Abstract
Augmented renal clearance (ARC) is a phenomenon of enhanced renal function seen in critically ill patients. ARC alters the disposition of renally eliminated medications currently used in the intensive care unit, resulting in underdosing and potential therapy failure. Our review addresses the rising concern of inadequate dosing in patients with ARC by summarizing the currently available evidence. To our knowledge, this guide is the first to provide clinicians with dose recommendation insights for renally eliminated agents in adult critically ill patients with ARC. A comprehensive literature search using MEDLINE, Embase, Cochrane Library, CINAHL, Scopus, and ProQuest Dissertations and Theses Global was conducted until 3 November 2021. Screening and data extraction were conducted in two steps: title and abstract screening followed by full-text review. Full text review resulted in a total of 51 studies included in this review. The results demonstrated the need for higher-than-standard doses for meropenem, imipenem, and vancomycin and reduced dosing intervals for ceftriaxone in patients with ARC. The potential need for increased dosing frequency in patients with ARC was also found for both enoxaparin and levetiracetam. In conclusion, ARC has been shown to influence the probability of target attainment in several medications requiring dosing changes to mitigate the risk of therapeutic failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Augmented renal clearance (ARC) is a phenomenon seen in critically ill patients potentially contributing to underdosing and treatment failure. |
The currently available evidence is not exhaustive of all drugs used in the ICU and other settings where patients are at a higher risk of developing ARC. |
Results demonstrated the need for higher doses for meropenem, imipenem, vancomycin, levetiracetam, and enoxaparin. |
1 Introduction
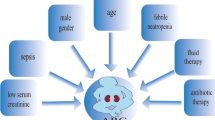
Augmented renal clearance (ARC) is a phenomenon seen in critically ill patients that has been increasingly recognized in the recent years. It is most often defined as a creatinine clearance (CrCl) > 130 ml/min/1.73 m2, which is most accurately based on measured CrCl using 8–24 h urine collection [1]. Although the exact mechanisms causing ARC are not fully understood, many have been hypothesized. ARC may perhaps be a physiologic response to acute injury such as traumatic brain injury or body temperature change. Renal clearance may also be enhanced owing to various treatments patients in the intensive care unit (ICU) receive, such as vasopressors and fluid resuscitation. It is also thought to be a consequence of the heightened sympathetic response associated with severe critical illness and systemic inflammatory responses such as in patients with traumatic brain injury and sepsis, as well as changes in vascular resistance, cardiac output, and blood flow to major organs, e.g., the kidneys, resulting in a hyperdynamic state and accelerated glomerular filtration rate. The prevalence of ARC has been reported to range between 14% and 80%, making it a common phenomenon [1]. The clinical relevance of ARC lies in the potential for enhancing the clearance of drugs primarily eliminated by the kidneys such as β-lactam antimicrobials and certain antiepileptic drugs, potentially leading to therapeutic failure and potentially poor outcomes in this especially vulnerable patient population.
Multiple ARC risk factors have been reported by research teams. As mentioned, ARC is more prevalent in the critical care setting, especially in trauma patients. Age appears to be the most important and widely verified risk factor for ARC. Patients of younger age (< 50 years of age) were at the highest risk of developing ARC. Additionally, patients with ARC tend to be males, with lower critical illness severity scores [1]. Therefore, it may be necessary to use risk assessment tools for more rapid identification of critically ill patients exhibiting ARC. A few tools have been developed for this purpose [2].
It is considered common practice to reduce doses in the presence of renal impairment; however, the scarcity of currently available evidence and the lack of a clear consensus supporting dosing requirements in the case of ARC have made it difficult for clinicians to optimize dosing regimens for patients with ARC. Multiple studies have demonstrated that ARC impacts the plasma levels of renally eliminated drugs commonly seen in a critical care setting, especially antimicrobials and antiepileptic drugs (AED). We discussed the phenomenon of ARC in our previous review [1]; however, multiple studies have been published since our initial review. Therefore, the objective of this review is to provide an update to summarize the current evidence pertaining to the influence of ARC on the disposition of renally eliminated medications commonly used in the ICU. We hope that this guide will provide clinicians with dosing recommendation insights for multiple renally eliminated agents used in patients with ARC.
2 Literature Search
2.1 Search Strategy
A comprehensive database search was conducted by the medical librarian (J.Y.K.) on 27 October 2020 in the following databases: MEDLINE (via Ovid), Embase (Ovid), Cochrane Library (Wiley), CINAHL, Scopus, and ProQuest Dissertations and Theses Global with no date or language limits. The search was updated on 3 November 2021 to capture newly published research following the original search. Keywords related to ARC in the critically ill were used to conduct the search (see Supplementary Table 1 for details on keywords used). We utilized, the web-based review screening tool “Covidence” for the screening process (www.covidence.org).
2.2 Study Inclusion and Exclusion Criteria
Human studies conducted in critically ill adult populations and reporting drug dosing or pharmacokinetics in the setting of ARC (those with creatinine clearance > 130 ml/min/1.73 m2) were included. Studies were further sorted on the basis of inclusion of specific medications. Studies focused on pediatrics, pregnant women, or studies conducted in populations with potentially altered renal elimination (e.g., cystic fibrosis, burn patients) were excluded. This is due to the physiological and pathological changes associated with these patient populations that would hinder the detection of ARC. In addition, reviews, editorials, case reports, preprints, and commentaries were also excluded.
2.3 Study Screening
Study screening and selection from the 27 October 2020 database search were conducted independently by A.S. and S.H.M. An updated study screening and selection from 3 November 2021, were conducted independently by S.H.M. and S.S. to include research published from October 2020 to November 2021. An initial title and abstract screening followed by a full-text review was conducted. Any conflicts were discussed among authors to reach a consensus.
2.4 Data Extraction
Data extracted included study design, study objectives, study population, drugs tested, method used, and study findings.
3 Results of Literature Search and Discussion
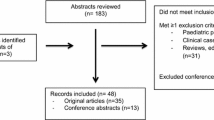
Literature search resulted in 3455 and 3941 articles across all databases on 27 October 2020, and 3 November 2021, respectively (Supplementary Table 2). A total of 1761 and 347 unique articles remained for screening from these comprehensive searches. Full text screening yielded 51 articles for inclusion (Supplementary Table 3).
Prospective observational studies constitute the main body of evidence in this review at 69% (n = 34) of the total evidence. Retrospective observational studies constitute 31% (n = 16) of the total evidence. This is in addition to a single prospective interventional study by Cojutti et al. discussing meropenem [3] Expectedly, the literature search concluded that renally eliminated drugs such as β-lactams and levetiracetam in patients with ARC needed alternate dosing regimens where a loading dose might be needed, an extended infusion strategy employed, or frequency or amount of dosing increased to achieve the same targets as in patients without ARC. Although the currently available evidence is not exhaustive of all drugs used in the ICU and other settings where patients are at a higher risk of developing ARC, it can be assumed that all renally eliminated drugs in this patient population will be at a higher risk of therapeutic failure or target non-attainment, and it would be prudent to take precautions to mitigate this risk.
Young age, male sex, and trauma are repeatedly defined in the literature as risk factors for ARC in those with apparently normal renal function and lower disease severity scores. Age, male sex, and trauma were associated with ARC with pooled OR (95% CI) of 0.95 (0.93–0.96), 2.36 (1.28–4.36), and 2.60 (1.21–5.58), respectively. Prevalence for neuro, trauma, mixed, and sepsis ICUs was 74 (55–87), 58 (48–67), 36 (31–41), and 33 (21–48), respectively [4].
3.1 Carbapenems
3.1.1 Meropenem
Meropenem is a member of the carbapenem class of antimicrobials. It has a broad spectrum of activity and exhibits time-dependent killing. It is also eliminated 70% by the kidneys [5]. In the case of critically ill patients, clinicians target a minimum of ≥ 50% fT > MIC (percent of time that free drug remains above the minimum inhibitory concentration, MIC), and the preferred target is ≥ 100% fT > MIC. In some cases, experts prefer to target ≥ 100% fT > 4 times the MIC [6,7,8]. Three prospective observational studies have described the impact of ARC on meropenem treatment [9,10,11]. In an early study, Kitzes-Cohen et al. reported that lower plasma concentrations of meropenem were seen with increasing kidney function [9]. Ehmann et al. corroborated this observation [10]. Additionally, standard meropenem doses were insufficient at achieving desired concentrations in the majority of patients with ARC [9, 10]. The authors suggested that a meropenem dosing of 2000 mg every 8 h will greatly enhance the probability of target attainment [9, 10]. A dose of 2000 mg every 8 h was also identified as an alternative strategy by Tamatsukuri et al. with the additional recommendation of administration via prolonged infusion over 180 min for each dose [11]. However, these studies were limited by a small sample size as well as a lack of correlation to clinical outcomes. An interventional study exploring the effects of therapeutic drug monitoring (TDM)-based optimization on treatment outcomes also exists [3]. Cojutti et al. found that, based on TDM, 30.1% of patients required dose adjustment, and this strategy resulted in a promising overall cure rate of 90%. In this study, mortality was also significantly associated with ARC (OR 10.846, CI 95% 1.534–76.672, P = 0.017) [3].
Conclusion: ARC results in lower plasma concentration of meropenem; this can lead to therapeutic failure and negative clinical outcomes. Because of this, higher dosing is recommended [9, 10]. A dose of 2000 mg every 8 h is likely needed; prolonged infusion may also improve drug exposure [9,10,11]. Therapeutic drug monitoring of meropenem could also be of benefit in these patients. Further studies focused on these strategies are needed.
3.1.2 Imipenem/Cilastatin
Imipenem is a carbapenem antimicrobial agent. It has a broad spectrum of activity and demonstrates time-dependent bacterial killing. It is also 70% renally eliminated [5]. In the case of critically ill patients, clinicians target a minimum of ≥ 50% fT > MIC, and the preferred target is ≥ 100% fT > MIC. In some cases, experts prefer to target ≥ 100% fT > 4 times the MIC [6,7,8]. A retrospective study demonstrated that, when patients received 500 mg every 6 h (2 g/day), frequent treatment failure and infrequent toxicity was documented [12]. The authors also found that, when patients received higher doses (3–4 g/day) instead of standard dosing (2 g/day), they reported fewer counts of treatment failures without any increase in toxicity. Huttner et al. performed a prospective observational study in which patients received standard doses of 500 mg four times daily as well [13]. They reported ARC as a predictor for undetectable trough levels, though this study was underpowered to determine any associations with clinical failure. These two studies suggest a possibility that standard dosing may be insufficient and increased doses could be warranted [12, 13].
Patel et al. 2021 conducted a prospective observational study to determine the probability of target attainment using various dosing regimens of imipenem/cilastatin/relebactam in patients with hospital-acquired bacterial pneumonia/ventilator acquired bacterial pneumonia [14]. ARC was defined as CrCl ≥ 150 ml/min calculated using the Cockcroft–Gault equation. A dose of imipenem/relebactam 500/250 mg every 6 h established a joint probability of target attainment (PTA) of 99% for a target trough of 30% fT > MIC [14]. Further studies analyzing the correlation between target trough concentration and clinical success are needed.
Conclusion: ARC may lead to lower concentrations of imipenem/cilastatin in some patients receiving standard doses of 500 mg every 6 h, which has the potential to result in therapeutic failure and negative effects on clinical outcomes [12, 13]. Increased doses could be considered for patients exhibiting ARC who are indicated for treatment with imipenem/cilastatin and experiencing clinical failure at standard doses. Doses of 1 g every 6 h could be considered for these individuals [12]. Further studies administering increased doses to patients with ARC in which safety and clinical outcomes are documented, are required. With regard to imipenem/relebactam, standard doses of 1.25 g (500 mg imipenem/500 mg cilastatin/250 mg relebactam) dosed every 6 h consistently showed high PTA for such doses in patients with ARC [14]. This may indicate that no further dose increases are necessary in patients with ARC being treated for infection with imipenem/relebactam. Further studies are needed to identify the relationship between target attainment and clinical success.
3.2 Cephalosporins
3.2.1 Ceftriaxone
Ceftriaxone is a third-generation cephalosporin antimicrobial. It has a relatively broad spectrum of activity, with specific efficacy against Gram-negative pathogens; it accomplishes bacterial killing in a time-dependent manner. Up to 67% of the drug is eliminated by the kidneys [5]. In the case of critically ill patients, clinicians target a minimum of ≥ 50% fT > MIC, and the preferred target is ≥ 100% fT > MIC. In some cases, experts prefer to target ≥ 100% fT > 4 times the MIC [7, 8, 15]. Three prospective observational studies describe ceftriaxone dosing in the context of ARC [16,17,18]. Increased kidney function leads to a decrease in plasma concentrations of ceftriaxone when standard dosing is used [17]. The insufficiency of standard dosing of ceftriaxone is further described by Ollivier et al. They found that CrCl > 150 ml/min is significantly associated with underdosing, defined as trough concentrations < 2 mg/L (OR 8.8, CI 95% 2.5–30.7, P < 0.01) [16]. The authors suggested that a reduced dosing interval of 2000 mg every 12 h would be better suited for target attainment [16]. However, these studies were limited by a small sample size and the inability to associate findings with clinical outcomes. Wong et al. found that ARC was a predictive factor for treatment failure. They suggested that ceftriaxone allowed for increased drug exposure throughout the dosing interval compared with other β-lactams, owing to the attainment of a strict target of 100% fT > 4× MIC in the majority of patients who received it [18]. A retrospective observational study evaluating the effects of an increased dosing regimen of ceftriaxone also exists [19]. Carrie et al. found that 2000 mg twice-daily dosing was effective at reducing therapeutic failure and relapse of infection without increased adverse effects [19].
Conclusion: Ceftriaxone may be a promising agent in improving target attainment, possibly owing to its high protein binding, which allows for prolonged half-life, ensuring that concentrations remain above target for the duration of the dosing interval [19]. However, it is not spared from reduced plasma concentrations in the setting of ARC, which can lead to higher rates of therapeutic failure [16, 17, 19]. Higher doses or reduced dosing intervals may be warranted [16]. Doses of 2000 mg every 12 h are likely required [16]. Further studies regarding increased doses of ceftriaxone, and its safety, in patients with ARC are necessary.
3.3 Aminoglycosides
3.3.1 Amikacin
Amikacin is a member of the aminoglycoside class of antimicrobials. It demonstrates concentration-dependent bacterial killing and is often used to treat severe Gram-negative infections. It is eliminated nearly 100% unchanged by the kidneys [5]. There is one retrospective observational study that has discussed amikacin’s use in patients with ARC [20]. Carrie et al. found that an increase in renal clearance is associated with an increased clearance of amikacin, which results in lower plasma concentrations. Using the Monte-Carlo simulation, the authors determined the standard loading dose of 25 mg/kg to be effective at reaching maximum blood concentration (Cmax)/MIC targets for most patients [20]. However, patients exhibiting CrCl > 130 ml/min may need higher-than-licensed doses of up to 35 mg/kg [20]. An increase in amikacin dose has been explored in a prospective observational study [21]. Arechiga-Alvarado et al. reported that an increase in creatinine clearance leads to lower concentrations of amikacin. Monte-Carlo simulations suggested that, for patients with ARC infected with pathogens with high minimum inhibitory concentrations, doses of up to 70 mg/kg could be warranted [21]. However, this study was limited by a small sample size, and both studies based the dosing recommendations only on simulation data. Furthermore, the safety of doses as high at 70 mg/kg needs to be explored in future studies. Both of the above studies investigated extended interval/once-daily dosing [20, 21].
Conclusion: ARC results in increased amikacin clearance and subsequent decreased plasma concentrations [20]. Low plasma concentrations of amikacin have the potential to increase instances of therapeutic failure, which would be detrimental in terms of increased mortality and emergence of resistant pathogens [20]. For this reason, higher-than-licensed doses may be needed to reach the recommended pharmacokinetic/pharmacodynamic (PK/PD) targets [20, 21]. An increase of amikacin dose may be effective at improving achievement of desired drug levels in patients with ARC [20, 21]. Further studies are required to assess the safety and clinical benefit of such dose increases.
3.4 Glycopeptides
3.4.1 Vancomycin
Vancomycin is a glycopeptide antimicrobial agent. It has a narrow spectrum of activity against primarily Gram-positive pathogens, including resistant strains of Staphylococcus. It is excreted by the kidneys as 80–90% unchanged drug [5]. The ideal monitoring parameter for vancomycin is area under the curve to minimum inhibitory concentration (AUC:MIC) ratio of greater than 400. However, in clinical practice, a steady-state trough value of 10–20 mg/L is often used as a surrogate target. Current standard dosing involves a loading dose of 25–30 mg/kg followed by a maintenance dose of 15 mg/kg administered at various intervals determined by the patients’ calculated CrCl. The recommended dosing interval for patients with CrCl > 80 ml/min is every 12 h [22].
Multiple studies have discussed the impacts of ARC on vancomycin plasma concentrations [23,24,25,26]. The clearance of vancomycin is drastically increased in patients with ARC ranging from 1.6 to up to 3.5 times the expected values [23, 26]. The authors also demonstrated that this was associated with subtherapeutic levels as well as a need for overall higher doses [26]. Multiple studies have suggested that standard dosing of vancomycin consistently results in subtherapeutic vancomycin levels and that higher doses are required [19, 24, 25, 27, 28]. To illustrate, Chen et al. found that only 19.23% of patients in the ARC group were able to achieve target trough levels of > 10 mg/L [24]. In addition, He et al. (2020) conducted a retrospective observational study and found that 77.7% of patients with ARC versus 68.8% of patient without ARC had subtherapeutic (< 10 mg/L) vancomycin trough concentrations when given vancomycin maintenance doses of 15 mg/kg every 12 h intravenous (IV) infusion. They also demonstrated that only 17.9% and 4.3% of patients with ARC were able to reach trough levels between 10–15 and 15–20 mg/L, respectively [25]. The authors suggested a dose of 46 mg/kg/day in patients with ARC to achieve trough levels of at least 10 mg/L [25]. Contrary to these studies, Zhao et al. found that standard doses of 1000 mg every 12 h would result in PTA (defined as targeted AUC:MIC ratio between 400 and 650 mg h/L) of 62.56% in patients with CrCl between 150 and 179 ml/min [29]. Results of this study were based solely on Monte-Carlo simulations with a mean patient total body weight (TBW) of 63.4 kg and thus may not be representative of adult populations with higher TBW [29]. Furthermore, two prospective observational studies have discussed the need for high loading doses for patients with ARC [30, 31]. Baptista et al. and Campassi et al. administered loading doses of 1000–1500 mg and 15 mg/kg, respectively with maintenance doses of 30 mg/kg/day [30, 31]. It was reported that only half of the patients with ARC were able to achieve therapeutic trough levels with this dosing strategy [30, 31]. The authors suggested a need for an increased loading dose of 2 g as well as a need for TDM for these patients [30]. A need for maintenance doses over 40 mg/kg/day was also reinforced by the findings of Helset et al. (2020). In their prospective observational study, they found that patients with ARC demonstrated an overall lower AUC:MIC despite receiving an average dose of 44.4 mg/kg/day [32].Two retrospective observational studies found that, when patients with ARC received doses of 1000 mg every 12 h, the majority were not able to achieve trough concentration > 10 mg/L [23, 27]. The authors suggested that increased frequency be considered. A retrospective study by Minkute et al. has reported that patients with ARC are at risk of underdosing (defined as trough below 5.2 mg/L) and that nearly double the standard dose is likely required. The authors further suggested that a decreased dosing interval to every 6–8 h be considered [28].
Lastly, both a prospective observational study and retrospective analysis have discussed the promising effects of the use of nomogram-based dosing in patients with ARC [33, 34]. Bapstista et al. (2014) administered dosing concurrent with a developed nomogram based on 8 h urine CrCl measurement. With this dosing strategy, all patients with ARC were able to achieve target trough levels within the first day of treatment [33]. This study was limited by a small sample size. However, Ishii et al. (2018) showed promising results when using a nomogram based on calculations of estimated glomerular filtration rate (eGFR) using the Japanese Society of Nephrology equation. This equation is essentially the Modification of Diet in Renal Disease Study (MDRD) equation multiplied by a Japanese coefficient of 0.741 [34]. This dosing resulted in no significant differences between trough concentrations of patients with ARC and those without ARC. Unfortunately, the nomogram was not detailed within the study.
Conclusion: Patients with ARC are at an increased risk for subtherapeutic trough concentrations of vancomycin; this reduced drug exposure has the potential to cause therapy failure and other negative clinical outcomes [23, 24, 26] . The most promising option may be dosing based on a nomogram for various renal functions [33, 34]. However, nomograms may be unavailable or not feasible in practice. On the basis of the data presented, an increase in loading dose and/or maintenance dose could also be considered to allow rapid and continuous achievement of desired vancomycin levels in patients with ARC. Loading doses of 2 g may be most effective at achieving target trough concentrations quickly [30]. Additionally, maintenance doses of 45 mg/kg/day would be a more realistic starting dose for patients with ARC [32]. Lastly, it is necessary that patients with ARC receive more frequent TDM with subsequent dose adjustment. Development and validation of a CrCl-based dosing nomogram is a promising step forward for patients with extremes of renal function requiring vancomycin therapy [33, 34]. Further studies administering increased doses or frequencies are needed to determine safety and efficacy.
3.5 Oxazolidinones
3.5.1 Linezolid
Linezolid is an oxazolidinone antimicrobial agent that exhibits concentration-dependent killing with time dependence, with the ideal monitoring parameter of AUC:MIC [22]. It is used primarily in the treatment of severe Gram-positive infections [35]. Linezolid is partially eliminated by the kidneys with about 30% of the unchanged drug excreted in the urine [5]. Currently, there is no clear definition for linezolid’s target parameters, an AUC24 h:MIC > 119 mg/L/h has been proposed [36, 37], an alternative target trough concentration ≥ MIC has been proposed [38].
A prospective observational study has addressed the impact of ARC on linezolid plasma concentrations and demonstrated the benefit of continuous infusion in this setting [35]. Barrasa et al. reported that, with increasing renal function, linezolid clearance is increased, which results in reduced plasma concentrations. Patients with ARC have a particularly low probability of target attainment; when receiving a standard dose of 600 mg every 12 h, no patients with ARC were able to achieve PK/PD targets [35]. However, 70% of patients with ARC who received linezolid as a continuous infusion of 50 mg/h, reached desired targets [35]. The authors also used Monte-Carlo simulation to determine optimal dosing regimens. They report the rate of target attainment could further be increased to 93% if a continuous infusion of 75 mg/h is used [35]. This study was limited by a lack of correlation with either dosing strategy with clinical outcomes.
Conclusion: Enhanced renal clearance results in lower plasma concentrations of linezolid, which has the potential to lead to therapeutic failure and subsequent negative clinical outcomes. Continuous infusion may allow for increased drug exposure in patients with ARC, which would allow for an increased probability of achieving and maintaining desired targets [35]. A dosing strategy including continuous infusion of 50–75 mg/h may be beneficial for target attainment in patients exhibiting ARC [35]. Further studies regarding the benefit of continuous infusion of linezolid on clinical outcomes of patients with ARC are needed.
3.6 β-lactams/β-lactamase Inhibitors Combination Antimicrobials
3.6.1 Piperacillin/Tazobactam
Piperacillin is a penicillin that belongs to the β-lactam class of antimicrobials. It is often administered in conjunction with tazobactam, a β-lactamase inhibitor. It has a broad spectrum of activity and, like other β-lactams, exhibits time-dependent bactericidal activity. Both piperacillin and tazobactam are eliminated by the kidneys, about 68% and 80%, respectively [5]. In the case of critically ill patients, clinicians target a minimum of ≥ 50% fT > MIC, and the preferred target is ≥ 100% fT > MIC. In some cases, experts prefer to target ≥ 100% fT > 4 times the MIC [6,7,8].
Three prospective observational studies have discussed the effects of ARC on PK/PD target attainment of piperacillin/tazobactam therapy [13, 39, 40]. Wu et al. (2019) demonstrated that patients with ARC were less likely to achieve targets of 50% fT > MIC and 100% fT > MIC. It has been suggested that critically ill patients should have treatment targets above these, specifically %T > 4× MIC [39]. This was examined by Carrie et al. (2018), where the authors found that, when targeting concentrations of > 4× MIC, CrCl > 170 ml/min was statistically associated with underdosing, adding that patients with therapeutic failure had significantly higher CrCl. The authors concluded that TDM is required to ensure adequate drug exposure in patients with ARC [40]. However, in this study, multiple β lactams were included as well as treatments consisting of multiple other antimicrobial agents [40]. Huttner et al. (2015) found that, with the administration of various β-lactams, including piperacillin/tazobactam at 4 g/0.5 g three times daily, patients with ARC were 3.3× more likely to have undetectable trough levels. Again, this finding was generalized to various β-lactams but suggests that this dose of piperacillin/tazobactam is likely not sufficient for patients who exhibit ARC [13].
A dose of 4 g/0.5 g every 8 h delivered by 3-min bolus infusions, was also unlikely to allow patients with ARC to attain PK/PD targets in a prospective observational study by Andersen et al. (2018). The authors suggested that increasing the frequency of administration to 4 g/0.5 g every 6 h or administering prolonged infusion, either over 3 h or continuously, would be more effective [41]. However, only four patients with ARC were included in this study [41]. They also concluded that administering 4 g/0.5 g every 6 h would provide 100% fT > MIC as long as MIC was 2.0 mg/L, so this recommendation would likely remain insufficient in the context of empirical dosing for high-MIC pathogens [41]. Every 6-h dosing was addressed in a prospective cohort study by Weber et al. (2019). It was reported that a dose of 4 g/0.5 g every 6 h was insufficient to attain targets of 50% and 100% fT > MIC in all patients, regardless of ARC, though increased CrCl was associated with lower trough levels [42]. This study was composed of a small number of patients with hematological malignancy, and findings cannot be generalized to all critical care settings but may suggest that even infusions of 4 g/0.5 g every 6 h may not be sufficient [42]. A cross-sectional study by Akers et al. (2014) showed that patients with high ARC scores had increased piperacillin/tazobactam clearance as well as reduced AUC, compared with the low-ARC-score group. They utilized PK simulation data to suggest that continuous infusion of 12 g/1.5 g per day or intermittent infusions of 4 g/0.5 g –6 g/0.75 g every 4 h or 6 g/0.75 g–8 g/1 g every 6 h would allow for target attainment above MIC of 16 mg/L [43]. This study included only 13 patients, and patients were only classified on the basis of ARC scores. CrCl was not used for comparison of groups [43]. Lastly, when patients received a 4 g/0.5 g loading dose, followed by 4 g/0.5 g every 6 h administered by 3-h infusion, half of the patients did not achieve the target (>16 mg/L); 80% of these patients had ARC [44]. This study was limited by a small sample size but suggests that a dosing frequency of every 6 h and prolonged infusion may not be sufficient to achieve desired targets for patients with ARC, especially those who are infected with high-MIC pathogens [44].
Three prospective observational studies have reported the effects of continuous infusion on piperacillin/tazobactam therapy in patients with ARC [19, 45, 46]. Carrie et al. (2018) administered 4 g/0.5 g loading and 16 g/2 g per day maintenance doses. With this dose, the rate of underexposure (defined as at least 1/3 concentration samples being under 16 mg/L) was higher in patients with ARC; however, the underexposure rate for the overall sample was only 19% [45]. The authors utilized simulation to determine that a continuous infusion of 20 g/2.5 g per day would allow for the highest probability of target attainment without excessive dosing (resulting in concentrations > 150 mg/L) [45]. Another study performed by Carrie et al. in 2019 administered the suggested increased dose to patients with hospital-acquired pneumonia/ventilator acquired pneumonia (HAP/VAP) and ARC [19]. They reported that, when maintenance doses were increased from 16 g/2 g per day (control group) to 20 g/2.5 g per day (treatment group), therapeutic failure was reduced by 13% [19]. Lastly, Dhaese et al. (2018) utilized Monte-Carlo simulation to determine that high-dose piperacillin/tazobactam, 4 g/0.5 g loading dose and 24 g/3 g per day as continuous infusion, would not allow patients with CrCl > 90 ml/min to reach targets of 100% fT > MIC (16 mg/L). The authors raise the question of whether additional agents should be added or a different therapy employed altogether [46]. Interestingly, a nested cohort substudy of the BLINGII trial showed no difference in clinical outcomes of patients with ARC when either continuous infusion or intermittent infusion was used [47]. However, this was a generalized finding for multiple β-lactams and not piperacillin/tazobactam specifically.
Conclusion: ARC results in lower levels of piperacillin/tazobactam, which has the potential to cause underexposure and lead to negative clinical outcomes. TDM, if available, should be considered for these patients to ensure adequate exposure to medication. Data for continuous infusion are promising. Perhaps continuous infusion of 16 g/2 g to 20 g/2.5 g per day following a 4 g/0.5 g loading dose would allow increased likelihood of target attainment for patients with ARC [45]. Further studies are required to determine efficacy and safety of such dose changes in patients with ARC. Additionally, larger studies are needed to determine impact on clinically significant outcomes.
3.6.2 Ceftolozane/Tazobactam
Ceftolozane/tazobactam is a cephalosporin/β-lactamase inhibitor combination product often used to treat Gram-negative infections resistant to other drugs. Common recommended dosing for treatment of infections ranges from 1.5 to 3 g every 8 h. Ceftolozane and tazobactam are renally eliminated as > 95% and > 80% unchanged drug, respectively [5].
Two prospective observational studies investigated the appropriateness of ceftolozane/tazobactam 3 g every 8 h dosing in patients with ARC [48, 49]. Nicolau et al. (2021) determined that 11/14 critically ill patients enrolled in the study demonstrated ARC (CrCl \(\ge\) 130mL/min). Eighty-two percent of patients with ARC demonstrated ceftolozane fT > MIC 4 μg/mL for up to 6 h after the dose was administered, and 64% of patients with ARC were able to demonstrate this for up to 8 h [48]. Sixty-four percent of patients with ARC demonstrated tazobactam fT > 1 μg/mL (threshold) for up to 4 h post-administration [48]. The authors concluded that adequate target levels were maintained for the 8-h interval between doses for ceftolozane/tazobactam. It should be noted that the generalizability of this study is reduced owing to its sample size (n = 14). Shorr et al. (2021) conducted a larger study using the patients enrolled in the phase-3 ASPECT-NP trial to investigate the same dose. Monte-Carlo simulations were developed on the basis of patients with hospital-acquired bacterial pneumonia (HABP)/ventilator-acquired bacterial pneumonia (VABP) with varying renal functions. ARC was defined as CrCl \(\ge\) 130 mL/min [49]. Over 99% of simulated patients achieved the ceftolozane target of 50% fT > MIC of 4 μg/mL in plasma in all renal function groups including ARC [49]. Eighty percent of patients achieved the tazobactam target of 35% fT > Ct of 1 μg/mL across all renal function groups including ARC [49]. Although a high PTA was shown for tazobactam in patients with ARC, PTA did trend down as ARC increased across groups. No statistical difference was shown in 28-day all-cause mortality between non-ARC and ARC groups treated with ceftolozane/tazobactam in intention-to-treat [0.2 (95% CI, −9.6 to 10.6)] and microbiologic-intention-to-treat groups [−1.4 (95% CI,−11.6 to 9.4)] [49]. The authors concluded that ceftolozane/tazobactam 3 g every 8 h is an appropriate dose for patients with ARC on the basis of these findings.
Conclusion: Doses of ceftolozane/tazobactam in critically ill adults of 3 g every 8 h have been shown to achieve high probability of target attainment in patients with ARC [48, 49]. Few clinical outcomes such as all-cause mortality have been shown to exhibit no difference in patients with or without ARC treated with ceftolozane and tazobactam [49]. Ceftolozane/tazobactam 3 g every 8 h is likely an appropriate dose for critically ill adults with ARC; however, further analysis comparing target concentration attainment with clinically relevant results such as infection resolution is needed.
3.7 Low-Molecular-Weight Heparins
3.7.1 Enoxaparin
Enoxaparin is a commonly used low-molecular-weight heparin; up to 40% of the drug is excreted by the kidneys [5]. For venous thromboembolism prophylaxis in moderate-to-high-risk patients, a peak factor Xa level of 0.2–0.4 units/mL or trough level of 0.1–0.2 units/mL is usually targeted [50]. A prospective observational study provides information about the effects of ARC on prophylactic enoxaparin dosing [51]. Patients with ARC exhibited target anti-factor Xa levels at hour 4, but these levels dropped significantly by hours 12 and 24 [51]. This suggests that the duration of activity of enoxaparin may be shortened by enhanced renal clearance in patients with ARC, possibly rendering the need for dose adjustment [51]. However, the significance of anti-factor Xa monitoring at 12 and 24 h is not fully known. This study is also limited by a small sample size, and data regarding the development of clots were not collected.
Conclusion: The duration of action of enoxaparin may be shortened in patients with ARC, which could potentially lead to an increased risk for clot formation. An increased frequency of dosing to 40 mg twice daily should be considered for clot prophylaxis in these patients. Further studies exploring a shortened dosing interval and the impacts on clot formation in critically ill patients with ARC are needed.
3.8 Anti-epileptics
3.8.1 Levetiracetam
Levetiracetam is an AED used to treat multiple seizure types as well as seizure prophylaxis in certain care settings. It is eliminated renally with 66% of the unchanged drug excreted in the urine [5]. Its efficacy in patients with ARC has been studied. Therapeutic drug monitoring of levetiracetam targets a plasma concentration between 12 and 46 mg/mL [52, 53].
A prospective observational study by Ong et al. in 2021 on neurosurgical ICU patients targeting a trough concentration of 6 mg/L showed Monte-Carlo simulations demonstrating PTA > 80% in patients with ARC who were dosed with levetiracetam 1000 mg every 8 h [54]. Three prospective observational studies further discussed levetiracetam administration in patients with ARC [55,56,57]. La et al. reported that a standard dose of 1000 mg twice daily resulted in subtherapeutic concentrations in patients with ARC. Two of the studies also utilized Monte-Carlo simulation to determine an optimized dosing strategy. May et al. determined that three-times-daily dosing is needed to reach desired plasma concentrations of levetiracetam. Three-times-daily dosing was also suggested by Spencer et al. as an alternative, as they reported an increased probability of target attainment when a dose of 500 mg every 8 h was simulated. They also documented similar findings for a dose of 1000 mg twice daily. Again, these studies were limited by small sample size and did not discuss development of seizures in ARC patients specifically. Additionally, dosing suggestions were based on simulation data alone. Two prospective observational studies conducted by Bilbao-Meseguer et al. and Sime et al. also demonstrated levetiracetam dosing in critically ill patients with ARC using doses as high as 6 g/day [58, 59]. Bilbao-Meseguer et al. 2021 performed Monte-Carlo simulations using data from adult ICU patients to demonstrate PTA using various dosing regimens in patients with CrCl ranges (80–240 ml/min). They found 500 mg BID to be inadequate in all critically ill patients with or without ARC and found doses as high as 1500 mg every 12 h to only guarantee target trough concentrations in those with CrCl > 80 ml/min. The study concluded doses as high as 1500–2000 mg every 8 h were required to achieve target trough concentrations in those with ARC [58]. Sime et al. 2021 also developed Monte-Carlo simulations and found patients with ARC had a PTA of 0 for trough concentrations of \(\ge\) 46 mg/L with doses as high as 6 g/day; however, these doses demonstrated a PTA ≤ 80% for a target trough concentration of 6 mg/L. These results should be interpreted with caution owing to the small sample sizes and wide range in target trough concentrations (6–46 mg/L) in both studies [58, 59]. Further safety data on dose regimens this high should be analyzed before implemented.
Conclusion: ARC results in lower plasma levels of levetiracetam, which could lead to therapeutic failure and the increased development of seizures in these patients. It appears that an increase in dose or frequency is needed. Dosing regimens of 500 mg every 8 h or 1000–2000 mg every 12 h can be currently recommended for seizure prophylaxis in patients exhibiting ARC as they may allow for the achievement of therapeutic plasma levels [1, 56, 57]. Additionally, loading doses may be used to further increase drug exposure. Though some studies suggest that further dose increases may be warranted up to 6 g/day [58, 59], further studies attempting to administer increased doses and report seizure occurrence and adverse event profiles in patients with ARC are necessary to determine the safety of efficacy of such doses.
3.9 Other Considerations
It is important to note that ARC is one of multiple pathophysiological changes due to critical illness that need to be taken into account when following drug therapy guidelines in various disease states. Numerous changes include but are not limited to altered plasma protein binding, extracorporeal membrane oxygenation (ECMO), alterations in gastric pH and the rate and extent of absorption of orally administered drugs, and reductions in hepatic blood flow or enzyme activity. These changes can consequently affect the pharmacokinetics of different drugs. Therefore, they should be taken into consideration before adopting unadjusted dosing regimens in critically ill patient settings, putting them at a risk for therapy failure, longer hospitalizations, and increased adverse drug events [60].
3.10 Limitations
This literature review is limited by the inherent drawbacks to nonsystematic reviews. In addition, some of the proposed dosing regimens are derived from pharmacokinetic simulations as opposed to controlled clinical trials. Although we aimed to provide clinicians with a summary of the available evidence to aid in the dosing of key renally eliminated drugs in critical care settings, there is a current lack of a clear consensus of high-quality critically appraised evidence to support the dosing regimens of some of the reported drugs such as linezolid and enoxaparin.
4 Conclusion
In conclusion, our review summarizes the evidence on medications relevant to the care of critically ill adults that may require alternate dosing regimens in patients with ARC, addressing an area of rising concern. ARC has been repeatedly shown to negatively influence the probability of target trough level attainment in many life-saving medications, potentially increasing the risk of therapeutic failure. We have provided a table with recommended doses for ten medications for critically ill adult patients with CrCl > 130 ml/min/1.73 m2 based on multiple studies (Table 1). Further research is required to investigate the correlation between target trough level attainment and clinical outcomes as well as the safety of higher doses such as those reported in our recommendation table.
References
Mahmoud SH, Shen C. Augmented Renal Clearance in Critical Illness: An Important Consideration in Drug Dosing. Pharmaceutics. 2017;9(3). doi:https://doi.org/10.3390/pharmaceutics9030036.
Molina KC, Hall ST, Barletta JF, Mangram AJ, Dzandu JK, Huang V. Utilization of augmented renal clearance in trauma intensive care scoring system to improve vancomycin dosing in trauma patients at risk for augmented renal clearance. Surg Infect. 2020;21(1):43–7. https://doi.org/10.1089/sur.2019.026.
Cojutti PG, Lazzarotto D, Candoni A, Dubbini MV, Zannier ME, Fanin R, et al. Real-time TDM-based optimization of continuous-infusion meropenem for improving treatment outcome of febrile neutropenia in oncohaematological patients: results from a prospective, monocentric, interventional study. J Antimicrob Chemother. 2020;75(10):3029–37. https://doi.org/10.1093/jac/dkaa267.
Hefny F, Stuart A, Kung JY, Mahmoud SH. Prevalence and risk factors of augmented renal clearance: a systematic review and meta-analysis. Pharmaceutics. 2022;14(2). https://doi.org/10.3390/pharmaceutics14020445
Lexicomp. UpToDate, Inc. , Waltham, MA. https://online.lexi.com/lco/action/login.
Al-Shaer MH, Alghamdi WA, Graham E, Peloquin CA. Meropenem, cefepime, and piperacillin protein binding in patient samples. Ther Drug Monit. 2020;42(1):129–32. https://doi.org/10.1097/FTD.0000000000000675.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–83. https://doi.org/10.1093/cid/ciu027.
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit Care. 2019;23(1):104. https://doi.org/10.1186/s13054-019-2378-9.
Kitzes-Cohen R, Farin D, Piva G, De Myttenaere-Bursztein SA. Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents. 2002;19(2):105–10.
Ehmann L, Zoller M, Minichmayr IK, Scharf C, Maier B, Schmitt MV, et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care. 2017;21:1–14. https://doi.org/10.1186/s13054-017-1829-4.
Tamatsukuri T, Ohbayashi M, Kohyama N, Kobayashi Y, Yamamoto T, Fukuda K, et al. The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. J Infect Chemotherap. 2018;24(10):834–40. https://doi.org/10.1016/j.jiac.2018.07.007.
Bricheux A, Lenggenhager L, Hughes S, Karmime A, Lescuyer P, Huttner A. Therapeutic drug monitoring of imipenem and the incidence of toxicity and failure in hospitalized patients: a retrospective cohort study. Clin Microbiol Infect 2019;25(3):383.e1-e4. doi:https://doi.org/10.1016/j.cmi.2018.11.020.
Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–92. https://doi.org/10.1016/j.ijantimicag.2014.12.017.
Patel M, Bellanti F, Daryani NM, Noormohamed N, Hilbert DW, Young K, et al. Population pharmacokinetic/pharmacodynamic assessment of imipenem/cilastatin/relebactam in patients with hospital-acquired/ventilator-associated bacterial pneumonia. Clin Transl Sci. 2021. https://doi.org/10.1111/cts.13158.
Jamal JA, Mat-Nor MB, Mohamad-Nor FS, Udy AA, Wallis SC, Lipman J, et al. Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;45(1):41–5. https://doi.org/10.1016/j.ijantimicag.2014.09.009.
Ollivier J, Carrie C, d'Houdain N, Djabarouti S, Petit L, Xuereb F et al. Are standard dosing regimens of ceftriaxone adapted for critically ill patients with augmented creatinine clearance? Antimicrob Agents Chemotherap. 2019;63(3). doi: https://doi.org/10.1128/AAC.02134-18
Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother. 2001;47(4):421–9.
Wong G, Briscoe S, McWhinney B, Ally M, Ungerer J, Lipman J, et al. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother. 2018;73(11):3087–94. https://doi.org/10.1093/jac/dky314.
Carrie C, Chadefaux G, Sauvage N, de Courson H, Petit L, Nouette-Gaulain K, et al. Increased beta-lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: a before and after study. Crit Care (London, England). 2019;23(1):379. https://doi.org/10.1186/s13054-019-2621-4.
Carrie C, Delzor F, Roure S, Dubuisson V, Petit L, Molimard M et al. Population pharmacokinetic study of the suitability of standard dosing regimens of amikacin in critically ill patients with open-abdomen and negative-pressure wound therapy. Antimicrob Agents Chemother. 2020;64(4). doi: https://doi.org/10.1128/AAC.02098-19
Arechiga-Alvarado NA, Medellin-Garibay SE, Milan-Segovia RDC, Ortiz-Alvarez A, Magana-Aquino M, Romano-Moreno S. Population Pharmacokinetics of amikacin administered once daily in patients with different renal functions. Antimicrob Agents Chemother. 2020;64(5). https://doi.org/10.1128/AAC.02178-19
Bugs & Drugs. ©1998-2020 Alberta Health Service. https://www.bugsanddrugs.org/.
Chu Y, Luo Y, Ji S, Jiang M, Zhou B. Population pharmacokinetics of vancomycin in Chinese patients with augmented renal clearance. J Infect Public Health. 2020;13(1):68–74. https://doi.org/10.1016/j.jiph.2019.06.016.
Chen Y, Liu L, Zhu M. Effect of augmented renal clearance on the therapeutic drug monitoring of vancomycin in patients after neurosurgery. J Int Med Res. 2020;48(10):300060520949076. https://doi.org/10.1177/0300060520949076.
He J, Yang ZT, Qian X, Zhao B, Mao EQ, Chen EZ, et al. A higher dose of vancomycin is needed in critically ill patients with augmented renal clearance. Transl Androl Urol. 2020;9(5):2166–71. https://doi.org/10.21037/tau-20-1048.
Hirai K, Ishii H, Shimoshikiryo T, Shimomura T, Tsuji D, Inoue K, et al. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther Drug Monit. 2016;38(6):706–10. https://doi.org/10.1097/FTD.0000000000000346.
Chu Y, Luo Y, Qu L, Zhao C, Jiang M. Application of vancomycin in patients with varying renal function, especially those with augmented renal clearance. Pharm Biol. 2016;54(12):2802–6. https://doi.org/10.1080/13880209.2016.1183684.
Minkute R, Briedis V, Steponaviciute R, Vitkauskiene A, Maciulaitis R. Augmented renal clearance—an evolving risk factor to consider during the treatment with vancomycin. J Clin Pharm Ther. 2013;38(6):462–7. https://doi.org/10.1111/jcpt.12088.
Zhao S, He N, Zhang Y, Wang C, Zhai S, Zhang C. Population pharmacokinetic modeling and dose optimization of vancomycin in Chinese patients with augmented renal clearance. Antibiotics (Basel). 2021;10(10). doi:https://doi.org/10.3390/antibiotics10101238.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–3. https://doi.org/10.1016/j.ijantimicag.2011.12.011.
Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Incremento da depuracao renal em pacientes gravemente enfermos: incidencia, fatores associados e efeitos no tratamento com vancomicina. 2014;26(1):13–20.
Helset E, Nordøy I, Sporsem H, Bakke VD, Bugge JF, Gammelsrud KW, et al. Factors increasing the risk of inappropriate vancomycin therapy in ICU patients: a prospective observational study. Acta Anaesthesiol Scand. 2020;64(9):1295–304. https://doi.org/10.1111/aas.13658.
Baptista JP, Roberts JA, Sousa E, Freitas R, Deveza N, Pimentel J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care (London, England). 2014;18(6):654. https://doi.org/10.1186/s13054-014-0654-2.
Ishii H, Hirai K, Sugiyama K, Nakatani E, Kimura M, Itoh K. Validation of a nomogram for achieving target trough concentration of vancomycin: accuracy in patients with augmented renal function. Ther Drug Monit. 2018;40(6):693–8. https://doi.org/10.1097/FTD.0000000000000562.
Barrasa H, Soraluce A, Uson E, Sainz J, Martin A, Sanchez-Izquierdo JA, et al. Impact of augmented renal clearance on the pharmacokinetics of linezolid: advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int J Infect Dis. 2020;93:329–38. https://doi.org/10.1016/j.ijid.2020.02.044.
Millard J, Pertinez H, Bonnett L, Hodel EM, Dartois V, Johnson JL, et al. Linezolid pharmacokinetics in MDR-TB: a systematic review, meta-analysis and Monte Carlo simulation. J Antimicrob Chemother. 2018;73(7):1755–62. https://doi.org/10.1093/jac/dky096.
Srivastava S, Magombedze G, Koeuth T, Sherman C, Pasipanodya JG, Raj P et al. Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother. 2017;61(8). doi:https://doi.org/10.1128/AAC.00751-17.
Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74(8):839–54. https://doi.org/10.1007/s40265-014-0222-8.
Wu CC, Tai CH, Liao WY, Wang CC, Kuo CH, Lin SW, et al. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: a prospective observational study. Infect Drug Resist. 2019;12:2531–41. https://doi.org/10.2147/IDR.S213183.
Carrie C, Petit L, d’Houdain N, Sauvage N, Cottenceau V, Lafitte M, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51(3):443–9. https://doi.org/10.1016/j.ijantimicag.2017.11.013.
Andersen MG, Thorsted A, Storgaard M, Kristoffersson AN, Friberg LE, Obrink-Hansen K. Population Pharmacokinetics of piperacillin in sepsis patients: should alternative dosing strategies be considered? Antimicrob Agents Chemother. 2018;62(5). doi:https:https://doi.org/10.1128/AAC.02306-17.
Weber N, Jackson K, McWhinney B, Ungerer J, Kennedy G, Lipman J, et al. Evaluation of pharmacokinetic/pharmacodynamic and clinical outcomes with 6-hourly empiric piperacillin-tazobactam dosing in hematological malignancy patients with febrile neutropenia. J Infect Chemother. 2019;25(7):503–8. https://doi.org/10.1016/j.jiac.2019.02.014.
Akers KS, Niece KL, Chung KK, Cannon JW, Cota JM, Murray CK. Modified augmented renal clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J Trauma Acute Care Surg. 2014;77(3 Suppl 2):S163–70. https://doi.org/10.1097/TA.0000000000000191.
Carlier M, Carrette S, Roberts JA, Stove V, Verstraete A, Hoste E, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care (London, England). 2013;17(3):R84. https://doi.org/10.1186/cc12705.
Carrie C, Legeron R, Petit L, Ollivier J, Cottenceau V, d’Houdain N, et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. J Crit Care. 2018;48:66–71. https://doi.org/10.1016/j.jcrc.2018.08.026.
Dhaese SAM, Roberts JA, Carlier M, Verstraete AG, Stove V, De Waele JJ. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int J Antimicrob Agents. 2018;51(4):594–600. https://doi.org/10.1016/j.ijantimicag.2017.12.015.
Udy AA, Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving beta-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017;49(5):624–30. https://doi.org/10.1016/j.ijantimicag.2016.12.022.
Nicolau DP, De Waele J, Kuti JL, Caro L, Larson KB, Yu B, et al. Pharmacokinetics and pharmacodynamics of ceftolozane/tazobactam in critically ill patients with augmented renal clearance. Int J Antimicrob Agents. 2021;57(4): 106299. https://doi.org/10.1016/j.ijantimicag.2021.106299.
Shorr AF, Bruno CJ, Zhang Z, Jensen E, Gao W, Feng HP, et al. Ceftolozane/tazobactam probability of target attainment and outcomes in participants with augmented renal clearance from the randomized phase 3 ASPECT-NP trial. Crit Care. 2021;25(1):354. https://doi.org/10.1186/s13054-021-03773-5.
Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: a Western Trauma Association critical decisions algorithm. J Trauma Acute Care Surg. 2020;89(5):971–81. https://doi.org/10.1097/TA.0000000000002830.
Abdel El Naeem HEM, Abdelhamid MHE, Atteya DAM. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt J Anaesth. 2017;33(1):113-7. doi:https://doi.org/10.1016/j.egja.2016.11.001.
Hernandez-Mitre MP, Medellin-Garibay SE, Rodriguez-Leyva I, Rodriguez-Pinal CJ, Zarazua S, Jung-Cook HH, et al. Population pharmacokinetics and dosing recommendations of levetiracetam in adult and elderly patients with epilepsy. J Pharm Sci. 2020;109(6):2070–8. https://doi.org/10.1016/j.xphs.2020.02.018.
Jarvie D, Mahmoud SH. Therapeutic drug monitoring of levetiracetam in select populations. J Pharm Pharm Sci. 2018;21(1s):149s-s176. https://doi.org/10.18433/jpps30081.
Ong CLJ, Goh PSJ, Teo MM, Lim TP, Goh KKK, Ang XY, et al. Pharmacokinetics of levetiracetam in neurosurgical ICU patients. J Crit Care. 2021;64:255–61. https://doi.org/10.1016/j.jcrc.2021.04.013.
La MK, Morbitzer KA, Cook A, Hatton-Kolpek J, Jordan JD, Nelson NR, et al. Levetiracetam pharmacokinetics and dose optimization for seizure prophylaxis in TBI. Neurocrit Care. 2018;29(1 Supplement):S106. https://doi.org/10.1007/s12028-018-0606-9.
May C, Arora S, Parli S, Bastin MT, Cook A. Levetiracetam pharmacokinetics in subarachnoid hemorrhage patients with augmented renal clearance: a Monte Carlo simulation. Pharmacotherapy. 2014;34(10):e261–2. https://doi.org/10.1002/phar.1497.
Spencer DD, Jacobi J, Juenke JM, Fleck JD, Kays MB. Steady-state pharmacokinetics of intravenous levetiracetam in neurocritical care patients. Pharmacotherapy. 2011;31(10):934–41. https://doi.org/10.1592/phco.31.10.934.
Bilbao-Meseguer I, Barrasa H, Asin-Prieto E, Alarcia-Lacalle A, Rodriguez-Gascon A, Maynar J et al. Population pharmacokinetics of levetiracetam and dosing evaluation in critically ill patients with normal or augmented renal function. Pharmaceutics. 2021;13(10). doi:https://doi.org/10.3390/pharmaceutics13101690.
Sime FB, Roberts JA, Jeffree RL, Pandey S, Adiraju S, Livermore A, et al. Population pharmacokinetics of levetiracetam in patients with traumatic brain injury and subarachnoid hemorrhage exhibiting augmented renal clearance. Clin Pharmacokinet. 2021;60(5):655–64. https://doi.org/10.1007/s40262-020-00979-8.
Forsberg J, Bedard E, Mahmoud SH. Bioavailability of orally administered drugs in critically ill patients. J Pharm Pract. 2022:8971900221100205. doi:https://doi.org/10.1177/08971900221100205.
Al-Hwiesh A, Alhwiesh A, Abdul-Rahman IS, Al-Harbi A, Mousa D, Skiker S, et al. Meropenem at recommended dose is a potential risk for seizure in hemodialysis patient. Saudi J Kidney Dis Transpl. 2020;31(6):1427–31. https://doi.org/10.4103/1319-2442.308364.
Izumisawa T, Kaneko T, Soma M, Imai M, Wakui N, Hasegawa H, et al. Augmented renal clearance of vancomycin in hematologic malignancy patients. Biol Pharm Bull. 2019;42(12):2089–94. https://doi.org/10.1248/bpb.b19-00652.
Villanueva RD, Talledo O, Neely S, White B, Celii A, Cross A, et al. Vancomycin dosing in critically ill trauma patients: the VANCTIC study. J Trauma Acute Care Surg. 2019;87(5):1164–71. https://doi.org/10.1097/TA.0000000000002492.
Vermis K, Steel E, Vandenbroucke J. Prevalence of augmented renal clearance in haematological patients and the impact on vancomycin dosing. J Oncol Pharm Pract. 2014;20(3 SUPPL. 1):7. https://doi.org/10.1177/1078155214523700.
Chu Y, Luo Y, Jiang M, Zhou B. Application of vancomycin in patients with augmented renal clearance. Eur J Hosp Pharm. 2020;27(5):276–9. https://doi.org/10.1136/ejhpharm-2018-001781.
Mikami R, Imai S, Hayakawa M, Sugawara M, Takekuma Y. Clinical applicability of urinary creatinine clearance for determining the initial dose of vancomycin in critically ill patients. J Infect Chemother. 2022;28(2):199–205. https://doi.org/10.1016/j.jiac.2021.10.008.
Weigel J, Egal M, Lima A, Koch B, Hunfeld NG, Van Gelder T, et al. Vancomycin is underdosed in patients with high estimated glomerular filtration rate. Intensive Care Med. 2014;40(1 SUPPL. 1):S252. https://doi.org/10.1007/s00134-013-3451-5.
Sridharan K, Pasha SAA, Qader AM, Hasan HM, ElSeirafi MM. Drug utilization in critically ill adults with augmented renal clearance compared to normal renal clearance: implications for use of antimicrobials with predominant renal excretion. Curr Clin Pharmacol. 2020. https://doi.org/10.2174/1574884715666200810095225.
Ramos A, Dogliotti A, Pires N, Lovesio C, Latasa D, Perezlindo M et al. Enoxaparin pharmacokinetics in patients with augmented renal clearance, preliminary results of a single center study. Crit Care. 2018;22(Supplement 1). https://doi.org/10.1186/s13054-018-1973-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There is no funding associated with this work.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Data availability
All data generated during this review are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Author contributions
Conceptualization and design, S.H.M.; database search, J.Y.K.; study screening and selection, S.H.M., A.S., S.S.; data extraction and summarization, F.H., C.M., S.S., A.S.; resolution of conflict in study selection and interpretation, S.H.M.; drafting the first version of the manuscript; C.M., S.S.; revision and approval of the final manuscript, all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hefny, F., Sambhi, S., Morris, C. et al. Drug Dosing in Critically Ill Adult Patients with Augmented Renal Clearance. Eur J Drug Metab Pharmacokinet 47, 607–620 (2022). https://doi.org/10.1007/s13318-022-00779-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00779-4