Abstract
Diabetes mellitus is associated with bone fragility. Although osteoblast maturation is disturbed in patients with diabetes mellitus, the involvement of high glucose (HG) in different stages of osteoblast maturation is unclear. We used MC3T3-E1 cells, a murine osteoblastic cell line. The cells were incubated in high glucose medium (16.5 and 27.5 mM) with three different time courses: throughout 21 days, only first 7 days (early stage) and only last 7 days (late stage). Mineralization assay showed that HG throughout 21 days increased mineralization compared with control (5.5 mM). In the time course experiment, HG increased mRNA expression of Alp, osteocalcin (Ocn), runt-related transcription factor 2 and osterix on days 3 and 5. By contrast, long-term treatment with HG (14 and 21 days) decreased expression of these osteoblastic markers. HG only during early stage enhanced mineralization, while HG only during late stage had no effects. HG increased the expression of bone morphogenetic protein (BMP) 4 and enhanced phosphorylation of Smad1/5/8. Treatment with a BMP receptor antagonist LDN193189 prevented the HG-induced mineralization during early stage of osteoblast differentiation, indicating that HG in the early stage promotes mineralization by BMP4. In conclusion, the study demonstrates that continuous HG treatment might enhance early osteoblast differentiation but disturbs osteoblast maturation, and that BMP-4-Smad signal might be involved in the HG-induced differentiation and mineralization of osteoblasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence has shown that diabetes mellitus (DM) is associated with bone fragility [1,2,3]. The underlying mechanism of DM-induced bone fragility seems to be very complex and remains unclear. Previous studies showed that accumulation of non-enzymatic collagen crosslink is involved in the DM-induced bone fragility by deteriorating bone quality [4]. Moreover, various factors, such as advanced glycation end products [5,6,7] and oxidative stress [7], suppress osteoblast differentiation and function. In addition, serum bone-specific alkaline phosphatase (ALP), a marker of premature osteoblasts, is normal or slightly increased, whereas serum osteocalcin (OCN), a marker of mature osteoblasts, is decreased in patients with type 2 DM [8,9,10,11], and which was recovered by treatment of DM [8, 9]. These findings indicate that DM may cause impairment of osteoblast maturation, and that DM may differently affect osteoblasts in early stage and late stage of osteoblast differentiation.
Glucose is an important nutrient for osteoblastogenesis. Guntur et al. reported that non-differentiated precursor cells prefer to use oxidative phosphorylation, and the cells obtain marked preference for glycolysis to generate adenosine triphosphate during differentiating to osteoblasts [12]. Wei et al. showed that glucose uptake inhibition through glucose transporter (GLUT) 1 in osteoblasts suppressed osteoblastogenesis by proteosomal degradation of runt-related transcription factor 2 (Runx2), a master regulator of osteoblastogenesis [13]. Li et al. also reported that Mst1/2 kinase is necessary for the expression of GLUT1, glucose uptake and bone formation [14]. Thus, glucose uptake seems to play crucial roles in osteoblastogenesis and bone formation. Bone morphogenetic proteins (BMPs) are known to be powerful osteo-inductive molecules which strongly promote osteoblastic differentiation and mineralization through Smad and non-Smad signals [15, 16]. Among them, several BMPs, such as BMP-2, 4 and 7, are reported to have potent osteoblastogenic ability [15, 16]. Karvande et al. reported that parathyroid hormone promoted glucose uptake, leading to upregulation of BMP-4 and osteoblast differentiation [17]. Therefore, BMP signals may be involved in osteoblastogenesis facilitated by glucose uptake.
Although glucose is important for osteoblastogenesis as described above, the effect of high glucose (HG) on osteoblastogenesis is still unclear. Previous in vitro studies reported that HG (11 to 33 mM) suppressed osteoblast differentiation and mineralization on days 21 to 28 [18,19,20,21,22,23]. By contrast, Zhen et al. showed that glucose 11 mM enhanced osteoblast differentiation and mineralization, whereas 22 and 44 mM inhibited them on day 28 in rat primary osteoblasts [24]. Liu et al. also reported that glucose 15.5 mM stimulated proliferation, osteoblast differentiation and mineralization and glucose 25.5 and 35.5 mM prevented on days 7 and 14 in a mouse osteoblastic cell line MC3T3-E1 [25]. These observations suggest that moderate HG promotes osteoblastogenesis, and considerable HG inhibits it. However, how HG influences osteoblast maturation is not fully understood so far. Further investigation is, thus, necessary to clarify the effects of HG on osteoblasts.
The present study aimed to investigate the effects of HG on osteoblast differentiation and mineralization in MC3T3-E1 cells. We also investigated the effect of HG in early and late stages of osteoblast differentiation, separately. In addition, the involvement of BMP-Smad signals was examined.
Materials and methods
Reagents
Cell culture medium and supplements were purchased from GIBCO-BRL (Rockville, MD, USA). Anti-β actin antibody was purchased from Sigma–Aldrich (St. Louis, MO, USA). Antibody against phospho-Smad1/5/8 was purchased from Merck Millipore (Burlington, MA, USA), and antibody against Smad1 was from Cell Signaling Technology (Beverly, MA, USA). A BMP type 1 receptor antagonist LDN193189 hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell cultures
A mouse clonal osteoblastic cell line MC3T3-E1 was purchased from the RIKEN Cell Bank. The cells were cultured in α-minimum essential medium (α-MEM), which contains 5.5 mM of glucose, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin in 5% CO2 at 37 °C. The medium was changed twice a week, and the cells were passaged when they were 80% confluence. For osteoblast differentiation and mineralization assay, MC3T3-E1 cells were cultured in α-MEM supplemented with 10% FBS, 1% penicillin–streptomycin, 10 mM β-glycerophosphate and 50 mg/L ascorbic acid for 21 days after reaching confluence. The medium was changed twice a week. For incubation with HG medium, l-glucose was added to osteoblastogenic medium so that final concentration of glucose became 16.5 mM and 27.5 mM. We used MC3T3-E1 cells passaged within 9 times.
To investigate the effects of glucose on osteoblasts, MC3T3-E1 cells were treated in different glucose concentration (5.5, 16.5 and 27.5 mM) and three different time course group during osteoblast differentiation. As shown in Fig. 1, in continuous group, the cells were continuously treated with glucose 5.5, 16.5 and 27.5 mM for 21 days (continuous). In early-stage group, the cells were treated with glucose 5.5, 16.5 and 27.5 mM for first 7 days, and then they were incubated with normal glucose (5.5 mM) for 14 days (early stage). In late-stage group, the cells were incubated with normal glucose medium for 14 days, and then they were treated with glucose 5.5, 16.5 and 27.5 mM for late 7 days (late stage).
Schematic protocol of high glucose treatment during osteoblastic differentiation. The cells were exposed to high glucose with three different protocols (continuous, early stage and late stage). The expression of mRNA was examined on days 3, 5, 7, 14 and 21 in continuous group. Mineralization assay was performed on day 21
Mineralization stainings
The mineralization of MC3T3-E1 cells was determined in 24-well plates using von Kossa and Alizarin red stainings. After reaching confluence, the cells were incubated in osteoblast differentiation medium with or without additional glucose for 21 days, the cells were stained with 2% AgNO3 and fixed with 2.5% NaS2O3 by the von Kossa method to detect phosphate deposits in bone nodules [26]. At the same time, the other plates were fixed with ice-cold 70% ethanol and stained with Alizarin red to detect calcification [26]. For quantification, cells stained with Alizarin red were destained with ethylpyridium chloride, then the extracted stain was transferred to a 96-well plate, and the absorbance at 595 nm was measured using a microplate reader.
Alkaline phosphatase staining
ALP staining was performed by standard protocol. After reaching confluence, the cells were incubated in osteoblast differentiation medium containing glucose 5.5, 16.5 and 27.5 mM for 4 days. Thereafter, the cells were rinsed in PBS, fixed in 4% formaldehyde, rinsed with PBS again, and then overlaid with the BCIP/NBT reagent (Sigma–Aldrich, St. Louis, MO, USA), followed by incubation at room temperature for 15 min in the dark.
Quantification of gene expression using real-time polymerase chain reaction (PCR)
Total RNA was extracted from the cultured MC3T3-E1 cells using Trizol reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer’s recommended protocol. Total RNA 2 µg was transcribed into double-stranded cDNA (cDNA synthesis kit; Invitrogen, San Diego, CA, USA). Then, we used SYBR Green chemistry to examine the mRNA expression of osteoblastogenic markers; Alp, type 1 collagen (Col-1), Ocn, Runx2 and osterix (Osx). A housekeeping gene, 36b4, was used to normalize the differences in the efficiencies of reverse transcription. The primer sequences are described in Table 1. Real-time PCR was performed using 50 ng of cDNA in a 25 μL reaction volume with Thermal Cycler Dice Real-Time System II (Takara Bio, Shiga, Japan). The double-stranded DNA-specific dye SYBR Green I was incorporated into the PCR buffer provided in the SYBR Green Real-time PCR Master Mix (Toyobo Co. Ltd., Tokyo, Japan) to enable quantitative detection of the PCR product. The PCR conditions were 95 °C for 15 min, 40 cycles of denaturation at 94 °C for 15 s, and annealing and extension at 60 °C for 1 min.
Western blot analysis
For western blot analysis, the cells were plated in 6-well plates and cultured. Triton X-100 lysis buffer (50 mM Tris, 1 mM EDTA, 1.5 mM MgCl2, 150 mM NaCl, 1% Triton X-100 and 10% glycerol) was mixed with protease inhibitor Cocktail (Sigma–Aldrich, St. Louis, MO, USA), phosphatase inhibitor Cocktail 2 (Sigma–Aldrich, St. Louis, MO, USA), phosphatase inhibitor Cocktail 3 (Sigma–Aldrich, St. Louis, MO, USA). The cells were washed with cold PBS, then Triton X-100 buffer containing the inhibitors was added. The cells were scraped, incubated on ice for 10 min and shaken for 30 min at 4 °C. The cell lysates were centrifuged at 14,000 rpm, 4 °C for 20 min. The supernatant was collected, and the protein concentration was quantified with Bradford Protein Assay (Thermo Fisher Scientific, MA, Waltham, USA). The protein concentrations were adjusted to the lowest one. Thereafter, equal volume of 2 × Laemmli Sample Buffer (BIO-RAD, Hercules, CA, USA) containing 5% β-Mercaptoethanol was added. The cell lysates were electrophoresed using 10% SDS-PAGE and transferred to a nitrocellulose membrane (BIO-RAD, Hercules, CA, USA). The blots were blocked with Tris-buffered saline (TBS) containing 1% Tween 20 (BIO-RAD, Hercules, CA, USA) and 3% BSA for 1 h at 4 °C. Thereafter, the blots were incubated overnight at 4 °C with gentle shaking with a primary antibody for Smad1 and phosphorylated Smad1/5/8 at a dilution of 1:1000 and primary antibodies for β actin at 1:5000. These blots were extensively washed with TBS containing 1% Tween 20 and were further incubated with a 1:5000 dilution of horseradish peroxidase-coupled IgG of specified animal species (rabbit or mouse) matched to the primary antibodies in TBS for 30 min at 4 °C. The blots were then washed in TBS containing 1% Tween 20 three times, and the signal was visualized using an enhanced chemiluminescence technique. The bands were quantified with a software Image J [27]. The results were described as relative to control.
Statistics
Results are expressed as means ± standard error (SE). Statistical evaluations for differences between groups were performed using one-way analysis of variance followed by Fisher’s protected least significant difference. For all statistical tests, a value of p < 0.05 was considered a statistically significant difference.
Results
The effects of high glucose on mineralization and expression of osteoblastic differentiation markers in MC3T3-E1 cells
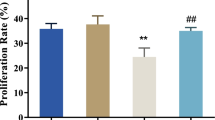
We examined the effect of HG on mineralization in MC3T3-E1 cells. von Kossa and Alizarin red staining showed that HG 16.5 mM and 27.5 mM increased mineralization (Fig. 2a). Quantification of Alizarin red staining showed that the increase of mineralization was significant in a dose-dependent manner (Fig. 2b). In addition, glucose 16.5 and 27.5 mM increased ALP staining (Fig. 2c). On the other hand, bromodeoxyuridine assay showed that HG 16.5 mM did not affect proliferation (supplemental fig. S1). These observations suggest that HG facilitates osteoblast differentiation and mineralization independent of proliferation.
The effects of high glucose on mineralization and the expression of osteoblastic differentiation markers at different time points in MC3T3-E1 cells. a von Kossa staining and Alizarin red staining on day 21. b Quantification of Alizarin red staining (n = 8). c ALP staining on day 4. d–h The mRNA expression of osteoblastic markers (Alp, Col-1, Ocn, Runx2 and Osx) (n ≧ 10). *p < 0.05, **p < 0.01, ***p < 0.001
Next, we examined the effects of continuous HG treatment on expression of osteoblastic differentiation markers at different time points. The cells were incubated in differentiation medium containing glucose 5.5, 16.5 and 27.5 mM, respectively, and mRNA expression of osteoblastic differentiation markers (Alp, Col-1, and Ocn) and crucial osteoblastogenic transcription factors (Runx2 and Osx) were examined on days 3, 5, 7, 14 and 21 (Fig. 1). HG 16.5 mM significantly increased the expression of Alp, Ocn and Osx on days 3 and 5 (Fig. 2d, e), and increased Runx2 on day 3 (Fig. 2d). In addition, HG 27.5 mM increased Alp and Runx2 on days 3 and 5 (Fig. 2d, e), and increased Osx on day 3 (Fig. 2d). The expression of Col-1 was not affected by either HG 16.5 or 27.5 mM on days 3 and 5 (Fig. 2d, e). The increases in osteoblastogenic markers were greater in HG 16.5 mM except for Runx2 on day 5 (Fig. 2d,e). By contrast, HG 16.5 and 27.5 mM significantly decreased Col-1 and Ocn on day 7 (Fig. 2f). Moreover, HG 16.5 and 27.5 mM significantly decreased all of the osteoblastic differentiation markers on days 14 and 21 (Fig. 2g, h).
The effects of high glucose on mineralization during early and late stages of osteoblast differentiation in MC3T3-E1 cells
Because the effects of HG on the expression of osteoblastic markers were different between early and late stages of osteoblast differentiation, we speculated that the effects of HG on osteoblast differentiation are different between premature and mature osteoblasts. To investigate our hypothesis, we examined the effects of HG during early and late stages of osteoblast differentiation on mineralization in MC3T3-E1 cells. The cells were incubated in differentiation medium containing glucose 5.5, 16.5 and 27.5 mM only during day 0 to 7 (early stage) or during day 14 to 21 (late stage) (Fig. 1). Remaining period was incubated in glucose 5.5 mM (Fig. 1). Alizarin red and von Kossa stainings showed that HG 16.5 and 27.5 mM only during early stage of osteoblastogenesis clearly enhanced mineralization (Fig. 3a). Quantification of Alizarin red staining showed that the increase was significant (Fig. 3b). By contrast, HG only during late stage did not affect mineralization (Fig. 3c, d).
The effect of high glucose on mineralization during the early and late stages of osteoblast differentiation in MC3T3-E1 cells. The effects of early stage and late stage HG on mineralization. a, c Mineralization assay on day 21. b, d Quantification of Alizarin red staining (n = 8). **p < 0.01, ***p < 0.001
The effects of high glucose on expression of osteoblastic differentiation markers in late stage of osteoblast differentiation
We investigated the effect of HG on expression of osteoblastic differentiation markers in mature osteoblasts. MC3T3-E1 cells were differentiated toward mature osteoblasts by incubating in differentiation medium containing glucose 5.5 mM for 14 days. Thereafter, the cells were incubated in differentiation medium containing glucose 5.5, 16.5 and 27.5 mM for 3 days, and mRNA expression was examined. Unlike early stage of MC3T3-E1 cells, HG 16.5 and 27.5 mM significantly decreased Col-1 and Ocn (Fig. 4b, c). The expression of Alp, Runx2 and Osx was not affected by HG (Fig. 4a, d, e). Taken together, these findings suggest that HG promotes osteoblast differentiation in premature osteoblasts, which contributes to the increase of mineralization. By contrast, HG prevents maturation of late-stage osteoblasts.
The effects of high glucose on the expression of osteoblastic differentiation markers in MC3T3-E1 cells in the late stage of osteoblast differentiation. a–e The cells were incubated in osteoblast differentiation medium containing glucose 5.5 mM for 14 days. Then incubated with glucose 5.5, 16.5 and 27.5 mM for 3 days, and the mRNA expression was examined (n = 17). *p < 0.05, **p < 0.01, ***p < 0.001
The involvement of BMP signals in HG-induced enhancement of mineralization in MC3T3-E1 cells
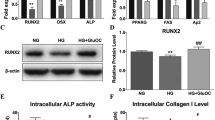
Because BMP-2, BMP-4 and BMP-7 are known to be potent inducers of osteoblastogenesis [16], we examined the effects of HG on BMPs expression. Real-time PCR showed that HG 16.5 and 27.5 mM significantly and dose-dependently increased the expression of BMP-4 (Fig. 5b) but not BMP-2 (Fig. 5a). BMP-7 mRNA was hardly expressed (data not shown). Next, we examined the effect of HG on phosphorylation of Smad1/5/8, a key molecule of BMP signals. Western blot analysis showed that HG clearly increases phosphorylated Smad1/5/8 and total Smad1 (Fig. 5c). Quantification of the bands showed that HG 16.5 and 27.5 mM significantly increased ratio of phospho-Smad1/5/8 and β-actin (Fig. 5e), and HG 27.5 mM significantly increased total Smad1/β-actin ratio (Fig. 5f). Reflecting the increase in total Smad1, the increase in the ratio of phosho-Smad1/5/8 and total Smad1 was slight but significant (Fig. 5d).
The involvement of BMP-4-Smad signal in HG-induced enhancement of osteoblast differentiation and mineralization in MC3T3-E1 cells. a, b The mRNA expression of Bmp-2 and Bmp-4 on day 3 (n ≧ 11). c–f) The cells were treated with glucose 5.5, 16.5 and 27.5 mM for 48 h, and protein levels were examined (n = 4). g The cells were treated with glucose 5.5 and 16.5 mM with or without a BMP receptor antagonist, LDN193189 (LDN), for 48 h, and the protein levels were examined. h, i The cells were treated with glucose 5.5 and 16.5 mM with or without LDN for 7 days. Then, the cells were normal glucose medium and Alizarin red staining was performed on day 21 (n = 3). **p < 0.01, ***p < 0.001
We examined the involvement of BMP-4-Smad signal in HG-induced enhancement of mineralization using a BMP type 1 receptor antagonist LDN193189 (LDN). Treatment with LDN 10–7 M alone slightly decreased phosphorylation of Smad1/5/8 (Fig. 5g), and LDN 10–7 M also prevented HG-induced phosphorylation of Smad1/5/8 (Fig. 5g). Thereafter, we examined the effect of BMP receptor blockade on mineralization. MC3T3-E1 cells were incubated in osteoblastogenic medium containing glucose 5.5 or 16.5 mM with or without LDN 10–7 M for 7 days, then the cells were incubated in differentiation medium containing glucose 5.5 mM for 14 days. Alizarin red staining was performed on day 21. LDN alone seemed to suppress mineralization by Alizarin red staining (Fig. 5h); however, the difference was not significant by its quantification (Fig. 5i). On the other hand, LDN prevented HG-induced enhancement of mineralization (Fig. 5h, i). These findings indicate that HG increases the expression of BMP-4 in early stage of osteoblastogenesis, which leads to the enhancement of mineralization.
Discussion and conclusion
The present study showed that HG in early stage of osteoblast differentiation enhanced mineralization via increasing BMP-4 expression and BMP-4-Smad signal in MC3T3-E1 cells. Moreover, HG increased osteoblastogenic markers in early stage and decreased them in late stage, respectively, indicating that HG may stimulate osteoblastogenesis in premature osteoblasts, whereas HG may prevent maturation of osteoblasts. Recent studies showed that mature osteoblasts predominantly use glucose to produce ATP [12], and that glucose uptake plays crucial roles in osteoblast differentiation and bone formation [13, 14, 17]. Especially, Karvande et al. reported that parathyroid hormone increased glucose uptake, leading to the increase in BMP-4 and bone formation [17]. Taken all together, BMP-4 might be a key molecule which links glucose uptake and osteoblastogenesis.
Several in vitro studies showed that HG prevented osteoblastogenesis and mineralization [18,19,20,21,22,23]. Most of these studies used markedly high concentrations of glucose (greater than or equal to 25 mM). In contrast, Zhen et al. and Liu et al. reported that moderately HG 11 mM and 15.5 mM enhanced and markedly HG suppressed osteoblast differentiation and mineralization in rat primary osteoblasts [24] and MC3T3-E1 cells [25], respectively. Taken together, it is considered that moderately, HG may enhance mineralization of osteoblasts, and markedly, HG may suppress it. Moderately, HG may be beneficial for osteoblastogenesis, but further increase in glucose levels may counteract by increasing oxidative stress [19] and suppression of phosphatidylinositol-3 kinase/Akt signals [19, 21, 23, 25].
In the present study, the increase in osteoblastic markers was greater in HG 16.5 mM than 27.5 mM. As to this point, we found that HG 16.5 and 27.5 mM significantly decreased intrinsic BMP antagonists, DAN and noggin (supplemental fig. S2). Of note, the decreases of DAN and noggin were greater in glucose 16.5 mM group than 27.5 mM (supplemental fig. S2). These alterations of intrinsic BMP antagonists might be involved in the variation of the markers. In addition, variability was also observed in the mRNA expression of Col-1. The mRNA levels of Col-1 (relative to control) reduced until day 14 and then increased on day 21. A previous study showed that glucose uptake in osteoblasts increased expression of type 1 collagen via activation of mTORC1 signals and which was independent of Runx2 [13]. Therefore, it is considered that the increase in Col-1 mRNA on day 21 might be mediated by mechanisms independent of Runx2.
In the study of Liu et al., moderately, HG 15.5 mM increased mRNA of Runx2, Osx and Ocn on days 3 or 7 in MC3T3-E1 cells [25], which is consistent with our study. By contrast, the study of Zheng et al. showed that moderately HG 11 mM increased Runx2 mRNA on day 28 in rat primary osteoblasts [24], which is different from the present study that longer treatment with HG suppressed the expression of osteoblastic markers. Although the reasons remain unknown, the discrepancy might be attributable to the difference of cell types (MC3T3-E1 cells and rat primary osteoblasts) or methods of osteoblast differentiation (containing dexamethasone or not).
A discrepancy was observed between the effect of HG on the mRNA expression of osteoblastic markers and mineralization in this study. HG 16.5 mM effectively increased osteoblastic markers than HG 27.5 mM. However, glucose 27.5 mM increased mineralization greater than 16.5 mM. The discrepancy might be attributable to differences between protein and mRNA expression levels. It has been reported that glucose uptake facilitates osteoblast differentiation by inhibiting proteasomal degradation of Runx2 [13]. Moreover, BMP signals are known to activate ERK1/2 [16] and it has been reported that ERK1/2 promotes osteoblastogenesis by phosphorylation of Runx2 [28,29,30]. These inhibition of degradation or phosphorylation of Runx2 protein might have contributed to the discrepancy between the mRNA expression and mineralization. However, the discrepancy of the other markers is not sufficiently explained, and further investigation is necessary.
HG is an important trait of DM. Previous studies showed that an early premature osteoblast marker ALP was increased and a mature osteoblast marker OCN was decreased in type 2 DM patients [8,9,10,11], and that short-term treatment of hyperglycemia improved the alterations of bone markers [8, 9]. These observations suggest that disturbance of osteoblast maturation may be involved in DM-induced bone fragility. The clinical characteristics of bone markers in type 2 DM are similar to the results of our study that HG increased ALP in early-stage premature osteoblasts and decreased OCN in late-stage mature osteoblasts. Therefore, HG itself may contribute to the DM-associated abnormality of bone remodeling. Bone mineral density (BMD) is decreased in patients with type 1 DM, characterized by complete depletion of insulin secretion, whereas BMD in patients with type 2 DM is often slightly elevated compared with an age-matched control population [1,2,3]. Although the increased BMD in type 2 DM is usually explained by overweight, HG may be partly involved in it.
There are several limitations. Effect of hyperosmolality caused by HG was not investigated in the study. Whether hyperosmolarity affects osteoblast differentiation and mineralization is controversial [23, 31,32,33,34]. Previous studies have reported that hyperosmolality caused by mannitol altered mRNA expression of Alp, Col-1 and Ocn [33, 34]. On the other hand, we and other researchers have shown that mannitol had no effect on osteoblast differentiation and mineralization [23, 31, 32]. Therefore, hyperosmolarity may not strongly alter the differentiation and mineralization of osteoblasts although there is a possibility that it affected the results of this study. Next, the inhibitory effect of HG on osteoblast differentiation markers was observed as early as day 7. Thus, late stage may correspond to days 7 to 21. Lastly, the study used a single mouse cell line. Therefore, it is necessary to confirm results of the present study using primary cultures and in vivo models.
In conclusion, HG increased osteoblastic differentiation and mineralization especially in premature osteoblasts, whereas it decreased osteoblastic markers in mature osteoblasts. BMP-4-Smad signal may be involved in the HG-induced osteoblastic differentiation and mineralization. These findings suggest that hyperglycemia may contribute to the mechanism of DM-induced bone fragility, and that treatment of hyperglycemia may improve the risk of fragility fracture in patients with DM.
References
Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int. 2007;18:427–44.
Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–92.
Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24:702–9.
Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–23.
Okazaki K, Yamaguchi T, Tanaka K, Notsu M, Ogawa N, Yano S, et al. Advanced glycation end products (AGEs), but not high glucose, inhibit the osteoblastic differentiation of mouse stromal ST2 cells through the suppression of osterix expression, and inhibit cell growth and increasing cell apoptosis. Calcif Tissue Int. 2012;91:286–96.
Notsu M, Yamaguchi T, Okazaki K, Tanaka K, Ogawa N, Kanazawa I, et al. Advanced glycation end product 3 (AGE3) suppresses the mineralization of mouse stromal ST2 cells and human mesenchymal stem cells by increasing TGF-beta expression and secretion. Endocrinology. 2014;155:2402–10.
Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–19.
Okazaki R, Totsuka Y, Hamano K, Ajima M, Miura M, Hirota Y, et al. Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J Clin Endocrinol Metab. 1997;82:2915–20.
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, et al. Adiponectin is associated with changes in bone markers during glycemic control in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:3031–7.
Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus—a systematic review. Bone. 2016;82:69–78.
Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover—a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137–R157157.
Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018;33:1052–65.
Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161:1576–91.
Li W, Deng Y, Feng B, Mak KK. Mst1/2 kinases modulate glucose uptake for osteoblast differentiation and bone formation. J Bone Miner Res. 2018;33:1183–95.
Sanchez-Duffhues G, Hiepen C, Knaus P, Ten Dijke P. Bone morphogenetic protein signaling in bone homeostasis. Bone. 2015;80:43–59.
Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005.
Karvande A, Kushwaha P, Ahmad N, Adhikary S, Kothari P, Tripathi AK, et al. Glucose dependent miR-451a expression contributes to parathyroid hormone mediated osteoblast differentiation. Bone. 2018;117:98–115.
Cao B, Liu N, Wang W. High glucose prevents osteogenic differentiation of mesenchymal stem cells via lncRNA AK028326/CXCL13 pathway. Biomed Pharmacother. 2016;84:544–51.
Dong K, Hao P, Xu S, Liu S, Zhou W, Yue X, et al. Alpha-lipoic acid alleviates high-glucose suppressed osteogenic differentiation of MC3T3-E1 cells via antioxidant effect and PI3K/Akt signaling pathway. Cell Physiol Biochem. 2017;42:1897–906.
Liu J, Yang J. Uncarboxylated osteocalcin inhibits high glucose-induced ROS production and stimulates osteoblastic differentiation by preventing the activation of PI3K/Akt in MC3T3-E1 cells. Int J Mol Med. 2016;37:173–81.
Wang R, Gao D, Zhou Y, Chen L, Luo B, Yu Y, et al. High glucose impaired estrogen receptor alpha signaling via beta-catenin in osteoblastic MC3T3-E1. J Steroid Biochem Mol Biol. 2017;174:276–83.
Bilotta FL, Arcidiacono B, Messineo S, Greco M, Chiefari E, Britti D, et al. Insulin and osteocalcin: further evidence for a mutual cross-talk. Endocrine. 2018;59:622–32.
Chen Y, Hu Y, Yang L, Zhou J, Tang Y, Zheng L, et al. Runx2 alleviates high glucose-suppressed osteogenic differentiation via PI3K/AKT/GSK3beta/beta-catenin pathway. Cell Biol Int. 2017;41:822–32.
Zhen D, Chen Y, Tang X. Metformin reverses the deleterious effects of high glucose on osteoblast function. J Diabetes Complications. 2010;24:334–44.
Liu Z, Jiang H, Dong K, Liu S, Zhou W, Zhang J, et al. Different concentrations of glucose regulate proliferation and osteogenic differentiation of osteoblasts via the PI3 kinase/Akt pathway. Implant Dent. 2015;24:83–91.
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab. 2009;296:E139–E146146.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, Franceschi RT. Interactions between extracellular signal-regulated kinase 1/2 and p38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res. 2012;27:538–51.
Li Y, Ge C, Long JP, Begun DL, Rodriguez JA, Goldstein SA, et al. Biomechanical stimulation of osteoblast gene expression requires phosphorylation of the RUNX2 transcription factor. J Bone Miner Res. 2012;27:1263–74.
Ge C, Cawthorn WP, Li Y, Zhao G, Macdougald OA, Franceschi RT. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J Cell Physiol. 2016;231:587–96.
Balint E, Szabo P, Marshall CF, Sprague SM. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001;28(1):21–8.
Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm Metab Res. 2007;39(12):871–5.
Zayzafoon M, Stell C, Irwin R, McCabe LR. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J Cell Biochem. 2000;79:301–10.
Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99:411–24.
Acknowledgements
This study was partly supported by a research grant from Eli Lilly Japan. The authors thank Keiko Nagira and Shizuko Aoki for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. Eli Lilly Japan had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Human and animal research statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takeno, A., Kanazawa, I., Tanaka, Ki. et al. High glucose promotes mineralization via bone morphogenetic protein 4-Smad signals in early stage of osteoblast differentiation. Diabetol Int 12, 171–180 (2021). https://doi.org/10.1007/s13340-020-00463-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-020-00463-5