Abstract
Distinct features of the pancreas of fulminant type 1 diabetes (FT1DM) include (1) enterovirus infection of the islets and exocrine acinar tissue. (2) Activated innate immune responses: MDA5 and RIG-I expression and TLR4 and TLR9 expression in the islets of FT1DM. (3) Combined activation of the STAT/JNK and NFkB pathways, resulting in Type I interferon (IFN) and proinflammatory cytokine (i.e., IFNγ) expression in islet beta cells and MHC class I hyper-expression. (4) Activation of dendritic cells followed by effector cell infiltration of CD8+ T cells and CD68+ macrophages, resulting in apoptosis and neurosis of islet cells and exocrine acinar cells. (5) Many chemo-attractants (i.e., CXCL10) and chemotactic activators (i.e., l-plastin) were induced by a viral infection. (6) Mutual stimulating effect of cytokines expressed in beta cells in autocrine and paracrine mechanisms may enhance beta-cell destruction through the STA1-caspase pathway. (7) Proteomics analysis using laser capture microdissection followed by mass spectrometry found 38 molecules in inflamed islets of FT1DM, which were not highlighted before. Our pathologically verified model of beta-cell destruction in FT1DM will contribute to anti-virus therapy of type 1 diabetes in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “fulminant type 1 diabetes (FT1DM)” is derived from the characteristic symptoms documented as “an abrupt onset of diabetic symptoms, thirst and weakness, and the point at which symptoms first developed can be easily determined” [1,2,3]. At admission to hospital, such patients usually show normal HbA1 levels and the extent of their hyperglycemia and ketosis is more severe than in acute-onset type 1 diabetes [1,2,3]. Recent pathological analysis demonstrated that the destructive process progresses rapidly through virus-induced innate and adaptive immune processes and specific host immunogenetic backgrounds [1,2,3,4,5,6,7,8,9] in addition to many molecules not highlighted in type 1 diabetes previously [10, 11].
Pathogenic virus
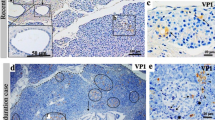
The virus that causes FT1DM is an acutely cytolytic virus that exhibits tropism mainly to islets and occasionally to exocrine acinar cells [12, 13]. It generally assumed that the causative viruses are belongs to the family Picornaviridae: enterovirus (EV), human herpes virus 6, influenza B virus and cytomegalovirus [14]. Immunohistochemical methods (IHC) and in situ hybridization (ISH) are used to detect EV, by staining freshly cut/prepared sections with the commonly used monoclonal antibody 5D8/1 with the optimized condition to obtain specific IHC staining VP1 in the islet cells (Fig. 1a) [15].
Enterovirus infection in fulminant type 1 diabetes (FT1DM). a Enterovirus (EV) envelope protein 1 (VP1, brown, arrows) staining is positive mainly in beta cells in FT1DM. Acinar cells (arrowheads) are also positive for VP1. b In situ hybridization demonstrate EV-RNA (brown dots) in exocrine pancreatic acinar cells in FT1DM. Numerous RNA dots in acinar cells suggest strong tropism of EV to exocrine acinar cells in FT1DM. c Enterovirus VP1 (red) was stained in the duodenal mucosa in a case with FT1DM
In FT1DM, EVs are found to have tropism both to the islets and exocrine acinar cells of the pancreas (Fig. 1a, b) [12, 15]. Interestingly, EV-positive acinar cells are pyknotic and sometimes de-granulated, and are eosinophilic by Hematoxylin–Eosin (HE) staining [12, 15]. EVs also reside on the duodenal mucosa (Fig. 1c) and are assumed to retrogradely spread from duodenal mucosa to the pancreas through pancreatic ducts or through the systemic circulation in FT1DM and acute-onset type 1 diabetes (AT1DM) [15].
Innate immune responses to viral invasion
The most profound feature of innate immunity against EV invasion in FT1DM is expression of the cytoplasmic pattern recognizing receptors, which include MDA5 and RIG-I receptors. Further, their membrane sensors TLR2, TLR4 and TLR9 are reported in FT1DM [13, 16, 17].
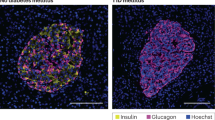
In FT1DM, MDA5, a cytoplasmic double stranded RNA sensor, is expressed in all islet cell subtypes, while RIG-I is expressed specifically in beta cells [16]. Downstream signals are the STAT1 and MyD88 pathways, which induce the expression of type I interferons (IFNα, β) and some proinflammatory cytokines and natural killer (NK) cells (Fig. 2a). The expression of type I IFNs induces class I MHC hyper-expression on the surface of beta cells (Fig. 2b). MHC-class I hyper-expression is a hallmark of migratory immune cells including CD8+ T cells and CD68+ macrophages. Dendritic cells (DCs), which connect innate and adaptive immunity, are activated and engulf beta-cell debris in the islets to present antigen to CD8+ T cells in the regional lymph nodes (Fig. 2c) [16, 17].
Presence of natural killer (NK) cells, dendritic cells (DCs), CD8+ T cells and MHC class-I expressing islet cells. a CD56+ natured killer (NK) cells (arrows) to the islet in FT1DM. b MHC class I hyper-expressed islet cells in FT1DM. Merge image with MHC class I glucagon (red) and insulin (blue). Note that hyper-expression of MHC class I is observed in alpha cells as well as beta cells. c CD11C+ dendritic cells (DCs) are infiltrated around and into the islets. d CD8+ T cell (brown) infiltration to the islets and acinar cells (arrowhead)
Adaptive immunity and destructive mechanisms of islet beta cells
The most predominant pathological feature of enterovirus-induced inflammation in the islets and exocrine pancreas is insulitis and exocrine pancreatic inflammation [12, 15]. The frequency of insulitis among islets is 60%, and most islets are infiltrated by CD8+ T cells, CD68+ macrophages and DCs [15] (Fig. 2d). Characteristically, DCs reside mainly around the islets (Fig. 2c). Beta cells expressing caspase 3 showed T-cell mediated cell damage [16]. Destruction of Fas-positive beta cells by Fas ligand-positive cell-mediated mechanisms is also involved [10, 16].
Another interesting finding regarding islet inflammation is the migratory characteristic of effector cells [19]. They extravasate from the vascular wall near the target islets (Fig. 3a–d), and migrate to the interstitial space (rather than the vasculature of the islets) to reach the target islets, suggesting that specific attracting chemokines may influence their trafficking movements to the target. In addition, the peri-islet region is the final destination of migrating immune cells, indicating that peri-insulitis is common in human type 1 diabetes (Fig. 3d). One of the attracting chemokines is C-X-C motif chemokine 10 (CXCL10), which is expressed on endangered beta cells, and specific ligand-bearing cells (CXCR3+ cells) or macrophages are attracted to and accumulate in the islets [12]. Interestingly, CXCL10 was also expressed on EV-positive acinar cells, suggesting that EV-induced adaptive mechanisms involve the exocrine pancreas attracting virus-specific T cells in FT1DM [15]. Detailed mechanisms of accelerated beta cells in FT1DM was reviewed previously [10].
Migration of CD68+ macrophages to target islets through interstitial space of pancreatic exocrine glands. Fibronectin (green) represents the basement membrane surrounding each gland. a Migration of CD68+ macrophages (red) to the target islet beta cells (blue), penetrating the vascular wall (green). b Magnified view of b in a. c Magnified view of c in a. d The CD68+ macrophages reached and surrounded the target islets in FT1DM
Recent mass spectrometry-based proteomic analysis of inflamed islets in FT1DM detected several proteins not highlighted by classical pathological observation [11] (Table 1).
Table 1 includes 38 molecules associated with cell repair, viral replication, anti-viral function, immune cell migration and viral infection. Plastin-2 (LCP1) was highly expressed on CD8+ T cells and CD68+ macrophages that aggressively infiltrated to or around the islets in FT1DM, indicating a sensitive marker of active migration of T cells or macrophages to the islets [11]. Thymidine phosphorylase (TYMP) is expressed in infiltrating MNCs to the islets of FT1DM, which presumably effector cells and the inhibitors of TYMP are potential targets of intervention of beta-cell destruction [11].
Structural alterations of islets
Structural alterations of islets frequently occur at the basement membranes (BMs), extra-cellular matrix (ECM) packing the islets and cell cluster named Acinar-cell cluster Touching Langerhans islets with Thin Interstitial Surrounding (ATLANTIS): the BMs and ECM were sometimes disrupted during islet inflammation (Fig. 4a) [18]. A detailed description of ATLANTIS is beyond the scope of this review. The mechanisms of structural change of islets and ATLANTIS are mainly related to infiltrating mononuclear cell (MNC), especially CD8+ T cells, DCs and CD68+ macrophages [18]. These cells infiltrate into islets by secreting proteases, which enable immune cells to penetrate the barriers (e.g., BMs and ECMs) surrounding the islets, and disrupt the microenvironment maintained by islets and ALANTIS. Another interesting feature of ATLANTIS in inflamed FT1DM is the increased expression of regenerating protein 1 (Reg 1α) in ATLANTIS. Reg 1α is located in ATLANTIS under non-inflammatory conditions. Under inflammatory conditions the cell cluster hyper-expresses Reg 1α, suggesting crucial roles of ATLANTIS to regenerate the islet cells in an inflammatory milieu of FT1DM [18, 19].
Damaged islet basement membrane (red) by immune cell infiltrated into the islets and hyper-expressing Reg 1alpha in the ATLANTIS cell (green), which touch with beta cells (blue). a Basement membranes (red, fibronectin) encapsulating islets and ATLANTIS cells were damaged by infiltrating effector cells. ATLANTIS cells (asterisks), which is touching with beta cells (blue), hyper-express regenerating protein alpha (Reg 1α) (green, asterisks). b Ki-67+ cells (red, arrows) were increased in beta cells representing active regeneration in the islets of FT1DM (18)
Regeneration of beta cells
Exocrine pancreatic inflammation
Distinct pathological feature of the pancreas with FT1DM is exocrine pancreatic inflammation never reported previously [15]. Enterovirus is the primary cause of exocrine inflammation (Fig. 1b), which is sometimes associated with clinical symptoms of FT1DM including abdominal pain and hyperamylasemia. Most striking features in pancreatic inflammation go through a similar immunological pathway with insulitis. Enterovirus triggers innate immunity followed by adaptive immunity to cell apoptotic death also in exocrine pancreas in FT1DM. CXCL10 is also expressed in the acinar cells accelerating the processes [15]. As a consequence, autoantibodies against amylase α 2A and heat shock protein 10 are reported in FT1DM [20, 21].
Conclusion
FT1DM is mainly caused by acute cytolytic EV infection to the islets and acinar cells. EVs initiate innate immune activation followed by adaptive immune activation. During innate and adaptive immune conditions, beta cells and acinar cells are affected by chemokine and cytokine exposure. It remains unclear whether EVs persistently infect the pancreas with established pathological features or not. Further study on long-standing features in the pancreas of FT1DM is required.
References
Kobayashi T. Immunology and immunogenetics of type I diabetes in Japan. IDF Bull. 1990;35:34–7.
Kobayashi T. Subtype of insulin-dependent diabetes mellitus (IDDM) in Japan: slowly progressive IDDM-the clinical characteristics and pathogenesis of the syndrome. Diabetes Res Clin Pract. 1994;24(Suppl):S95–S9999.
Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000;342:301–7.
Tanaka S, Kobayashi T, Momotsu T. A novel subtype of Type 1 diabetes mellitus. N Engl J Med. 2000;342:1835–7.
Tanaka S, Kobayashi T, Nakanishi K, Koyama R, Okubo M, Murase T, Odawara M, Inoko H. Association of HLA-DQ genotype in autoantibody-negative and rapid onset type 1 diabetes. Diabetes Care. 2002;25:2302–7.
Kawabata Y, Nishida N, Awata T, Kawasaki E, Imagawa A, Shimada A, Osawa H, Tanaka S, Takahashi K, Nagata M, Yasuda H, Uchigata Y, Kajio H, Makino H, Yasuda K, Kobayashi T, Hanafusa T, Tokunaga K, Ikegami H. Genome-Wide association study confirming a strong effect of HLA and identifying variants in CSAD/lnc-ITGB7-1 on chromosome 12q13.13 associated with susceptibility to fulminant type 1 diabetes. Diabetes. 2019;68:665–75.
Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H. Different contribution of class II HLA in fulminant and typical autoimmune type 1 diabetes mellitus. Diabetologia. 2005;48:294–300.
Tsutsumi C, Imagawa A, Ikegami H, Makino H, Kobayashi T, Hanafusa T, Japan Diabetes Society Committee on Type 1 Diabetes Research. Class II HLA genotype in fulminant type 1 diabetes: a nationwide survey with reference to glutamic acid decarboxylase antibodies. J Diabetes Investig. 2012;3:62–9.
Kawabata Y, Ikegami H, Awata T, Imagawa A, Maruyama T, Kawasaki E, Tanaka S, Shimada A, Osawa H, Kobayashi T, Hanafusa T, Tokunaga K, Makino H. on behalf of the committee on type 1 diabetes, Japan diabetes society: Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52:2513–21.
Tanaka S, Aida K, Nishida Y, Kobayashi T. Pathophysiological mechanisms involving aggressive islet cell destruction in fulminant type 1 diabetes. Endocr J. 2013;60:837–45.
Nishida Y, Aida K, Kihara M, Kobayashi T. Antibody-validated proteins in inflamed islets of fulminant type 1 diabetes profiled by laser-capture microdissection followed by mass spectrometry. PLoS ONE. 2014;9:e107664.
Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, Suzuki M, Shimura H, Takizawa S, Takahashi M, Akiyama D, Arai-Yamashita S, Furuya F, Kawaguchi A, Kaneshige M, Katoh R, Endo T, Kobayashi T. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated β-cell failure in fulminant type 1 diabetes. Diabetes. 2009;58:2285–91.
Shibasaki S, Imagawa A, Tauriainen S, et al. Expression of toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–9.
Yoneda S, Imagawa A, Fukui K, Uno S, Kozawa J, Sakai M, Yumioka T, Iwahashi H, Shimomura I. A histological study of fulminant type 1 diabetes mellitus related to human cytomegalovirus reactivation. J Clin Endocrinol Metab. 2017;102:2394–400.
Takita M, Jimbo E, Fukui T, Aida K, Shimada A, Oikawa Y, Yagihashi S, Miura J, Babazono T, Kobayashi T. Unique inflammatory changes in exocrine and endocrine pancreas in enterovirus-induced fulminant type 1 diabetes. J Clin Endocrinol Metab. 2019;104:4282–94.
Aida K, Nishida Y, Tanaka S, Maruyama T, Shimada A, Awata T, Suzuki M, Shimura H, Takizawa S, Ichijo M, Akiyama D, Furuya F, Kawaguchi A, Kaneshige M, Itakura J, Fujii H, Endo T, Kobayashi T. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates β-cell death in fulminant type 1 diabetes. Diabetes Diabetes. 2011;60:884–9.
Kobayashi T, Nishida Y, Tanaka S, Aida K. Pathological changes in the pancreas of fulminant type 1 diabetes and slowly progressive insulin-dependent diabetes mellitus (SPIDDM): innate immunity in fulminant type 1 diabetes and SPIDDM. Diabetes Metab Res Rev. 2011;27:965–70.
Aida K, Saitoh S, Nishida Y, Yokota S, Ohno S, Mao X, Akiyama D, Tanaka S, Awata T, Shimada A, Oikawa Y, Shimura H, Furuya F, Takizawa S, Ichijo M, Ichijo S, Itakura J, Fujii H, Hashiguchi A, Takasawa S, Endo T, Kobayashi T. Distinct cell clusters touching islet cells induce islet cell replication in association with over-expression of regenerating gene (REG) protein in fulminant type 1 diabetes. PLoS ONE. 2014;9:e95110.
Aida K, Kobayashi T, Takeshita A, Jimbo E, Nishida Y, Yagihashi S, Hosoi M, Fukui T, Sugawara A, Takasawa S. Crucial role Reg 1 from acinar-like cell cluster touching with islets (ATLANTIS) on mitogenesis of beta cells in EMC virus-induced diabetic mice. Biochem Biophys Res Commun. 2018;503:963–9.
Endo T, Takizawa S, Tanaka S, Takahashi M, Fujii H, Kamisawa T, Kobayashi T. Amylase α-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes. 2009;58:732–7.
Takizawa S, Endo T, Wanjia X, Tanaka S, Takahashi M, Kobayashi T. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant type 1 diabetes. Biochem Biophys Res Commun. 2009;386:192–6.
Acknowledgements
The authors would like to thank the late Taro Maruyama, Saitama Social Insurance Hospital, for his generous assistance in obtaining the study samples, Erika Jimbo, Okinaka Memorial Institute for Medical Research, for her excellent technical assistance with immunostaining of samples, and Fumie Takano, Okinaka Memorial Institute for Medical Research, for her excellent secretarial assistance.
Funding
This work was supported by a research grant from Japan Society for the Promotion of Science KAKENHI (Grant 2459319).
Author information
Authors and Affiliations
Contributions
TK, KA and ST conducted the immunohistochemical staining and discussed, reviewed, and edited the manuscript. TK contributed to the planning and discussions and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest in this work.
Ethical approval
All procedures used in this study were approved by the ethics committees of the University of Yamanashi and Toranomon Hospital.
Informed consent
Written, informed consent was obtained from the next of kin on behalf of the autopsied patients.
Statement on human research
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation of Toranomon Hospital and University of Yamanashi and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kobayashi, T., Tanaka, S. & Aida, K. Unique pathological changes in the pancreas of fulminant type 1 diabetes. Diabetol Int 11, 323–328 (2020). https://doi.org/10.1007/s13340-020-00462-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-020-00462-6