Abstract
Quercetin, a naturally occurring flavonoid, has been credited with a wide spectrum of therapeutic properties. However, the oral use of quercetin is limited due to its poor water solubility, low bioavailability, rapid metabolism, and rapid plasma clearance. Quercetin has been studied extensively when used with various nanodelivery systems for enhancing quercetin bioavailability. To enhance its oral bioavailability and efficacy, various quercetin-loaded nanosystems such as nanosuspensions, polymer nanoparticles, metal nanoparticles, emulsions, liposomes or phytosomes, micelles, solid lipid nanoparticles, and other lipid-based nanoparticles have been investigated in in-vitro cells, in-vivo animal models, and humans. Among the aforementioned nanosystems, quercetin phytosomes are attracting more interest and are available on the market. The present review covers insights into the possibilities of harnessing quercetin for several therapeutic applications and a special focus on anticancer applications and the clinical benefits of nanoquercetin formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Quercetin is a plant flavonoid used mainly as an anticancer agent due to its antioxidant properties, but also as an anti-microbial agent, anti-osteoporotic agent, anti-fungal agent, anti-psoriatic agent, anti-neurodegenerative agent, anti-inflammatory agent, and in the treatment of cardiovascular diseases. |
The bioavailability and therapeutic efficacy of quercetin are limited by its low water solubility, limited permeability, high enzymatic degradation, and the lack of bioenhanced formulations on the market. |
Among various approaches, the nanodrug delivery strategy provides significant improvements in the solubilization and bioenhancement of quercetin, with the added advantage of targeted delivery whenever desired. |
Marketed quercetin nanoformulations and quercetin phytosomes have attracted huge attention around the world. |
1 Introduction
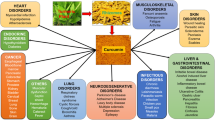
Quercetin is a plant flavonoid obtained from vegetables, grains, and fruits. It has attained immense importance in the past few years due to its multifarious therapeutic applications [1, 2]; for example, it can be used as an anti-microbial agent, anti-osteoporotic agent, anticancer agent, anti-fungal, anti-psoriatic, and anti-neurodegenerative, and anti-inflammatory agent, as well as in the treatment of cardiovascular diseases [3]. The potential use of quercetin in the treatment of viral infections such as COVID-19 has also proposed [3, 4]. The various mechanisms of action and possible applications of quercetin are depicted in Fig. 1 [5].
Various uses and mechanisms of action of quercetin. BAX Bcl-2-associated X protein, TLR toll-like receptor, DAF abnormal Dauer formation, PI3K phosphoinositide 3-kinase, Akt protein kinase B, ROS reactive oxygen species, NF-κB nuclear factor kappa B, TNF-α tumor necrosis factor α, IL interleukins, GSH glutathione, SOD superoxide dismutase, iNOS inducible nitric oxide synthase, AChE acetylcholinesterase, AMPK adenosine monophosphate-activated protein kinase, PPAR-γ PPAR peroxisome proliferator-activated receptor
2 Challenges Associated with the Conventional Administration of Quercetin
The structure of quercetin is shown in Fig. 2. Quercetin is classified as a BCS class IV drug based on its poor solubility (0.00215 g/L at 25 °C) in aqueous media and limited permeability through the gastrointestinal epithelium [6]. Quercetin is unstable in the presence of heat and oxygen and also undergoes photolytic degradation [7, 8]. Another challenge to delivering quercetin is its rapid metabolization into glucuronide and sulfate conjugates, which limits systemic circulation and therapeutic efficacy [9]. Studies were carried out by administering quercetin either via intraperitoneal injection or orally (via the gavage method or drinking water) and the intravenous injection route at various doses ranging from 100 µg to 200 mg/kg/day for between 10 and 90 days [10]. The suggested reviews [11,12,13,14] provide additional details on routes of administration of quercetin. Gastrointestinal factors are reported to influence the delivery of quercetin. Oral bioavailability is influenced significantly by food and in particular fat intake, as well as the gastrointestinal tract pH [15]. Collectively, factors including its low water solubility, high metabolic rate, inactive metabolic products, and rapid clearance from plasma [16,17,18] limit the bioavailability and therapeutic efficacy of quercetin, implying the need to develop nanoformulation strategies to improve quercetin's bioavailability and efficacy.
3 Bioenhancement Strategies for Quercetin
Various approaches have been evaluated for quercetin bioenhancement [12]: for instance, coadministration with piperine [19] or chemical modification (e.g., esterification) or the design of prodrugs [20]. Nevertheless, an important focus has been the development of solid dispersions using various carriers. An amorphous solid dispersion (ASD) generally comprises emulsifying components with drug particles less than 1000 nm in size [21]. Using an ASD is an exciting approach for improving enhancement due to its rapid dissolution and simplicity of preparation [22,23,24]. An ASD of quercetin in a 1:1 weight ratio with combination carriers of Pluronic F-127 and polyvinylpyrrolidone K30 (5:95) significantly improved quercetin solubility with its enhanced dissolution performance [25]. Chitosan oligosaccharide (amorphous) was reported as a promising hydrophilic matrix for a quercetin ASD. The ASD formulation showed enhanced in vitro dissolution performance and oral bioavailability compared to pure quercetin [26]. Further, a quercetin solid dispersion with hydrophilic carriers of hydroxypropyl methylcellulose along with poloxamer 188 as a surfactant exhibited an enhanced dissolution rate with a 61-fold higher oral bioavailability than the pure drug [27]. Other polymers explored for the design of quercetin solid dispersions with rapid dissolution and/or enhanced bioavailability include polyvinylpyrrolidone [28], cellulose esters [29], polyethylene glycol 1000 [30], hydroxypropyl methylcellulose [31], polyvinylpyrrolidone K30 [32], poloxamer 188 [33], and combination of polyvinylpyrrolidone or hydroxypropyl methylcellulose with Pluronic F-127 [34, 35].
4 Nanoformulation Approaches for Oral Bioenhancement
Nanoparticles (NPs) are small particles 1–1000 nm in size [36] which exhibit significantly enhanced dissolution rates due to their large surface area and also the bioavailability of water-insoluble drugs [37]. These nanosystems can be prepared from many materials, including lipids, polymers, metals, proteins [38, 39], and combinations [11, 40]. All of these materials display good chemical stability, enhanced drug loading, controlled drug release, enhanced bioavailability, and excellent biocompatibility [41]. Figure 3 shows nanodrug delivery strategies for oral bioenhanced quercetin formulations. These NPs can encapsulate drug molecules and carry them to various target sites in the body, as their nanosize permits them to cross biological barriers and target specific cells and tissues [42]. Another strategy that could particularly target the lung and breast is lymph-mediated oral uptake [43, 44]. Moreover, NPs can protect drugs from degradation and metabolism, thereby improving their bioavailability [45]. Among various approaches, nano approaches present significant advantages in solubilization and bioenhancement, with the added advantage of targeted delivery whenever desired. This review focuses on various nano approaches for quercetin bioenhancement, which could also have targeting applications. Table 1 summarizes the physicochemical properties and other outcomes of quercetin nanoformulations, whereas Table 2 represents a summary of oral pharmacokinetic parameters of quercetin nanoformulations.
4.1 Nanosuspensions
Nanosuspensions are dispersions of active hydrophobic substances that are nanometrically dispersed in water using stabilizers (surfactants) and produced by various methods [70]. Generally, nanosuspensions are prepared by either the top-down or the bottom-up process [71]. In the bottom-up process, the active moiety with or without carrier(s) is solubilized in an organic solvent. It is then precipitated by addition to an aqueous phase acting as an anti-solvent along with a stabilizer to enable precipitation at a nanosize [72]. This is followed by the elimination of organic solvents. This process is simple, cost-effective, and requires a low energy input [73]. The top-down process involves breaking down the bulk material into NPs in the presence of a high-energy input such as high-pressure homogenization [74].
Improved solubility of quercetin nanosuspensions—nine times higher than that of quercetin—is reported. This improved solubility is attributed to the reduced particle size and enhanced surface area available for dissolution [75]. Quercetin nanosuspensions prepared by evaporative precipitation in an aqueous solution (EPAS, a bottom-up technique) and by high-pressure homogenization (HPH, a top-down process) were compared. The nanosuspension produced by the EPAS process displayed an improved solubility and dissolution rate when compared with the HPH process. These observations were related to the unchanged crystalline state of quercetin during the top-down manufacturing process, whereas a crystalline to amorphous phase change was induced during the bottom-up process, implying the role of the preparation method in the properties of the nanosuspension [76, 77]. Nanosuspensions of quercetin prepared by wet milling combined with lyophilization displayed a 26-fold improvement in dissolution as well as a 3.35-fold enhancement in quercetin permeability [78]. Quercetin nanosuspensions prepared by a solvent displacement method were studied for efficacy against A. aegypti larvae. A high and concentration-dependent larvae mortality was reported for nanosuspensions of quercetin at 100 ppm (44%) and 500 ppm (100%) at 48 h. Pure quercetin showed a maximum mortality of ~ 50% irrespective of concentration [79]. A quercetin nanosuspension formulation revealed a 70-fold solubility enhancement, a 7-fold reduction in clearance rate, and a > 10-fold increase in AUC0–∞ compared with a control suspension in a rat model [80]. In another study, the enhanced cellular uptake of a quercetin nanosuspension was attributed to the small particle size, which facilitated high cellular uptake and bioavailability [81]. Further, quercetin nanosuspensions have shown significantly higher anticancer activity against human breast cancer cells [82, 83].
4.2 Liposomes
Liposomes are phospholipid-based vesicular systems [84]. Phospholipids, which have hydrophobic and hydrophilic portions, align as lamellar structures, which form liposomes [85]. These can protect active pharmaceutical ingredients from their external surroundings, increase water solubility, and facilitate targeted delivery due to their morphology, which resembles cellular membranes [86]. Liposomes are classified based on size, preparation method, and lamellarity [87]. The manufacturing method dictates the formation of unilamellar, multilamellar, or multivesicular vesicles [88]. The lipid film hydration and ether/ethanol injection, solvent dispersion, mechanical dispersion, and detergent removal methods are the most common techniques used to load both hydrophilic and hydrophobic drugs into liposomes. Extensive details are provided in the reviews [89,90,91,92]. Due to their safety and efficacy, liposomes are the most commonly used nanoformulations [93].
Liposomal quercetin prepared by the ethanol injection method displayed extended drug release and suppressed the levels of reactive oxygen species induced by UVB irradiation [94]. Lecithin, cholesterol, and PEG containing flexible liposomes generated by a simple solid dispersion method induced apoptosis by arresting the cell cycle in A2780s and A2780cp cells [47]. Liposomal quercetin formulations prepared by thin-film hydration revealed effective accumulation in tumor tissues, suppression of tumor growth, and prolonged survival time in tumor-bearing mice. This demonstrated the application of quercetin liposomes for tumor-targeted drug delivery in vivo [95]. Poloxamer 188, tween 80, cholesterol, soy lecithin, and glyceryl behenate (ATO)-containing liposomes generated by low-temperature emulsification evaporation exhibited a prolonged in vivo circulation time [65].
Quercetin is loaded into peptide-functionalized liposomes by the thin-film hydration method. These targeted formulations showed a threefold increase in cell toxicity, higher apoptosis, and S-phase cell-cycle arrest in A549 cell lines. When targeted to the lungs by pulmonary administration, they exhibited significantly increased anticancer activity in orthotopic lung tumor-bearing mice as well as an increase in the lifespan of mice [96]. Quercetin liposomes of phosphatidylcholine and cholesterol also exhibited oral hepatoprotective activity in rats and 50 times more antioxidant activity compared to plain quercetin [48]. Further, the superior therapeutic effect of quercetin PEGylated liposomes seen in streptozotocin-induced diabetic nephropathy was attributed to the higher quercetin concentrations in plasma compared to quercetin [97]. In another study, quercetin liposomes of phosphatidylcholine and cholesterol demonstrated cognition-enhancing and anxiolytic effects [98].
4.3 Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
SLNs are an example of a colloidal lipid carrier delivery system; they are prepared using biodegradable, physiological, and biocompatible solid lipids [99]. SLNs display good chemical stability, enhanced drug loading, controlled drug release, enhanced bioavailability, and excellent biocompatibility [41]. The reviews [100,101,102] detail the various techniques utilizing SLN formulations. However, a major concern with SLNs is the expulsion of encapsulated drugs from the carrier over time; this challenge led to the development of NLCs [103].
NLCs are made of solid lipids combined with liquid lipids acting as the matrix [104]. The combination of solid and liquid lipids gives imperfections that can entrap more molecules than SLNs, thus enabling a high entrapment efficiency. NLCs overcome the challenge presented by drug expulsion from SLNs, thereby providing a major advantage [105]. Both SLNs and NLCs provide solubility enhancement, improved bioavailability and permeability, a prolonged half-life, fewer adverse effects, and targeted tissue delivery [106].
Quercetin SLNs prepared by emulsification followed by low-temperature solidification with glyceryl monostearate as the solid lipid displayed increased quercetin gastrointestinal absorption in rats [67]. Quercetin SLNs prepared using an ultrasonication method with a combination of tripalmitin and lecithin as the lipid core coated with chitosan allowed a faster release than pure quercetin, with enhanced uptake in Caco-2 cells [107]. Quercetin NLCs prepared using soya lecithin, medium-chain triglyceride, and glyceryl monostearate showed higher solubility, good stability, and enhanced apoptosis in MCF-7 and MDA-MB-231 cells [49]. NLCs of quercetin promoted neuroprotective effects in vitro in a model of Alzheimer’s disease by inhibiting fibril formation [50].
4.4 Microemulsions (MEs), Nanoemulsions, and Self-microemulsifying Drug Delivery Systems (SMEDDS)
Microemulsions are thermodynamic stable, optically isotropic clear systems [108] comprising oil, a surfactant, a cosurfactant, and an aqueous phase [109, 110]. The ability of microemulsions to increase the solubility of water-insoluble BCS class II and IV drugs as well as enhance absorption facilitates bioavailability enhancement [111, 112]. ME can also be prepared by the phase-inversion temperature method [113, 114]. MEs provide manifold advantages like easy preparation, transparency, protection from degradation, low viscosity, and a high solubilization capacity.
Nanoemulsions—also known as ultrafine emulsions, submicron emulsions, and mini-emulsions—are kinetically stable nanodispersions of two immiscible liquids which are stabilized by surfactant(s) to form a single phase [115,116,117,118]. A nanoemulsion offers various merits such as improved dissolution and enhanced oral bioavailability [119]. Yet another very effective approach for enhancing the solubility and bioavailability of hydrophobic moieties utilizes SMEDDS [120]. These are microemulsions without an aqueous phase, which improves the stability of the formulation. SMEDDS are isotropic mixtures of surfactant, oil, and cosurfactant that spontaneously form microemulsions in GIT fluid/aqueous media with gastric motility/mixing [121, 122]. SMEDDSs can be added to finished dosage forms such as capsules or other solid dosage forms [123, 124].
The significantly increased solubility of another quercetin ME in comparison with plain quercetin in aqueous media with good ileum absorption was demonstrated [125]. Reduced IL-4 and IL-5 levels, reduced P-selectin expression, and decreased mucus secretion in the lung have also been demonstrated with a quercetin ME [51]. Nanoemulsions showed improved quercetin bioavailability and permeability [52]. A quercetin nanoemulsion prepared by aqueous-phase titration showed improved in vitro permeability through artificial intestinal membranes as well as enhanced oral bioavailability [53]. Co-delivery of pemetrexed and a quercetin-based nanoemulsion improved oral bioavailability and exhibited superior tumor growth inhibition in A549 tumor-bearing mice models compared with controls [126]. Quercetin SMEDDS showed rapid in vitro release, with a ninefold increase in AUC compared to free quercetin [127]. They also provided significantly enhanced solubility and 1-month stability at 25 °C along with enhanced transport across the Caco-2 cell monolayer [128].
4.5 Polymeric NPs
Natural and synthetic polymers that are biodegradable are used to prepare polymeric NPs of size <1000 nm [129]. They are classified as either nanocapsules, wherein the drug is present in the core, or as nanospheres, where the drug is distributed uniformly in the polymeric matrix [130, 131]. Polymeric NPs may show increased reactivity, sensitivity, and stability compared to liposomes. Their enhanced membrane permeability (attributed to their nanosize) and an ability to target a specific organ by attaching ligands to their surfaces make polymeric NPs attractive drug carriers [132].
Nanoprecipitation is considered the simplest approach for preparing drug-loaded NPs; in this, drug-loaded polymeric particles are precipitated out of organic solvents following addition to aqueous media. Other approaches for the fabrication of drug-loaded polymeric NPs include crosslinking, homogenization, solvent evaporation/diffusion, and spray drying. Other green techniques based on microwaves and aqueous solvents are also reported [133, 134]. Various polymers used for quercetin polymeric NPs include polylactic acid [135], poly(lactic-co-glycolic acid) (PLGA), chitosan [58], fucoidan [55], zein [56], zein-hydroxypropyl-β-cyclodextrin [54], and PEGylated PLGA conjugated with folic acid [136].
Quercetin-loaded polylactic acid NPs with high drug encapsulation efficiency exhibited controlled release, suggesting promise in terms of their utilization in newer therapies [137]. pH-sensitive NPs were synthesized that displayed high drug release in acidic media, leading to proposed applications in cancer therapy, considering the acidic environment of the tumor site [138]. Using cholate-modified polymer-lipid hybrid NPs (cPLNs), a bile salt transport pathway was evaluated for the oral delivery of quercetin. Quercetin cPLNs exhibited a 375.12% bioenhancement compared to quercetin suspensions [139]. PLGA-TPGS (D-α-tocopheryl polyethylene glycol 1000 succinate) quercetin NPs developed for oral delivery exhibited superior inhibition of triple-negative breast cancer cells. Remarkable anti-tumor efficacy was observed in 4T1-bearing mice, and fewer lung metastatic colonies were detected [57].
4.6 Micelles
Micelles are drug delivery systems with a size range of 5–100 nm wherein a surfactant or block copolymer forms self-assembled aggregates [140, 141]. They are aqueous dispersions in which the block copolymer concentration is greater than the critical micelle concentration [142]. Micelles can be prepared by dissolution, emulsion technique, dialysis, solvent evaporation, as well as lyophilization [143]. Micelles are small in size and can facilitate an increased cellular uptake, improved therapeutic potential, and sustained drug release [144, 145].
Pluronic P123/TPGS mixed micelles enabled improved solubility and bioactivity of quercetin [146]. PEG-modified quercetin micelles demonstrated enhanced solubility and K562 (human erythromyelogenous leukemia) cells were arrested at the G2/M phase [147] Quercetin micelles of γ-benzyloxy-substituted poly(ε-caprolactone) have proven to have anticancer and antioxidative activity against HepG2 and H9c2 [60, 61]. Quercetin-loaded mixed micelles showed high uptake inside the cells and exhibited sustained drug release. Results indicated that TPGS facilitated enhanced apoptosis, which resulted in an improvement in lung cancer treatment [34].
Quercetin-loaded sodium taurocholate-Pluronic P123 micelles demonstrated sustained release in both simulated gastric and intestinal fluids. This formulation displayed 1.8-fold and 1.6-fold higher Cmax and AUC0–24 values compared to the free quercetin, respectively [148]. Quercetin-loaded LipoMicel® (liquid micelle matrix) exhibited a ninefold enhancement in Cmax and an eightfold increase in AUC0–24 compared to free quercetin [149]. In another pharmacokinetic study, orally administered lecithin-stabilized polymeric micelles significantly increased the relative bioavailability by 360% and showed an absolute bioavailability of 5.13% compared to quercetin [59]. While most studies have evaluated the anticancer efficacy of quercetin, Brahmeshwar Mishra et al. developed quercetin-loaded bio-enhanced and prolonged-release Soluplus® micelles for the management of diabetes and demonstrated an enhanced anti-diabetic effect [40].
4.7 Dendrimers
Dendrimers are especially recognized for their monodispersity, hyperbranched nature, polyvalence, nanoscale size, biocompatibility, and stability [150]. Dendrimers comprise a hydrophobic cavity that acts as an initiator, surrounding interior generations of repeating units, and outermost exterior terminal functional groups [151]. Convergent and divergent methods are the two common approaches for dendrimer synthesis [152]. Polyamidoamine (PAMAM) was the first family of dendrimers to be commercialized, and they are the most commonly exploited [153]. Due to their nanoscale size, dendrimers can adapt paracellular or transcellular pathways to cross cell barriers, making them attractive carriers for nanodrug delivery [154]. Investigations proved that the solubility of quercetin improved when PAMAM was used as a carrier [155]. Quercetin magnetite/poly-aminoester dendrimer with poly(ε-caprolactone) improved the release of quercetin at pH 5.8 (80%) when compared with pH 7.4 (65%) [64]. Quercetin with linear PEG-PLGA polymer caused significantly increased cancer cell death in 66 GB cell lines [156]. Dendrimeric quercetin formulations were also found effective in terms of anti-inflammatory [62], anti-bacterial [63], and neuroprotective [157] activity.
4.8 Magnetic NPs
Due to their magnetic properties, magnetic NPs are readily targeted to the target sites with the help of an externally applied magnetic field [158]. Magnetic NPs are less than 10–20 nm in size, exhibit the properties of a giant paramagnetic atom, and exhibit a rapid response to external magnetic fields. They also exhibit only trace residual magnetism and coercivity [159]. This is crucial to prevent agglomeration [160].
A quercetin-loaded pH-sensitive superparamagnetic drug carrier (Fe3O4) surface coated with polyamidoamine-b-PEG-folate (hyperbranched) demonstrated high aqueous solubility [161]. Iron oxide was functionalized with folic acid to target overexpressed folic acid receptors on brain adenocarcinoma cells (U87). Results of MTT assay and cell uptake studies confirmed that these magnetic NPs are useful for cancer therapy [162]. Similarly, quercetin-loaded PLGA-MNPs demonstrated anticancer activity against viable A549 cells and were safe after being injected into mice [163]. In another study, superparamagnetic quercetin Fe3O4 NPs were also found to be cytotoxic to MCF-7 breast cancer cell lines, which was confirmed by morphological changes observed under a fluorescence microscope [164].
4.9 Gold NPs
Quercetin was effectively harnessed to prepare gold NPs of size 20–45 nm by reduction [165]. High anticancer activity was exhibited by the quercetin-functionalized gold NPs, with an anti-angiogenic effect demonstrated in a chorioallantoic membrane assay [166]. However, these NPs exhibited no cytotoxicity to human fibroblasts (L929 cells) [167]. An in-vivo reduction in tumor volume seen in the 4T1 tumor mouse model was attributed to altered expression of genes related to apoptosis [168]. Furthermore, quercetin gold NPs demonstrated higher antioxidant activity compared to free quercetin [167]. Quercetin-conjugated gold NPs were also studied for their efficacy against leishmaniasis [169].
4.10 Miscellaneous Quercetin Nanosystems
Silver nanocubes prepared using extract of the leaf of Peltophorum pterocarphum relied on quercetin-3-O-β-d-galactopyranoside to act as the reducing agent. They showed promising antifungal activity compared to the commercial antifungal agent fluconazole [170]. Quercetin mesoporous silica NPs anchored with folic acid caused apoptosis due to cell cycle arrest in breast cancer cell lines [171, 172]. In melanoma cells, the activity of titanium dioxide nanotubes containing quercetin on melanoma cells was ascribed to enhanced cleaved caspase-3 levels and enhanced apoptosis compared to titanium dioxide nanotubes or quercetin alone [173]. Quercetin-poly(lactide-co-glycolide)-folic acid targeted nanocapsules showed selective uptake and cytotoxicity towards cancer cells where folate was over-expressed. High accumulation at tumors and active targeting were confirmed following intravenous administration in IGROV-1 or HeLa tumor-bearing mice [136]. Treatment with quercetin was effective at overcoming the harmful effects of multi-walled carbon nanotubes, which included inflammatory and oxidative as well as immunotoxic effects [174]. On the other hand, quercetin-loaded protein NPs based on natural proteins (albumin, gelatin, hemoglobin) are an attractive alternative to synthetic polymers in drug delivery applications due to their safety, biodegradability, biocompatibility, unique self-assembly, and hydrophobic interaction properties [38, 39, 175].
5 Quercetin Clinical Trials
Clinical trials investigating the therapeutic effects of quercetin are on the rise. Inflammation is a major contributor to the progress of many chronic diseases, namely, cancer, diabetes, and heart disease [148]. Several clinical trials have investigated the anti-inflammatory properties of quercetin. One randomized controlled trial (RCT) involving 50 participants with rheumatoid arthritis found that supplementation with quercetin reduced inflammatory markers and improved joint mobility compared to a placebo (NCT05371340).
Quercetin has also been studied for its potential to improve cardiovascular health [176]. A meta-analysis of 17 RCTs found that quercetin supplementation significantly reduced blood pressure, especially in those with high blood pressure (NCT01839344). Another RCT involving obese individuals with type 2 diabetes found that quercetin supplementation improved blood lipid profiles compared to a placebo (NCT00065676).
Cancer is a key cause of mortality and morbidity worldwide [177]. Quercetin nanoformulations have been extensively evaluated for anticancer efficacy. Importantly, while free quercetin has demonstrated some activity against various anticancer cell lines, quercetin nanoformulations have demonstrated superior activity. This application of quercetin nanoformulations for the treatment of a variety of cancers is evident from Table 3, which lists the cell lines evaluated for various cancers. Quercetin has been studied for its potential anticancer effects, given its ability to affect cancer cell death and inhibit the growth of tumors. Several clinical trials have investigated quercetin as an adjuvant therapy for cancer treatment (NCT03493997, NCT05456022, NCT03476330, NCT01538316, NCT05724329, NCT01912820). A pilot RCT involving patients with sarcoidosis and idiopathic pulmonary fibrosis found that quercetin supplementation improved quality of life and reduced inflammation markers compared to a placebo (NCT00512967). Another RCT involving 40 patients with prostate cancer found that quercetin supplementation improved oxidative stress markers and inflammation compared to a placebo (NCT03493997). Quercetin has also been studied for its potential to improve immune function. One RCT study with quercetin supplementation showed increased natural killer cell activity and attenuated the incidence of COVID-19 infection compared to a placebo (NCT04853199).
In summary, quercetin has been studied for its possible therapeutic effects in a variety of health conditions. Clinical trials have provided evidence that quercetin supplementation may have anti-inflammatory, cardiovascular, anticancer, and immune-enhancing effects. However, further research is important to fully harness quercetin as a therapeutic and to arrive at optimal dosages of quercetin for different health conditions [257].
6 Marketed Products of Quercetin Nanoformulations
Quercefit® is a lecithin-based water-soluble quercetin formulation that produced a 20-fold-increase in plasma levels of quercetin without any notable side effects after oral administration of the quercetin nanoformulation in human volunteers [258]. On the same note, each tablet of Quevir®, a dietary supplement, contains 500 mg of quercetin phytosome. Here, quercetin is in a food-grade delivery system with sunflower phospholipids, which increases its oral absorption up to 20-fold [259]. Other marketed formulations are Thorne’s Quercetin Phytosome® [260], Codeage’s Quercetin Phytosome® [261], One Planet Nutrition’s Nano Quercetin® [262], and Quercetin LipoMicel® [149].
7 Future Perspective and Conclusion
Clinical trials to date have employed free quercetin, which, despite serious solubility and bioavailability limitations, exhibits great promise. Nanoformulations of quercetin shown great promise for anticancer activity compared with free quercetin and combinations of drugs. Through their inherent targeting property, nanoformulations could provide a remarkable improvement in therapy, possibly even at lower doses. Considering the high dose of quercetin, the oral route appears to be the most practical. Among all the nanoformulations, quercetin phytosomes—which are available on the market—are attracting the most interest because of the enhanced plasma concentration levels of quercetin (20-fold more bioavailable compared to free quercetin) after the oral administration of a single dose in humans. However, more clinical safety and efficacy studies are needed to study the safety and effectiveness of quercetin nanoformulations. Targeting organs like the lung and breast through lymph-mediated uptake increases the oral bioavailability. Quercetin-loaded nanosystems could be one more opportunity to harness the beneficial effects of quercetin nanoformulations for breast cancer as well as various lung afflictions. More research into quercetin nanoformulations is imperative to harness the various applications of this wonderful nutraceutical.
References
Batiha GE, Beshbishy AM, Ikram M, Mulla ZS, El-Hack ME, Taha AE, Algammal AM, Elewa YH. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods. 2020;9(3):374.
Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother Res. 2021;35(3):1230–6.
Yao C, Xi C, Hu K, Gao W, Cai X, Qin J, Lv S, Du C, Wei Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol J. 2018;15:1–3.
Pierro FD, Khan A, Bertuccioli A, Maffioli P, Derosa G, Khan S, Khan BA, Nigar R, Ujjan I, Devrajani BR. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol. 2021;67(2):190–5.
Wadhwa K, Kadian V, Puri V, Bhardwaj BY, Sharma A, Pahwa R, Rao R, Gupta M, Singh I. New insights into quercetin nanoformulations for topical delivery. Phytomedicine. 2022;14: 100257.
Murakami T. A minireview: usefulness of transporter-targeted prodrugs in enhancing membrane permeability. J Pharm Sci. 2016;105(9):2515–26.
Cunico LP, Cobo AM, Al-Hamimi S, Turner C. Solubility and thermal degradation of quercetin in CO2-expanded liquids. Molecules. 2020;25(23):5582.
Wang W, Sun C, Mao L, Ma P, Liu F, Yang J, Gao Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Tech. 2016;56:21–38.
Filipa Brito A, Ribeiro M, Margarida Abrantes A, Salome Pires A, Jorge Teixo R, Guilherme Tralhao J, Filomena BM. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem. 2015;22(26):3025–39.
Papakyriakopoulou P, Velidakis N, Khattab E, Valsami G, Korakianitis I, Kadoglou NP. Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals. 2022;15(8):1019.
Tomou EM, Papakyriakopoulou P, Saitani EM, Valsami G, Pippa N, Skaltsa H. Recent advances in nanoformulations for quercetin delivery. Pharmaceutics. 2023;15(6):1656.
Khursheed R, Singh SK, Wadhwa S, Gulati M, Awasthi A. Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discov Today. 2020;25(1):209–22.
Nam JS, Sharma AR, Nguyen LT, Chakraborty C, Sharma G, Lee SS. Application of bioactive quercetin in oncotherapy: from nutrition to nanomedicine. Molecules. 2016;21(1):108.
Vinayak M, Maurya AK. Quercetin loaded nanoparticles in targeting cancer: recent development. Anti-Cancer Agent ME. 2019;19(13):1560–76.
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–47.
Terao J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem Pharmacol. 2017;139:15–23.
Kandemir K, Tomas M, McClements DJ, Capanoglu E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci Tech. 2022;119:192–200.
Manzoor MF, Hussain A, Sameen A, Sahar A, Khan S, Siddique R, Aadil RM, Xu B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: a review. Ultrason Sonochem. 2021;78: 105686.
Singh A, Verma BK, Pandey S. Exploring natural bioenhancers to enhancing bioavailability: an overview. Int J Pharm. 2021;12(2):24–31.
Shinkar DM, Amrutkar SV, Pingale PL. Case study: Indian herbal bioenhancers. In: Pingale PL, editor. Drug delivery technology: herbal bioenhancers in pharmaceuticals. Berlin: de Gruyter; 2022. p. 239.
Krause KP, Müller RH. Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation. Int J Pharm. 2001;214(1–2):21–4.
Allam AN, Komeil IA, Fouda MA, Abdallah OY. Preparation, characterization and in vivo evaluation of curcumin self-nano phospholipid dispersion as an approach to enhance oral bioavailability. Int J Pharm. 2015;489(1–2):117–23.
Tan KW, Tang SY, Thomas R, Vasanthakumari N, Manickam S. Curcumin-loaded sterically stabilized nanodispersion based on non-ionic colloidal system induced by ultrasound and solvent diffusion-evaporation. Pure Appl Chem. 2016;88(1–2):43–60.
Zhang Q, Polyakov NE, Chistyachenko YS, Khvostov MV, Frolova TS, Tolstikova TG, Dushkin AV, Su W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018;25(1):198–209.
Ghanem AS, Ali HS, El-Shanawany SM, Ibrahim ES. Solubility and dissolution enhancement of quercetin via preparation of spray dried microstructured solid dispersions. Thai J Pharm Sci. 2013;37(1):12–24.
Han J, Tong M, Li S, Yu X, Hu Z, Zhang Q, Xu R, Wang J. Surfactant-free amorphous solid dispersion with high dissolution for bioavailability enhancement of hydrophobic drugs: a case of quercetin. Drug Dev Ind Pharmacy. 2021;47(1):153–62.
Li SJ. Study on preparation of quercetin solid dispersions and its bioavailability in rats. Chin Tradit Herb Drugs. 2017;24:4229–34.
Li B, Konecke S, Harich K, Wegiel L, Taylor LS, Edgar KJ. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohyd Polym. 2013;92(2):2033–40.
Gilley AD, Arca HC, Nichols BL, Mosquera-Giraldo LI, Taylor LS, Edgar KJ, Neilson AP. Novel cellulose-based amorphous solid dispersions enhance quercetin solution concentrations in vitro. Carbohyd Polym. 2017;157:86–93.
Van Hecke E, Benali M. Solid dispersions of quercetin-PEG matrices: miscibility prediction, preparation and characterization. Food Biosci. 2022;49: 101868.
Fan N, He Z, Ma P, Wang X, Li C, Sun J, Sun Y, Li J. Impact of HPMC on inhibiting crystallization and improving permeability of curcumin amorphous solid dispersions. Carbohyd Polym. 2018;181:543–50.
Bunlung S, Nualnoi T, Issarachot O, Wiwattanapatapee R. Development of raft-forming liquid and chewable tablet formulations incorporating quercetin solid dispersions for treatment of gastric ulcers. Saudi Pharm J. 2021;29(10):1143–54.
Chen ZP, Sun J, Chen HX, Xiao YY, Liu D, Chen J, Cai H, Cai BC. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia. 2010;81(8):1045–52.
Zhao MH, Yuan L, Meng LY, Qiu JL, Wang CB. Quercetin-loaded mixed micelles exhibit enhanced cytotoxic efficacy in non-small cell lung cancer in vitro. Exp Ther Med. 2017;14(6):5503–8.
Choi MK, Lee J, Song IS. Pharmacokinetic modulation of substrate drugs via the inhibition of drug-metabolizing enzymes and transporters using pharmaceutical excipients. J Pharm Investig. 2023;53(1):1–8.
Strambeanu N, Demetrovici L, Dragos D, Lungu M. Nanoparticles: Definition, classification and general physical properties. In: Lungu M, Neculae A, Bunoiu M, Biris C, editors. Nanoparticles’ promises and risks. Cham: Springer; 2014. p. 3–8.
Khor CM, Ng WK, Chan KP, Dong Y. Preparation and characterization of quercetin/dietary fiber nanoformulations. Carbohyd Polym. 2017;161:109–17.
Saha C, Kaushik A, Das A, Pal S, Majumder D. Anthracycline drugs on modified surface of quercetin-loaded polymer nanoparticles: a dual drug delivery model for cancer treatment. PLoS ONE. 2016;11(5): e0155710.
Majumder D, Roychoudhry S, Kundu S, Dey SK, Saha C. Hydrophobic quercetin encapsulated hemoglobin nanoparticles: formulation and spectroscopic characterization. J Biomol Struct Dyn. 2022;40(20):9860–9.
Singh J, Mittal P, Vasant Bonde G, Ajmal G, Mishra B. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus®/P407 micelles in diabetes treatment. Artif Cells Nanomed Biotechnol. 2018;46:S546–55.
Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part II—Ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2017;110:58–69.
Rabanel MJ, Aoun V, Elkin I, Mokhtar M, Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19(19):3070–102.
Bachhav SS, Dighe VD, Kotak D, Devarajan PV. Rifampicin lipid-polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int J Pharm. 2017;532(1):612–22.
Bachhav SS, Dighe VD, Devarajan PV. Exploring Peyer’s patch uptake as a strategy for targeted lung delivery of polymeric rifampicin nanoparticles. Mol Pharm. 2018;15(10):4434–45.
Zhao J, Yang J, Xie Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int J Pharm. 2019;570: 118642.
Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Ye B. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006;12(10):3193–9.
Long Q, Xie Y, Huang Y, Wu Q, Zhang H, Xiong S, Liu Y, Chen L, Wei Y, Zhao X, Gong C. Induction of apoptosis and inhibition of angiogenesis by PEGylated liposomal quercetin in both cisplatin-sensitive and cisplatin-resistant ovarian cancers. J Biomed Nanotechnol. 2013;9(6):965–75.
Shaji J, Iyer S. Double-loaded liposomes encapsulating Quercetin and Quercetin beta-cyclodextrin complexes: Preparation, characterization and evaluation. Asian J Pharm. 2012;6(3):218–26.
Sun M, Nie S, Pan X, Zhang R, Fan Z, Wang S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf B Biointerfaces. 2014;113:15–24.
Pinheiro RG, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, Reis S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur J Pharm Biopharm. 2020;148:105314.
Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LF, Lemos-Senna E, Calixto JB. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res. 2010;61(4):288–97.
Chen W, Ju X, Aluko RE, Zou Y, Wang Z, Liu M, He R. Rice bran protein-based nanoemulsion carrier for improving stability and bioavailability of quercetin. Food Hydrocoll. 2020;108:106042.
Alsabeelah N, Kumar V. Formulation and optimization of quercetin nanoemulsion for enhancing its dissolution rate, bioavailability and cardioprotective activity. J Clust Sci. 2022;34:1893–906.
Penalva R, Gonzalez-Navarro CJ, Gamazo C, Esparza I, Irache JM. Zein nanoparticles for oral delivery of quercetin: pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomedicine: NBM. 2017;13(1):103–10.
Barbosa AI, Costa Lima SA, Reis S. Application of pH-Responsive Fucoidan/Chitosan Nanoparticles to Improve Oral Quercetin Delivery. Molecules. 2019;24(2):346. https://doi.org/10.3390/molecules24020346.
Tapia-Hernández JA, Del-Toro-Sánchez CL, Cinco-Moroyoqui FJ, Ruiz-Cruz S, Juárez J, Castro-Enríquez DD, Barreras-Urbina CG, López-Ahumada GA, Rodríguez-Félix F. Gallic acid-loaded zein nanoparticles by electrospraying process. J Food Sci. 2019;84(4):818–31.
Zhou Y, Chen D, Xue G, Yu S, Yuan C, Huang M, Jiang L. Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv. 2020;10(57):34517–26.
Baksi R, Singh DP, Borse SP, Rana R, Sharma V, Nivsarkar M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed Pharmacother. 2018;106:1513–26.
Chang CE, Hsieh CM, Huang SC, Su CY, Sheu MT, Ho HO. Lecithin-stabilized polymeric micelles (LsbPMs) for delivering quercetin: pharmacokinetic studies and therapeutic effects of quercetin alone and in combination with doxorubicin. Sci Rep. 2018;8(1):1–1.
Patra A, Satpathy S, Shenoy AK, Bush JA, Kazi M, Hussain MD. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int J Nanomedicine. 2018;13:2869.
Soltantabar P, Calubaquib EL, Mostafavi E, Biewer MC, Stefan MC. Enhancement of loading efficiency by coloading of doxorubicin and quercetin in thermoresponsive polymeric micelles. Biomacromol. 2020;21(4):1427–36.
Madaan K, Lather V, Pandita D. Evaluation of polyamidoamine dendrimers as potential carriers for quercetin, a versatile flavonoid. Drug Deliv. 2016;23(1):254–62.
Rehman K, Ali I, El-Haj BM, Kanwal T, Maharjan R, Saifullah S, Imran M, Simjee SU, Shah MR. Synthesis of novel biocompatible resorcinarene based nanosized dendrimer-vesicles for enhanced anti-bacterial potential of quercetin. J Mol Liq. 2021;341: 116921.
Khoee S, Hemati K. Synthesis of magnetite/polyamino-ester dendrimer based on PCL/PEG amphiphilic copolymers via convergent approach for targeted diagnosis and therapy. Polymer. 2013;54(21):5574–85.
Gang W, Jie WJ, Ping ZL, Ming DS, Ying LJ, Lei W, Fang Y. Liposomal quercetin: evaluating drug delivery in vitro and biodistribution in vivo. Expert Opin Drug Deliv. 2012;9(6):599–613.
Wong MY, Chiu GN. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomedicine: NBM. 2011;7(6):834–40.
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44.
Bose S, Du Y, Takhistov P, Michniak-Kohn B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int J Pharm. 2013;441(1–2):56–66.
Han J, Tong M, Li S, Yu X, Hu Z, Zhang Q, Xu R, Wang J. Surfactant-free amorphous solid dispersion with high dissolution for bioavailability enhancement of hydrophobic drugs: a case of quercetin. Drug Dev Ind Pharm. 2021;47(1):153–62.
Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10(3):134.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380(1–2):216–22.
Gera S, Sampathi S, Maddukuri S, Dodoala S, Junnuthula V, Dyawanapelly S. Therapeutic potential of naringenin nanosuspension: in vitro and in vivo anti-osteoporotic studies. Pharmaceutics. 2022;14(7):1449.
Yadav GV, Singh SR. Nanosuspension: A promising drug delivery system. Pharmacophore. 2012;3(5):217–43.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16.
Kakran M, Shegokar R, Sahoo NG, Al Shaal L, Li L, Müller RH. Fabrication of quercetin nanocrystals: comparison of different methods. Eur J Pharm Biopharm. 2012;80(1):113–21.
Karadag A, Ozcelik B, Huang Q. Quercetin nanosuspensions produced by high-pressure homogenization. J Agr Food Chem. 2014;62(8):1852–9.
Gao L, Liu G, Wang X, Liu F, Xu Y, Ma J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int J Pharm. 2011;404(1–2):231–7.
Ma Y, Cong Z, Gao P, Wang Y. Nanosuspensions technology as a master key for nature products drug delivery and in vivo fate. Eur J Pharm Sci. 2023;185: 106425.
Pessoa LZ, Duarte JL, Ferreira RM, Oliveira AE, Cruz RA, Faustino SM, Carvalho JC, Fernandes CP, Souto RN, Araújo RS. Nanosuspension of quercetin: preparation, characterization and effects against Aedes aegypti larvae. Rev Bras Farmacogn. 2018;28:618–25.
Sun M, Gao Y, Pei Y, Guo C, Li H, Cao F, Yu A, Zhai G. Development of nanosuspension formulation for oral delivery of quercetin. J Biomed Nanotech. 2010;6(4):325–32.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Li H, Li M, Fu J, Ao H, Wang W, Wang X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021;28(1):1226–36.
Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20(20):2572–82.
Gupta MK, Sansare V, Shrivastava B, Jadhav S, Gurav P. Comprehensive review on use of phospholipid based vesicles for phytoactive delivery. J Liposome Res. 2022;32(3):211–23.
Subramani T, Ganapathyswamy H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J Food Sci Technol. 2020;57(10):3545–55.
Hussein HA, Abdullah MA. Novel drug delivery systems based on silver nanoparticles, hyaluronic acid, lipid nanoparticles and liposomes for cancer treatment. Appl Nanosci. 2022;12(11):3071–96.
Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. Preparation, characterization and applications of liposomes: state of the art. J Colloid Sci Biotechnol. 2012;1(2):147–68.
Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18(3):241–68.
Dua JS, Rana AC, Bhandari AK. Liposome: methods of preparation and applications. Int J Pharm Stud Res. 2012;3(2):14–20.
Dwivedi C, Verma S. Review on preparation and characterization of liposomes with application. Int J Sci Innov Res. 2013;2:486–508.
Nsairat H, et al. Recent advances in using liposomes for delivery of nucleic acid-based therapeutics. OpenNano. 2023;11: 100132.
Maja L, Željko K, Mateja P. Sustainable technologies for liposome preparation. J Supercrit Fluids. 2020;165: 104984.
Ambrosio N, Voci S, Gagliardi A, Palma E, Fresta M, Cosco D. Application of biocompatible drug delivery nanosystems for the treatment of naturally occurring cancer in dogs. J Funct Biomater. 2022;13(3):116.
Liu D, Hu H, Lin Z, Chen D, Zhu Y, Hou S, Shi X. Quercetin deformable liposome: preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J Photochem Photobiol B: Biol. 2013;127:8–17.
Tang L, Li K, Zhang Y, Li H, Li A, Xu Y, Wei B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep. 2020;10(1):2440.
Riaz MK, Zhang X, Wong KH, Chen H, Liu Q, Chen X, Zhang G, Lu A, Yang Z. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int J Nanomed. 2019;14:2879–902.
Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020;121: 109604.
Priprem A, Watanatorn J, Sutthiparinyanont S, Phachonpai W, Muchimapura S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomedicine: NBM. 2008;4(1):70–8.
Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349.
Lingayat VJ, Zarekar NS, Shendge RS. Solid lipid nanoparticles: a review. Nanosci Nanotechnol Res. 2017;4(2):67–72.
Bansal AK, Munjal B. Preparation of solid lipid nanoparticles for enhancement of oral bioavailability of curcumin. In: The Electronic Conference on Pharmaceutical Sciences; 2011 Mar 1–31; online.
Akbari J, Saeedi M, Ahmadi F, Hashemi SM, Babaei A, Yaddollahi S, Rostamkalaei SS, Asare-Addo K, Nokhodchi A. Solid lipid nanoparticles and nanostructured lipid carriers: a review of the methods of manufacture and routes of administration. Pharm Dev Technol. 2022;27(5):525–44.
Teja VC, Chowdary VH, Raju YP, Surendra N, Vardhan RV, Reddy BK. A glimpse on solid lipid nanoparticles as drug delivery systems. J Glob Trends Pharm Sci. 2014;5(2):1649–57.
Chutoprapat R, Kopongpanich P, Chan LW. A mini-review on solid lipid nanoparticles and nanostructured lipid carriers: topical delivery of phytochemicals for the treatment of acne vulgaris. Molecules. 2022;27(11):3460.
Ngwuluka NC, Kotak DJ, Devarajan PV. Design and characterization of metformin-loaded solid lipid nanoparticles for colon cancer. AAPS PharmSciTech. 2017;18:358–68.
Karunakar G, Patel NP, Kamal SS. Nano structured lipid carrier based drug delivery system. J Chem Pharm Res. 2016;8(2):627–43.
Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS PharmSciTech. 2017;18:875–83.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2012;64:175–93.
Kurita T, Makino Y. Novel curcumin oral delivery systems. Anticancer Res. 2013;33(7):2807–21.
Heuschkel S, Goebel A, Neubert RH. Microemulsions—modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603–31.
Kale SN, Deore SL. Emulsion micro emulsion and nano emulsion: a review. Sys Rev Pharm. 2017;8(1):39.
Vimalson DC. Techniques to enhance solubility of hydrophobic drugs: an overview. Asian J Pharm. 2016;10(2):39–47.
Rajpoot K, Tekade RK. Microemulsion as drug and gene delivery vehicle: an inside story. In: Tekade RK, editor. Advances in pharmaceutical product development and research, drug delivery systems. Cambridge: Academic Press; 2019. p. 455–520.
Waghmare SG, Nikhade RR, Hadke MA. Microemulsion and its applications novel approach towards the drug delivery. World J Pharm Res. 2015;5(1):477–502.
Shinde RL, Devarajan PV. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017;24(1):152–61.
Sharma N, Mishra S, Sharma S, Deshpande RD, Sharma RK. Preparation and optimization of nanoemulsions for targeting drug delivery. Int J Drug Dev Res. 2013;5(4):37–48.
Thakur N, Garg G, Sharma PK, Kumar N. Nanoemulsions: a review on various pharmaceutical application. Glob J Pharmacol. 2012;6(3):222–5.
Wilking JN, Graves SM, Chang CB, Meleson K, Lin MY, Mason TG. Dense cluster formation during aggregation and gelation of attractive slippery nanoemulsion droplets. Phys Rev Lett. 2006;96(1): 015501.
Mahadev M, Nandini HS, Ramu R, Gowda DV, Almarhoon ZM, Al-Ghorbani M, Mabkhot YN. Fabrication and evaluation of quercetin nanoemulsion: a delivery system with improved bioavailability and therapeutic efficacy in diabetes mellitus. Pharmaceuticals. 2022;15(1):70.
Mande PP, Bachhav SS, Devarajan PV. Solid dispersion of curcumin as polymeric films for bioenhancement and improved therapy of rheumatoid arthritis. Pharm Res. 2016;33:1972–87.
Chandrakar A, Sahu B, Sahu H, Dewangan J, Kumar N, Singh R, Gupta R, Kumar D, Sahu B, Dewangan K, Kaushal R. Review on the formulation considerations needed to produce a stable Self micro Emulsifying Drug Delivery System (SMEDDS). Res J Pharm Technol. 2017;10(5):1563–70.
Khairnar DA, Darekar AB, Saudagar RB. A review on self-micro emulsifying drug delivery system: evident to improve the oral bioavailability of hydrophobic drugs. Asian J Pharm Technol. 2016;6(2):131–4.
Sharma S, Khinch MP, Sharma N, Agrawal D, Gupta MK. Approaches to development of solid-self micron emulsifying drug delivery system: formulation techniques and dosage forms—a review. Asian J Pharm Res Dev. 2013;1(5):146–56.
Khan BA, Bakhsh S, Khan H, Mahmood T, Rasul A. Basics of self micro emulsifying drug delivery system. J Pharm Altern Med. 2012;1(1):13–9.
Gao Y, Wang Y, Ma Y, Yu A, Cai F, Shao W, Zhai G. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Colloids Surf B Biointerfaces. 2009;71(2):306–14.
Pangeni R, Panthi VK, Yoon IS, Park JW. Preparation, characterization, and in vivo evaluation of an oral multiple nanoemulsive system for co-delivery of pemetrexed and quercetin. Pharmaceutics. 2018;10(3):158.
Jaisamut P, Wanna S, Limsuwan S, Chusri S, Wiwattanawongsa K, Wiwattanapatapee R. Enhanced oral bioavailability and improved biological activities of a quercetin/resveratrol combination using a liquid self-microemulsifying drug delivery system. Planta Med. 2021;87(04):336–46.
Poorani G, Uppuluri S, Uppuluri KB. Formulation, characterization, in vitro and in vivo evaluation of castor oil based self-nano emulsifying levosulpiride delivery systems. J Microencapsul. 2016;33(6):535–43.
Hanemann T, Szabó DV. Polymer-nanoparticle composites: from synthesis to modern applications. Materials. 2010;3(6):3468–517.
Mohanraj VJ, Chen YJ. Nanoparticles—a review. Trop J Pharm Res. 2006;5(1):561–73.
Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36(7):887–913.
Mallakpour S, Behranvand VJ. Polymeric nanoparticles: Recent development in synthesis and application. Express Polym Lett. 2016;10(11):895.
Ipar VS, Dsouza A, Devarajan PV. Enhancing curcumin oral bioavailability through nanoformulations. Eur J Drug Metab Pharmacokinet. 2019;44:459–80.
Nasir A, Kausar A, Younus A. A review on preparation, properties and applications of polymeric nanoparticle-based materials. Polym Plast Technol Eng. 2015;54(4):325–41.
Kumari A, Yadav SK, Pakade YB, Singh B, Yadav SC. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf B: Biointerfaces. 2010;80(2):184–92.
El-Gogary RI, Rubio N, Wang JT, Al-Jamal WT, Bourgognon M, Kafa H, Naeem M, Klippstein R, Abbate V, Leroux F, Bals S. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano. 2014;8(2):1384–401.
Kumari A, Kumar V, Yadav SK. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: a green approach. PLoS ONE. 2012;7(7): e41230.
Khoee S, Rahmatolahzadeh R. Synthesis and characterization of pH-responsive and folated nanoparticles based on self-assembled brush-like PLGA/PEG/AEMA copolymer with targeted cancer therapy properties: a comprehensive kinetic study. Eur J Med Chem. 2012;50:416–27.
Yin J, Hou Y, Song X, Wang P, Li Y. Cholate-modified polymer-lipid hybrid nanoparticles for oral delivery of quercetin to potentiate the antileukemic effect. Int J Nanomed. 2019;14:4045–57.
Deng C, Jiang Y, Cheng R, Meng F, Zhong Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects. Nano Today. 2012;7(5):467–80.
Lu Y, Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453(1):198–214.
Mondon K, Gurny R, Möller M. Colloidal drug delivery systems—recent advances with polymeric micelles. Chimia. 2008;62(10):832.
Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, Cho JM, Yun G, Lee J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9(6):304–16.
Li C, Guan H, Li Z, Wang F, Wu J, Zhang B. Study on different particle sizes of DOX-loaded mixed micelles for cancer therapy. Colloids Surf B: Biointerfaces. 2020;196: 111303.
Cagel M, Tesan FC, Bernabeu E, Salgueiro MJ, Zubillaga MB, Moretton MA, Chiappetta DA. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–28.
Zhao L, Shi Y, Zou S, Sun M, Li L, Zhai G. Formulation and in vitro evaluation of quercetin loaded polymeric micelles composed of pluronic P123 and D-a-tocopheryl polyethylene glycol succinate. J Biomed Nanotech. 2011;7(3):358–65.
Khonkarn R, Mankhetkorn S, Hennink WE, Okonogi S. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur J Pharm Biopharm. 2011;79(2):268–75.
Lu Z, Bu C, Hu W, Zhang H, Liu M, Lu M, Zhai G. Preparation and in vitro and in vivo evaluation of quercetin-loaded mixed micelles for oral delivery. Biosci Biotechnol Biochem. 2018;82(2):238–46.
Solnier J, Chang C, Roh K, Du M, Kuo YC, Hardy M, Lyon M, Gahler R. Quercetin LipoMicel—a novel delivery system to enhance bioavailability of quercetin. J Nat Health Prod Res. 2021;3(2):1–8.
Abbina S, Vappala S, Kumar P, Siren EM, La CC, Abbasi U, Brooks DE, Kizhakkedathu JN. Hyperbranched polyglycerols: recent advances in synthesis, biocompatibility and biomedical applications. J Mater Chem B. 2017;5(47):9249–77.
Tripathy S, Das MK. Dendrimers and their applications as novel drug delivery carriers. J Appl Pharm Sci. 2013;3(9):142–9.
Lyu Z, Ding L, Huang AT, Kao CL, Peng L. Poly (amidoamine) dendrimers: Covalent and supramolecular synthesis. Mater Today Chem. 2019;13:34–48.
Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. Pharm Bioallied Sci. 2014;6(3):139.
Onoue S, Yamada S, Chan HK. Nanodrugs: pharmacokinetics and safety. Int J Nanomedicine. 2014;9:1025.
Ramos MC, Horta BA. Drug-loading capacity of PAMAM dendrimers encapsulating quercetin molecules: a molecular dynamics study with the 2016H66 force field. J Chem Inf Model. 2021;61(2):987–1000.
Choi J, Moquin A, Bomal E, Na L, Maysinger D, Kakkar A. Telodendrimers for physical encapsulation and covalent linking of individual or combined therapeutics. Mol Pharm. 2017;14(8):2607–15.
Gan BH, Gaynord J, Rowe SM, Deingruber T, Spring DR. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem Soc Rev. 2021;50(13):7820–80.
Kianfar E. Magnetic nanoparticles in targeted drug delivery: a review. J Supercond Nov Magn. 2021;34(7):1709–35.
Chandradass J, Jadhav AH, Kim KH, Kim H. Influence of processing methodology on the structural and magnetic behavior of MgFe2O4 nanopowders. J Alloys Compd. 2012;517:164–9.
Prabhahar MJ, Jaisingh J, Arun Prakash VR. Role of magnetite (Fe3O4)-titania (TiO2) hybrid particle on mechanical, thermal and microwave attenuation behaviour of flexible natural rubber composite in X and Ku band frequencies. Mater Res Express. 2020;7(1):016106.
Rezaei SJ, Malekzadeh AM, Ramazani A, Niknejad H. pH-sensitive magnetite nanoparticles modified with hyperbranched polymers and folic acid for targeted imaging and therapy. Curr Drug Deliv. 2019;16(9):839–48.
Akal ZÜ, Alpsoy L, Baykal A. Superparamagnetic iron oxide conjugated with folic acid and carboxylated quercetin for chemotherapy applications. Ceram Int. 2016;42(7):9065–72.
Verma NK, Crosbie-Staunton K, Satti A, Gallagher S, Ryan KB, Doody T, McAtamney C, MacLoughlin R, Galvin P, Burke CS, Volkov Y. Magnetic core-shell nanoparticles for drug delivery by nebulization. J Nanobiotechnology. 2013;11(1):1–2.
Kumar SR, Priyatharshni S, Babu VN, Mangalaraj D, Viswanathan C, Kannan S, Ponpandian N. Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. J Colloid Interface Sci. 2014;436:234–42.
Nathiya S, Durga M, Thiyagarajan D. Quercetin, encapsulated quercetin and its application—a review. Int J Pharm Pharm Sci. 2014;6(10):20–6.
Sadalage PS, Patil RV, Havaldar DV, Gavade SS, Santos AC, Pawar KD. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J Nanobiotechnology. 2021;19(1):1–7.
Milanezi FG, Meireles LM, de Christo Scherer MM, de Oliveira JP, da Silva AR, de Araujo ML, Endringer DC, Fronza M, Guimarães MC, Scherer R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm J. 2019;27(7):968–74.
Yilmaz M, Karanastasis AA, Chatziathanasiadou MV, Oguz M, Kougioumtzi A, Clemente N, Kellici TF, Zafeiropoulos NE, Avgeropoulos A, Mavromoustakos T, Dianzani U. Inclusion of quercetin in gold nanoparticles decorated with supramolecular hosts amplifies its tumor targeting properties. ACS Appl Bio Mater. 2019;2(7):2715–25.
Das S, Roy P, Mondal S, Bera T, Mukherjee A. One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids Surf B Biointerfaces. 2013;107:27–34.
Palaniswamy M. Size dependent application of biologically synthesized silver nanoparticles against bacterial skin pathogens. Asian J Pharm Clin Res. 2017;10(10):192–5.
Tagde P, Kulkarni GT, Mishra DK, Kesharwani P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J Drug Deliv Sci Technol. 2020;56: 101613.
Das A, Konyak PM, Das A, Dey SK, Saha C. Physicochemical characterization of dual action liposomal formulations: anticancer and antimicrobial. Heliyon. 2019;5(8): e02372.
Gulla S, Lomada D, Araveti PB, Srivastava A, Murikinati MK, Reddy KR, Inamuddin, Reddy MC, Altalhi T. Titanium dioxide nanotubes conjugated with quercetin function as an effective anticancer agent by inducing apoptosis in melanoma cells. J Nanostructure Chem. 2021;11:721–34.
Sallam AA, Ahmed MM, El-Magd MA, Magdy A, Ghamry HI, Alshahrani MY, Abou El-Fotoh MF. Quercetin-ameliorated, multi-walled carbon nanotubes-induced immunotoxic, inflammatory, and oxidative effects in mice. Molecules. 2022;27(7):2117.
Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12(7):604.
Perez-Vizcaino F, Duarte J, Andriantsitohaina R. Endothelial function and cardiovascular disease: effects of quercetin and wine polyphenols. Free Radic Res. 2006;40(10):1054–65.
Razzaghi H, Quesnel-Crooks S, Sherman R, Joseph R, Kohler B, Andall-Brereton G, Ivey MA, Edwards BK, Mery L, Gawryszewski V, Saraiya M. Leading causes of cancer mortality—Caribbean region, 2003–2013. Morb Mortal Wkly Rep. 2016;65(49):1395–400.
Yang Z, Liu Y, Liao J, Gong C, Sun C, Zhou X, Wei X, Zhang T, Gao Q, Ma D, Chen G. Retracted: Quercetin induces endoplasmic reticulum stress to enhance c DDP cytotoxicity in ovarian cancer: involvement of STAT 3 signaling. FEBS J. 2015;282(6):1111–25.
Gao X, Wang B, Wei X, Men K, Zheng F, Zhou Y, Zheng Y, Gou M, Huang M, Guo G, Huang N. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4(22):7021–30.
Afroze SH, Peddaboina C, Mcdowell AB, Ashraf AZ, Mccormick TC, Newell-Rogers MK, Zawieja DC, Kuehl TJ, Uddin MN. Differential effects of in vitro treatment with cinobufotalin on three types of ovarian cancer cells. Anticancer Res. 2018;38(10):5717–24.
Li N, Sun C, Zhou B, Xing H, Ma D, Chen G, Weng D. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer. PLoS ONE. 2014;9(7): e100314.
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, Wei X, Ma D, Ye F, Gao QL. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 2017;22:544–57.
Shen F, Herenyiova M, Weber G. Synergistic down-regulation of signal transduction and cytotoxicity by tiazofurin and quercetin in human ovarian carcinoma cells. Life Sci. 1999;64(21):1869–76.
Teekaraman D, Elayapillai SP, Viswanathan MP, Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem Biol Interact. 2019;300:91–100.
Cote B, Carlson LJ, Rao DA, Alani AW. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J Control Release. 2015;213:128–33.
Du G, Lin H, Wang M, Zhang S, Wu X, Lu L, Ji L, Yu L. Quercetin greatly improved therapeutic index of doxorubicin against 4T1 breast cancer by its opposing effects on HIF-1α in tumor and normal cells. Cancer Chemother Pharmacol. 2010;65:277–87.
Manouchehri JM, Kalafatis M, Lindner D. Evaluation of the efficacy of TRAIL plus quercetin as a potential breast carcinoma therapeutic. Cancer Res. 2016;76:1295–1295.
Huang C, Lee SY, Lin CL, Tu TH, Chen LH, Chen YJ, Huang HC. Co-treatment with quercetin and 1,2,3,4,6-penta-O-galloyl-β-D-glucose causes cell cycle arrest and apoptosis in human breast cancer MDA-MB-231 and AU565 cells. J Agr Food Chem. 2013;61(26):6430–45.
Gulati N, Laudet B, Zohrabian VM, Murali RA, Jhanwar-Uniyal ME. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26(2A):1177–81.
Lv L, Liu C, Chen C, Yu X, Chen G, Shi Y, Qin F, Ou J, Qiu K, Li G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget. 2016;7(22):32184.
Cao L, Yang Y, Ye Z, Lin B, Zeng J, Li C, Liang T, Zhou K, Li J. Quercetin-3-methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int J Mol Med. 2018;42(3):1625–36.
Khorsandi L, Orazizadeh M, Niazvand F, Abbaspour MR, Mansouri E, Khodadadi AJ. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl Lek Listy. 2017;118(2):123–8.
Zhao X, Wang Q, Yang S, Chen C, Li X, Liu J, Zou Z, Cai D. Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur J Pharmacol. 2016;781:60–8.
Zuo J, Jiang Y, Zhang E, Chen Y, Liang Z, Zhu J, Zhao Y, Xu H, Liu G, Liu J, Wang W. Synergistic effects of 7-O-geranylquercetin and siRNAs on the treatment of human breast cancer. Life Sci. 2019;15(227):145–52.
Li S, et al. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed Pharmacother. 2018;100:441–7.
Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol. 2011;68:1161–72.
Ranganathan S, Halagowder D, Sivasithambaram ND. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE. 2015;10(10): e0141370.
Wang H, Tao L, Qi K, Zhang H, Feng D, Wei W, Kong H, Chen T, Lin Q. Quercetin reverses tamoxifen resistance in breast cancer cells. J Buon. 2015;20(3):707–13.
Lepik D, Jaks V, Kadaja L, Värv S, Maimets T. Electroporation and carrier DNA cause p53 activation, cell cycle arrest, and apoptosis. Anal Biochem. 2003;318(1):52–9.
Park M, Chae HD, Yun J, Jung M, Kim YS, Kim SH, Han MH, Shin DY. Constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated G2-M arrest. Cancer Res. 2000;60(3):542–5.
Srinivasan A, Thangavel C, Liu Y, Shoyele S, Den RB, Selvakumar P, Lakshmikuttyamma A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol Carcinog. 2016;55(5):743–56.
Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI, Teng YD, Cotanche DA, Snyder EY. Expression profile of an operationally-defined neural stem cell clone. Ex Neurol. 2005;194(2):320–32.
Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994;54(9):2424–8.
Guo W, Yu H, Zhang L, Chen X, Liu Y, Wang Y, Zhang Y. Effect of hyperoside on cervical cancer cells and transcriptome analysis of differentially expressed genes. Cancer Cell Int. 2019;19:1–4.
Bądziul D, Jakubowicz-Gil J, Paduch R, Głowniak K, Gawron A. Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol Cell Biochem. 2014;392:213–27.
Lv M, Shen Y, Yang J, Li S, Wang B, Chen Z, Li P, Liu P, Yang J. Angiomotin family members: oncogenes or tumor suppressors? Int J Biol Sci. 2017;13(6):772.
Luo CL, Liu YQ, Wang P, Song CH, Wang KJ, Dai LP, Zhang JY, Ye H. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed Pharmacother. 2016;82:595–605.
Yang MD, Lai KC, Lai TY, Hsu SC, Kuo CL, Yu CS, Lin ML, Yang JS, Kuo HM, Wu SH, Chung JG. Phenethyl isothiocyanate inhibits migration and invasion of human gastric cancer AGS cells through suppressing MAPK and NF-κB signal pathways. Anticancer Res. 2010;30(6):2135–43.
Ho CC, Lai KC, Hsu SC, Kuo CL, Ma CY, Lin ML, Yang JS, Chung JG. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human gastric cancer AGS cells via suppressing ERK signal pathways. Hum Exp Toxicol. 2011;30(4):296–306.
Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, Lin YF, Chung JG. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ Toxicol. 2018;33(11):1168–81.
Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20(13):3177.
Lee HH, Lee S, Shin YS, Cho M, Kang H, Cho H. Anti-cancer effect of quercetin in xenograft models with EBV-associated human gastric carcinoma. Molecules. 2016;21(10):1286.
Hsieh HL, Yu MC, Cheng LC, Chu MY, Huang TH, Yeh TS, Tsai MM. Quercetin exerts anti-inflammatory effects via inhibiting tumor necrosis factor-α-induced matrix metalloproteinase-9 expression in normal human gastric epithelial cells. World J Gastroenterol. 2022;28(11):1139.
Yamashita H, Kitayama J, Shida D, Yamaguchi H, Mori K, Osada M, Aoki S, Yatomi Y, Takuwa Y, Nagawa H. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation1. J Surg Res. 2006;130(1):80–7.
Chen M, Duan C, Pan J. Quercetin increases doxorubicin-induced apoptosis through oxidative DNA damage in KATO III gastric cancer cells. Iran Red Crescent Med J. 2021;23(4):1286–1296.
Zhang JY, Lin MT, Zhou MJ, Yi T, Tang YN, Tang SL, Yang ZJ, Zhao ZZ, Chen HB. Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules. 2015;20(6):11524–34.
Lee M, Son M, Ryu E, Shin YS, Kim JG, Kang BW, Sung GH, Cho H, Kang H. Quercetin-induced apoptosis prevents EBV infection. Oncotarget. 2015;6(14):12603.
Mukherjee A, Khuda-Bukhsh AR. Quercetin down-regulates IL-6/STAT-3 signals to induce mitochondrial-mediated apoptosis in a nonsmall-cell lung-cancer cell line, A549. J Pharmacopuncture. 2015;18(1):19.
Moon JH, Eo SK, Lee JH, Park SY. Quercetin-induced autophagy flux enhances TRAIL-mediated tumor cell death. Oncol Rep. 2015;34(1):375–81.
Chuang CH, Yeh CL, Yeh SL, Lin ES, Wang LY, Wang YH. Quercetin metabolites inhibit MMP-2 expression in A549 lung cancer cells by PPAR-γ associated mechanisms. J Nutr Biochem. 2016;33:45–53.
Xingyu Z, Peijie M, Dan P, Youg W, Daojun W, Xinzheng C, Xijun Z, Yangrong S. Quercetin suppresses lung cancer growth by targeting Aurora B kinase. Cancer Med. 2016;5(11):3156–65.
Baby B, Antony P, Vijayan R. Interactions of quercetin with receptor tyrosine kinases associated with human lung carcinoma. Nat Prod Res. 2018;32(24):2928–31.
Yousuf M, Khan P, Shamsi A, Shahbaaz M, Hasan GM, Haque QM, Christoffels A, Islam A, Hassan MI. Inhibiting CDK6 activity by quercetin is an attractive strategy for cancer therapy. ACS Omega. 2020;5(42):27480–91.
Li H, Tan L, Zhang JW, Chen H, Liang B, Qiu T, Li QS, Cai M, Zhang QH. Quercetin is the active component of Yang-Yin-Qing-Fei-Tang to induce apoptosis in non-small cell lung cancer. Am J Chin Med. 2019;47(04):879–93.
Xu D, Chi G, Xu D. Transcriptional regulation of miR-483-3p mediated by IL-6/STAT3 axis promoted epithelial-mesenchymal transition and tumor stemness in glioma. Aging (Albany NY). 2020;12:27480–27491.
Sacks D, Baxter B, Campbell BC, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–32.
Wang Q, Chen Y, Lu H, Wang H, Feng H, Xu J, Zhang B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life. 2020;72(5):1012–22.
Gokbulut AA, Apohan E, Baran Y. Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology. 2013;18(3):144–50.
Kogoshi H, Sato T, Koyama T, Nara N, Tohda S. γ-Secretase inhibitors suppress the growth of leukemia and lymphoma cells. Oncol Rep. 2007;18(1):77–80.
Lotfi N, Yousefi Z, Golabi M, Khalilian P, Ghezelbash B, Montazeri M, Shams MH, Baghbadorani PZ, Eskandari N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: an update. Front Immunol. 2023;14:1077531.
Avci CB, Yilmaz S, Dogan ZO, Saydam G, Dodurga Y, Ekiz HA, Kartal M, Sahin F, Baran Y, Gunduz C. Quercetin-induced apoptosis involves increased hTERT enzyme activity of leukemic cells. Hematology. 2011;16(5):303–7.
Kim SH, Yoo ES, Woo JS, Han SH, Lee JH, Jung SH, Kim HJ, Jung JY. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur J Pharmacol. 2019;860: 172568.
Lee WJ, Hsiao M, Chang JL, Yang SF, Tseng TH, Cheng CW, Chow JM, Lin KH, Lin YW, Liu CC, Lee LM. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch Toxicol. 2015;89:1103–17.
Brisdelli F, Coccia C, Cinque B, Cifone MG, Bozzi A. Induction of apoptosis by quercetin: different response of human chronic myeloid (K562) and acute lymphoblastic (HSB-2) leukemia cells. Mol Cell Biochem. 2007;296:137–49.
Chen FY, Cao LF, Wan HX, Zhang MY, Cai JY, Shen LJ, Zhong JH, Zhong H. Quercetin enhances adriamycin cytotoxicity through induction of apoptosis and regulation of mitogen-activated protein kinase/extracellular signal-regulated kinase/c-Jun N-terminal kinase signaling in multidrug-resistant leukemia K562 cells. Mol Med Rep. 2015;11(1):341–8.
Naimi A, Entezari A, Hagh MF, Hassanzadeh A, Saraei R, Solali S. Quercetin sensitizes human myeloid leukemia KG-1 cells against TRAIL-induced apoptosis. J Cell Physiol. 2019;234(8):13233–41.
Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G, Nikitovic D. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep. 2017;38(2):819–28.
Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6(1):1–3.
Ueda K, Ito E, Karayama M, Ohsaki E, Nakano K, Watanabe S. KSHV-infected PEL cell lines exhibit a distinct gene expression profile. Biochem Biophys Res Commun. 2010;394(3):482–7.
Aresté C, Blackbourn DJ. Modulation of the immune system by Kaposi’s sarcoma-associated herpesvirus. Trends Microbiol. 2009;17(3):119–29.
Granato M, Rizzello C, Montani MS, Cuomo L, Vitillo M, Santarelli R, Gonnella R, D’Orazi G, Faggioni A, Cirone M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J Nutr Biochem. 2017;41:124–36.
Alvarez MC, Maso V, Torello CO, Ferro KP, Saad ST. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin Epigenetics. 2018;10:1–1.
Kumar R, Saini KS, Kumar A, Kumar S, Ramakrishna E, Maurya R, Konwar R, Chattopadhyay N. Quercetin-6-C-β-D-glucopyranoside, natural analog of quercetin exhibits anti-prostate cancer activity by inhibiting Akt-mTOR pathway via aryl hydrocarbon receptor. Biochimie. 2015;119:68–79.
Shoskes DA. Treatment response to conventional and novel therapies in chronic prostatitis. Curr Urol Rep. 2003;4(4):311–5.
Al-Jabban SM, Zhang X, Chen G, Mekuria EA, Rakotondraibe LH, Chen QH. Synthesis and anti-proliferative effects of quercetin derivatives. Nat Prod Commun. 2015;10(12):2113–8.
Mousavi N, Rahimi S, Emami H, Kazemi AH, Kashi RM, Heidarian R. The effect of quercetin nanosuspension on prostate cancer cell line LNCaP via Hedgehog signaling pathway. Rep Biochem Mol Biol. 2021;10(1):69.
Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, Singh PR, Srinivasan N, Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25(11):1132–9.
Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J Surg Oncol. 2018;16(1):1–2.
Yang F, Song L, Wang H, Wang J, Xu Z, Xing N. Quercetin in prostate cancer: chemotherapeutic and chemopreventive effects, mechanisms and clinical application potential. Oncol Rep. 2015;33(6):2659–68.
Su Z, Liu T, Hong G. Quercetin suppress prostatic cancer biological activity in vitro and vivo study. J Biomater Tissue Eng. 2018;8(7):949–61.
Seeni A, Takahashi S, Takeshita K, Tang M, Sugiura S, Sato SY, Shirai T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac J Cancer Prev. 2008;9(1):7–14.
Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22(3):409–14.
Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007;6(9):2591–9.
Siegelin MD, Reuss DE, Habel A, Rami A, Von Deimling A. Quercetin promotes degradation of survivin and thereby enhances death-receptor–mediated apoptosis in glioma cells. Neuro Oncol. 2009;11(2):122–31.
Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, Tse AK, Kwan HY, Yu H, Yu ZL. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:1–2.
Catanzaro D, Ragazzi E, Vianello C, Caparrotta L, Montopoli M. Effect of quercetin on cell cycle and cyclin expression in ovarian carcinoma and osteosarcoma cell lines. Nat Prod Commun. 2015;10(8):1365–8.
Suh DK, Lee EJ, Kim HC, Kim JH. Induction of G 1/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch Pharm Res. 2010;33:781–5.
Saneja A, Kaushik P, Kaushik D, Kumar S, Kumar D. Antioxidant, analgesic and anti-inflammatory activities of Santalum album Linn. Planta Med. 2009;75(04):102.
Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P. Improved oral absorption of quercetin from quercetin phytosome®, a new delivery system based on food grade lecithin. Eur J Drug Metab Pharmacokinet. 2019;44:169–77.
Di Pierro F, Khan A, Iqtadar S, Mumtaz SU, Chaudhry MN, Bertuccioli A, Derosa G, Maffioli P, Togni S, Riva A, Allegrini P. Quercetin as a possible complementary agent for early-stage COVID-19: concluding results of a randomized clinical trial. Front Pharmacol. 2023;13:1096853.
Thorne. Quercetin Phytosome. Available at: https://www.thorne.com/products/dp/quercetin-phytosome. Accessed 23 Jun 2023.
Codeage. Nanofood liposomal liquid quercetin phytosome. Available at: https://www.codeage.com/products/liposomal-quercetin-phytosome-liquid. Accessed 23 Jun 2023.
One Planet Nutrition. Nano Quercetin. Available at: https://www.oneplanetnutrition.com/index.php/shop#!/Nano-Quercetin-240-Caps-250-mg/p/478601707. Accessed 23 Jun 2023.
Acknowledgments
Vanashree H. Chaudhari is thankful to the Indian Council of Medical Research, Government of India, for the fellowship provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Esha S. Attar, Vanashree H. Chaudhari, Chaitanya G. Deokar, Sathish Dyawanapelly, and Padma V. Devarajan report that they have no conflict of interest to declare.
Author Contributions
Esha S. Attar and Vanashree H. Chaudhari: conceptualization, writing—original draft, writing—review, and editing. Chaitanya G. Deokar: writing—original draft. Sathish Dyawanapelly: visualization, writing—review, and editing. Padma V. Devarajan: conceptualization, supervision, writing—review, and editing.
Ethical Approval
Not applicable.
Funding
No funding was received in the preparation of this manuscript.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data are included in the manuscript.
Code Availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Attar, E.S., Chaudhari, V.H., Deokar, C.G. et al. Nano Drug Delivery Strategies for an Oral Bioenhanced Quercetin Formulation. Eur J Drug Metab Pharmacokinet 48, 495–514 (2023). https://doi.org/10.1007/s13318-023-00843-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00843-7