [4, 5]. Therefore, it is interesting to study the effects of different components on microemulsion formation and characteristics.
The selection of oil phase is very important since it influences the solubility of an active ingredient for incorporation into microemulsions. Both natural and synthetic oils can be used to form the microemulsion systems. In the case of natural oils, both types and chain lengths of their composed fatty acids could affect the microemulsion formation and the size of the microemulsion region in the phase diagram. Many findings have reported on the development of microemulsions from natural oils such as babassu oil containing lauric and myristic acids [6], soy bean oil containing oleic and linoleic acids [7, 8], and coconut oil (CO) containing lauric acid [9]. Therefore, these observations clearly indicate that natural oils can provide microemulsions after being mixed with surfactant and water, if the component types and amounts in the systems are suitable.
It was of interest to use CO, rice bran oil (RBO), or palm oil (PO) as an oil phase in microemulsions since they are obtained from local plants in Southeast Asia countries, leading to sustainable sources. Naturally, these oils have different fatty acid compositions. CO is extracted from the copra of coconut palm (Cocos nucifera). It has high content of a medium-chain fatty acid, lauric acid. CO is extensively used for food applications such as margarine, frying oil, and ice cream. Its health benefits for the skin application include antioxidant and moisturizing effects [10–13]. RBO is extracted from rice bran or the brown outer layer of rice (Oryza sativa) kernel which is a by-product generated during the milling process. RBO is a rich source of linoleic and oleic acids. The benefits of RBO include its uses for cooking, providing health benefits as an antioxidant, lowering plasma cholesterol levels, and regulating the immune system [14, 15]. PO is extracted from the mesocarp of the fruit of oil palm tree (Elaeis guineensis). It contains high amounts of palmitic and oleic acids which are saturated and unsaturated fatty acids, respectively. PO is widely used for cooking and for manufacturing of many products such as ice cream, candles, lubricants, biodiesel, toothpaste, and other cosmetics [16–18]. However, these three oils were reported that they had difficulty to form microemulsions. For example, they could not form microemulsions in systems composed of decylglucoside as a surfactant, sorbitan monolaurate or sorbitan monooleate as a cosurfactant, and water as an aqueous phase [19]. Hence, it was of interest to find suitable microemulsion formulations of these oils using ingredients in generally recognized as safe (GRAS) class such as Tween80 and Span80 as nonionic surfactants and propylene glycol (PG) as a cosolvent.
The objectives of this study were to investigate the phase behavior of nonionic systems using different natural oils as oil phase and to study the effects of cosolvent concentrations in aqueous phase on microemulsion formation. Additionally, some microemulsions were selected to be physically characterized for being further used as cosmeceutical delivery systems.
Materials and methods
Materials
CO was prepared in-house by a wet extraction process from coconut milk. RBO was purchased from Thai Edible Oil Co., Ltd. (Samut Prakan, Thailand). PO was purchased from Oleen Co., Ltd. (Samut Sakhon, Thailand). Tween80 (polyoxyethylene (20) sorbitan monooleate), Span80 (sorbitan monooleate), and PG were purchased from P.C. Drug Center Co., Ltd. (Bangkok, Thailand). Distilled water was used throughout the experiments. All the chemicals were used as received without any modifications.
Investigation of types and amounts of fatty acids in natural oils
Three natural oils, i.e., CO, RBO, and PO, were analyzed for identifying their fatty acid compositions by gas chromatography with flame ionization detector (GC-FID; Agilent, USA) as described in a previous report [20] with some modifications. The flow rate of the carrier gas (helium) was 1 ml/min and the split ratio was 50:1. Hydrogen gas and air were used for FID with flow rates of 30 and 300 ml/min, respectively. Separation was carried out on Select Biodiesel for FAME length of 30 m. GC capillary column used was 30 m length, 0.25 mm internal diameter, and 0.25 μm film thickness (Agilent, USA). The injector and detector temperatures were set at 290 and 300 °C, respectively.
Study of the effects of natural oils on microemulsion formation
A pseudoternary phase diagram was constructed by titration method to define the microemulsion region. Briefly, each surfactant mixture (Smix) was prepared by mixing Tween80 and Span80 at various weight ratios of 1:0, 1:1, 2:1, 3:1, 1:2, 1:3, and 2:3. Afterwards, oil (CO, RBO, or PO) was added into each Smix at weight ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1. Subsequently, each obtained mixture of oil and Smix was titrated dropwise with distilled water under vigorous agitation. Finally, the end point of titration was determined when turbid liquid was visually observed. All component amounts providing clear microemulsions were calculated as %w/w and plotted on a triangular graph to define the microemulsion region in each pseudoternary phase diagram. The size of each microemulsion region was evaluated as the percentage of the total area of the phase diagram by cut-and-weight method [21].
Study of the effects of cosolvent concentrations on microemulsion formation
The aqueous phase was prepared by mixing water with a cosolvent, PG, at various water:PG ratios, i.e., 5:1, 3:1, 1:1, and 1:0. Then, each obtained mixture was used instead of distilled water for the construction of the pseudoternary phase diagrams by the method described above.
Preparation of selected microemulsions
Three identical points were selected from the largest microemulsion region of four systems composed of different oil and aqueous phases for the preparation and characterization of the microemulsions. Hence, twelve formulations as exhibited in Table 1 were investigated. All microemulsions were prepared by simply mixing at room temperature.
Characterization of selected microemulsions
The type of microemulsions was identified by staining and conductivity measurement methods. A water soluble dye and an oil soluble dye were dropped into each microemulsion to evaluate the diffusion rates of these two dyes. A faster diffusion of the water soluble dye indicated an oil-in-water (o/w) type while a faster diffusion of the oil soluble dye indicated a water-in-oil (w/o) type. The conductivity values were measured in triplicate by an electrical conductivity meter (ADWA AD32, ADWA Instruments, Hungary) at room temperature.
Microemulsion microstructure was observed using a transmission electron microscope (TEM; JEM-2010, JEOL, Japan). A sample was dropped on a Formvar carbon film on 200-mesh copper grid and left at room temperature until dry. Eventually, the dried sample on the grid was examined by TEM at magnification of ×50,000. Particle size and polydispersity index (PdI) values were determined without any dilution to avoid phase separation by dynamic light scattering (ZetaPALS, Brookhaven Instruments Corporation, USA) at room temperature.
Viscosity values were measured by Brookfield rheometer model DV-III ultra (Brookfield Engineering Laboratories, Inc., USA). The SC4-31 spindle was used with a variety of five speeds from 40 to 95 rpm for which the detected %torque was close to 100. Experiments were performed in triplicate at room temperature. Subsequently, rheograms were plotted for determining the rheological behavior. Viscosity values of the studied oils were also measured by the identical method.
Results and discussion
Types and amounts of fatty acids in natural oils
The types and amounts of fatty acids in CO, RBO, and PO as analyzed by GC-FID are shown in Table 2. The results exhibited that the components were in agreement with the previous report [8] for both types and proportions of fatty acids. However, some slight deviations in the amounts of each fatty acid were found due to the different sources of the oils. The main fatty acids in CO were medium chain such as lauric and myristic acids but those in RBO and PO were long chain. There were high amounts of oleic and linoleic acids in RBO while palmitic and oleic acids were the main long-chain fatty acids in PO.
Effects of natural oils on microemulsion formation
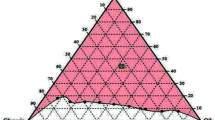
It was found that the systems containing only Tween80 (Smix ratio = 1:0) could not form microemulsions with any of the studied oils (CO, RBO, and PO) while mixtures of Tween80 and Span80 could provide microemulsions. The results indicated that a single surfactant was not suitable to microemulsify the natural oils and water, but the combination of surfactants could reduce the surface tension better than a single surfactant, resulting in effectiveness to generate microemulsions [22]. The pseudoternary phase diagrams of the systems of water, various natural oils, and different ratios of Smix were constructed as exhibited in Fig. 1. The largest microemulsion region of the systems prepared from CO and PO was 11.65 and 9.24 %, respectively, when Smix at weight ratio of 1:1 was used. However, the system from RBO provided the largest microemulsion region of 9.84 % when Smix at weight ratio of 2:1 was used. The reasons were unclear. However, it might be related to affinity between Smix and oils. The solubilization capacity of the surfactant and the structural similarity between the tails of surfactant and fatty acid chains of the oil were reported that they affected the formation of microemulsions [23]. Hydrophilic-lipophilic balance (HLB) is a parameter for identifying the solubilization capacity of a surfactant or of a surfactant blend. In this study, the largest microemulsion regions of the systems prepared from CO, RBO, and PO were found at HLB values of Smix at 9.65, 11.43, and 9.65, respectively, as exhibited in Fig. 2. Additionally, the microemulsion regions of the systems prepared from the studied oils were in agreement with the previous study reported that medium-chain fatty acids provided lager microemulsion regions than long-chain fatty acids since the last ones were too bulky to penetrate into the interfacial film [24]. Hence, the microemulsion regions of the systems prepared from CO were larger than those from RBO and PO at all HLB values as shown in Fig. 2.
Effects of cosolvent amounts on microemulsion formation
Among the studied oils, PO provided the smallest microemulsion regions. Therefore, it was a challenge to increase the size of the microemulsion regions prepared from PO by adding a cosolvent, PG, in aqueous phase. When the systems composed of PO, 1:1 Smix, and various water:PG mixtures were investigated, water:PG mixtures at weight ratios of 1:0, 5:1, 3:1, and 1:1 gave microemulsion regions of 9.24, 10.41, 11.56, and 15.33 %, respectively, as exhibited in Fig. 3. It could be noted that when water:PG mixtures were used instead of pure water, the microemulsion regions increased directly depending on PG concentrations. Higher amounts of PG in water:PG mixtures provided larger sized microemulsion regions. The results indicated that a cosolvent did have an effect on the microemulsion region since it might increase the flexibility of interfacial film and decrease the polarity or dielectric constant of aqueous phase [25, 26].
Characteristics of selected microemulsions
All products obtained from the selected formulations were clear yellowish liquids. The diffusion rate of the oil soluble dye was faster than that of the water soluble dye in all formulations (data not shown). Additionally, the conductivity values of all formulations were 0.00 mS/cm. These results indicated that all studied formulations were w/o type. The w/o microemulsions were obtained since in each system, the amount of aqueous phase was lower than that of oil phase [27] and the total HLB value of Smix was 9.65 causing negative curvature of the interfacial film [28]. This microemulsion type could be applied in cosmetics for removing oily makeups [3]. Furthermore, w/o microemulsions in both original and gel forms were reported to be useful carriers of nicotinamide, a skin-lightening agent [29, 30]. The w/o microemulsions were also suitable carriers for topical delivery of benzophenone-3, a sunscreen, when compared to o/w ones [31]. Hence, the obtained microemulsions should have a high tendency to provide cosmetic and cosmeceutical benefits.
Globule structures were seen by TEM as demonstrated for P1 in Fig. 4, confirming for discontinuous microemulsions. The droplet size of P1 was 17.0 ± 0.2 nm, indicating the nanosize particles. In addition, the droplet sizes of microemulsions typically range from 10 to 140 nm [32]. The PdI of P1 was 0.969 ± 0.055, exhibiting very broad size distribution. Various and high PdI values were previously reported for some microemulsion systems. For instance, systems composed of various ratios of isopropyl myristate, polyoxyethylene (4) lauryl ether, isopropyl alcohol, and water provided microemulsions with PdI values from 0.318 to 0.917 [33]. Systems composed of oleic acid, Tween20, diethylene glycol monoethyl ether, and water at various ratios provided microemulsions with PdI values from 0.25 to 0.89 [34]. This phenomenon was proposed to be caused by the polydispersion and aggregation of the internal droplets [33, 34].
It could be seen in Fig. 5 that the rheograms of the studied formulations showed straight lines, exhibiting Newtonian flow. This flow type is one of the specific characteristics of microemulsions [1]. The mean viscosity values of the samples were in the range of 247.53 to 690.35 cP as shown in Fig. 6. When comparing the formulations containing the same oil, viscosity values were directly dependent upon the concentrations of Smix. It might be due to the intrinsic properties of Tween80 and Span80. Decrease in viscosity was also found in microemulsions having a decrease in Tween80 when the systems were composed of isopropyl myristate, Tween80, PG, water, and amphotericin B [35]. At the same amounts of Smix, the viscosity values of microemulsions containing RBO and PO were similar and higher than those of microemulsions containing CO. The viscosity values of RBO, PO, and CO were 62.27 ± 0.25, 65.40 ± 0.30, and 44.43 ± 0.06 cP, respectively. Hence, when microemulsions contained the same amounts of Smix, their viscosity values were related to the viscosity values of the oil phase. A cosolvent, PG, affected viscosity values of microemulsions containing PO. After PG was added to the aqueous phase (PPG1–PPG3), viscosity values decreased when compared to those of their counterparts (P1–P3). Decrease of viscosity could be obviously seen in PPG1 compared with P1 rather than in PPG2 compared with P2 and in PPG3 compared with P3. It could be explained by that amount of aqueous phase in P1 and PPG1 was 10 % while that in the other formulations was 5 %. The droplet size of P1was 17.0 ± 0.2 nm while that of PPG1 was 9.6 ± 0.4 nm. A decrease in dispersed droplet size resulted in a decrease in viscosity in w/o microemulsions [36].
Conclusions
The natural oils were able to provide microemulsions when mixed with blends of nonionic surfactants having suitable HLB values. The largest microemulsion regions of the systems prepared from CO, RBO, and PO were observed at HLB values of Smix of 9.65, 11.43, and 9.65, respectively. The addition of PG to the aqueous phase increased the microemulsion regions of the systems prepared from PO and was dependent on the cosolvent concentrations. All selected formulations were w/o microemulsions and their characteristics were related to the types and concentrations of the components. The obtained data can be used in the future for the development of cosmeceutical formulations.
References
Boonme P. Applications of microemulsions in cosmetics. J Cosmet Dermatol. 2007;6(4):223–8.
Patravale VB, Mandawgade SD. Novel cosmetic delivery systems: an application update. Int J Cosmet Sci. 2008;30:19–33.
Kim EJ, Kong BJ, Kwon SS, Jang HN, Park SN. Preparation and characterization of w/o microemulsion for removal of oily make-up cosmetics. Int J Cosmet Sci. 2014;36(6):606–12.
Boonme P, Songkro S, Junyaprasert VB. Effects of polyhydroxy compounds on the formation of microemulsions of isopropyl myristate, water and Brij®97. Mahidol Univ J Pharm Sci. 2004;31(1-2):8–13.
Abdulkarim MF, Abdullah GZ, Sakeena M, Chitneni M, Yam MF, Mahdi ES, et al. Study of pseudoternary phase diagram behaviour and the effect of several Tweens and Spans on palm oil esters characteristics. Int J Drug Del. 2011;3(1):95–100.
Pessoa RS, França EL, Ribeiro EB, Lanes PKD, Chaud NGA, Moraes LCA, et al. Microemulsion of babassu oil as a natural product to improve human immune system function. Drug Des Devel Ther. 2015;9:21–31.
Polizelli MA, Telis VRN, Amaral LQ, Feitosa E. Formation and characterization of soy bean oil/surfactant/water microemulsions. Colloids Surf A Physicochem Eng Asp. 2006;281(1-3):230–6.
Dubois V, Breton S, Linder M, Fanni J, Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol. 2007;109(7):710–32.
Mo C, Li X. Microstructure and structural transition in coconut oil microemulsion using semidifferential electroanalysis. J Colloid Interface Sci. 2007;312(2):355–62.
Agyemang-Yeboah F. Health benefits of coconut (Cocos nucifera Linn.) seeds and coconut consumption. In: Preedy VR, Watson RR, Patel VB, editors. Nuts and seeds in health and disease prevention. San Diego: Academic; 2011. p. 361–7.
Yeap SK, Beh BK, Ali NM, Yusof HM, Ho WY, Koh SP, et al. Antistress and antioxidant effects of virgin coconut oil in vivo. Exp Ther Med. 2015;9(1):39–42.
Evangelista MTP, Abad-Casintahan F, Lopez-Villafuerte L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind, clinical trial. Int J Dermatol. 2014;53(1):100–8.
Amri IN. The lauric (coconut and palm kernel) oils. In: Gunstone FD, editor. Vegetable oils in food technology: composition, properties and uses. 2nd ed. West Sussex: Wiley-Blackwell; 2011. p. 169–97.
Friedman M. Rice brans, rice bran oils, and rice hulls: composition, food and industrial uses, and bioactivities in humans, animals, and cells. J Agric Food Chem. 2013;61(45):10626–41.
Sugano M, Tsuji E. Rice bran oil and cholesterol metabolism. J Nutr. 1997;127(3):521s–4.
Ahmad K, Ho CC, Fong WK, Toji D. Properties of palm oil-in-water emulsions stabilized by nonionic emulsifiers. J Colloid Interface Sci. 1996;181(2):595–604.
Sarmidi MR, El-Enshasy H, Hamid MA. Oil palm: the rich mine for pharma, food, feed and fuel industries. Am Eurasian J Agric Environ Sci. 2009;5(6):767–76.
Mba OI, Dumont MJ, Ngadi M. Palm oil: processing, characterization and utilization in the food industry—a review. Food Biosci. 2015;10:26–41.
Pakpayat N, Boonme P. Effects of various co-surfactants and oils on microemulsion formation in decylglucoside system. Adv Mater Res. 2013;747:653–6.
Rohman A, Man YBC. Palm oil analysis in adulterated sesame oil using chromatography and FTIR spectroscopy. Eur J Lipid Sci Technol. 2011;113(4):522–7.
Junyaprasert VB, Boonsaner P, Leatwimonlak S, Boonme P. Enhancement of the skin permeation of clindamycin phosphate by Aerosol OT/1-butanol microemulsions. Drug Dev Ind Pharm. 2007;33(8):874–80.
Li P, Ghosh A, Wagner RF, Krill S, Joshi YM, Serajuddin ATM. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int J Pharm. 2005;288:27–34.
Mahdi ES, Sakeena MHF, Abdulkarim MF, Abdullah GZ, Sattar MA, Noor AM. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des Devel Ther. 2011;5:311–23.
Roohinejad S, Oey I, Wen J, Lee SJ, Everett DW, Burritt DJ. Formulation of oil-in-water β-carotene microemulsions: effect of oil type and fatty acid chain length. Food Chem. 2015;174:270–8.
Yaghmur A, Aserin A, Garti N. Phase behavior of microemulsions based on food-grade nonionic surfactants: effect of polyols and short-chain alcohols. Colloids Surf A Physicochem Eng Asp. 2002;209(1):71–81.
Boonme P, Boonthongchuay C, Limsuwan T, Amnuaikit T, Wongpoowarak W. Effects of cosolvent in water phase on microemulsion regions of nonionic systems and antioxidant efficacy of topical nicotinamide microemulsion. Malaysian J Pharm. 2014;1(11):189.
Eccleston GM. Microemulsions. In: Swarback J, Boylan JC, editors. Encyclopedia of pharmaceutical technology, vol. 9. New York: Marcel Dekker; 1994. p. 375–421.
Mehta SK, Kaur G. Microemulsions: thermodynamic and dynamic properties. In: Tadashi M, editor. Thermodynamics. Rijeka: Intech; 2011. p. 381–406.
Boonme P, Suksawad N, Songkro S. Characterization and release kinetics of nicotinamide microemulsion-based gels. J Cosmet Sci. 2012;63(6):397–406.
Boonme P, Boonthongchuay C, Wongpoowarak W, Amnuaikit T. Evaluation of nicotinamide microemulsion on the skin penetration enhancement. Pharm Dev Technol. 2014. doi:10.3109/10837450.2014.971378.
Songkro S, Lo NL, Tanmanee N, Maneenuan D, Boonme P. In vitro release, skin permeation and retention of benzophenone-3 from microemulsions (o/w and w/o). J Drug Deliv Sci Technol. 2014;24(6):703–11.
Souto EB, Teeranachaideekul V, Boonme P, Müller RH, Junyaprasert VB. Lipid-based nanocarriers for cutaneous administration of pharmaceutics. In: Nalwa HS, editor. Encyclopedia of nanoscience and nanotechnology, vol. 15. Los Angeles: American Scientific Publishers; 2011. p. 479–91.
Acharya A, Sanyal SK, Moulik SP. Formation and characterization of a pharmaceutically useful microemulsion derived from isopropyl myristate, polyoxyethylene (4) lauryl ether (Brij-30), isopropyl alcohol and water. Curr Sci. 2001;81(4):362–70.
Hathout RM, Woodman TJ, Mansour S, Mortada ND, Geneidi AS, Guy RH. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 2010;40:188–96.
Butani D, Yewale C, Misra A. Amphotericin B topical microemulsion: formulation, characterization and evaluation. Colloids Surf B: Biointerfaces. 2014;116:351–8.
Fanun M. Microemulsions with mixed nonionic surfactants. In: Fanun M, editor. Microemulsions: properties and applications. Florida: CRS Press; 2008. p. 87–142.
Acknowledgments
The study was supported by the Nanotec-PSU Center of Excellence on Drug Delivery System, Thailand. The authors gratefully acknowledge Dr. Brian Hodgson for editing and assistance with the English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wuttikul, K., Boonme, P. Formation of microemulsions for using as cosmeceutical delivery systems: effects of various components and characteristics of some formulations. Drug Deliv. and Transl. Res. 6, 254–262 (2016). https://doi.org/10.1007/s13346-016-0279-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0279-x