Abstract
Background and Objective
MAMA decoction (MD) is an antimalarial product prepared from the leaves of Mangifera indica L. (Anacardiaceae), Alstonia boonei De Wild (Apocynaceae), Morinda lucida Benth (Rubiaceae) and Azadirachta indica A. Juss (Meliaceae). A previous report showed that MD enhanced the efficacy of amodiaquine (AQ) in malaria-infected mice, thus suggesting a herb–drug interaction. The present study hence evaluated the effect of MD on the disposition of AQ in mice with a view to investigating a possible pharmacokinetic interaction.
Methods
In a 3-period study design, three groups of mice (n = 72) were administered oral doses of AQ (10 mg/kg/day) alone, concurrently with MD (120 mg/kg/day), and in the 3rd period, mice were given AQ after a 3-day pre-treatment with MD. Blood samples were collected between 0 and 96 h for quantification of AQ and its major metabolite, desethylamodiaquine, by a validated high-performance liquid chromatography method.
Results
Maximum concentrations of AQ increased by 12% with the concurrent dosing of MD and by 85% in the group of mice pre-treated with MD. The exposure and half-life of desethylamodiaquine increased by approximately 11% and 21%, respectively, with concurrent administration. Corresponding increases of approximately 20% and 33% of desethylamodiaquine were also observed in mice pre-treated with MD.
Conclusion
MD influenced the pharmacokinetics of AQ and desethylamodiaquine, its major metabolite. The increase in the half-life and systemic exposure of AQ following its co-administration with MD may provide a basis for the enhanced pharmacological effect of the combination in an earlier study in Plasmodium-infected mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The study investigated the pharmacokinetic interaction between an antimalarial herbal product, MAMA decoction, and amodiaquine. |
MAMA decoction increased the half-life and systemic exposure of amodiaquine and that of its main metabolite, desethylamodiaquine. |
This may explain the enhanced effectiveness of the combined administration of amodiaquine with MAMA decoction in malaria-infected mice, in an earlier study. |
1 Introduction
The incidence of concurrent administration of herbal preparations with orthodox drugs is common globally [1]. In many parts of Africa, the use of herbal remedies as a first-line therapy is prevalent as well as self-medication with orthodox drugs for several ailments [2,3,4]. Malaria is one of such endemic ailments confronting the people of Africa where approximately 92% of global cases occur, accounting for 93% of mortality worldwide in 2018 [5].
Herbal remedies for malaria are popular in Africa, largely due to ease of access, cultural acceptability and relative affordability. Several herbal antimalarial preparations have been documented [6,7,8]. The concomitant intake of herbal preparations with orthodox drugs carries attendant benefits or risks. Some herbal medicines have been known to alter drug disposition, as shown with significant changes in parameters such as the area under the time-plasma-concentration curve (AUC), peak concentration (Cmax) and elimination half-life (T½) [9,10,11].
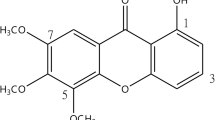
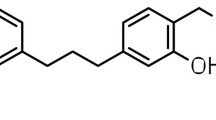
MAMA decoction (MD), a herbal antimalarial product, comprising the leaves of Morinda lucida, Azadirachta indica, Alstonia boonei and Mangifera indica, has been reported to possess significant chemosuppressive, prophylactic and curative antimalarial activities [12, 13]. An earlier study, however, revealed that the combination of MD with amodiaquine (AQ) resulted in an increased malaria parasite clearance and longer survival of experimental rodents [14]. Currently, AQ is a long-acting partner drug in the WHO-recommended artemisinin combination therapy. The antimalarial activity of AQ has been largely attributed to its major metabolite in humans, desethylamodiaquine, which has a higher concentration–time profile and longer half-life than the parent drug [15, 16].
Further to the reported beneficial pharmacodynamic interaction between MD and AQ, it is important to understand the possible underlying pharmacokinetic interaction. Previous studies have shown that herbal drugs have demonstrated some effects on the pharmacokinetics of orthodox drugs in rodents. For example, the tablet of Gingko biloba leaf significantly inhibited the metabolism of amlodipine while Azadirachta indica and Niprisan®, an herbal anti-sickling drug, also altered the pharmacokinetics of chloroquine [17,18,19]. In addition, the concurrent administration of the herbal supplement, Moringa oleifera, resulted in an increase in the AUC of AQ but caused a reduction in the Cmax of desethylamodiaquine [20].
Consequently, the present study describes the pharmacokinetics of orally administered AQ and the effects of concurrent administration of, as well as pre-treatment with, MD on the pharmacokinetics of AQ in mice.
2 Materials and Methods
2.1 Chemicals
AQ hydrochloride dihydrate, quinidine, HPLC-grade methanol and diethyl ether were purchased from Sigma (St. Louis, MO, USA). Triethylamine and ortho-phosphoric acid were from BDH Chemicals Ltd. (Dorset, Poole, UK), while desethylamodiaquine was obtained from TLC Pharmaceutical Standards (Newmarket, Ontario, Canada).
2.2 Preparation of MD
The collection of the component plants and preparation of MD were carried out as previously described by Adepiti et al. [12]. In brief, fresh leaves of Mangifera indica L. (Anacardiaceae), Alstonia boonei De Wild (Apocynaceae), Morinda lucida Benth (Rubiaceae) and Azadirachta indica A. Juss (Meliaceae) were collected from Obafemi Awolowo University Campus, Ile-Ife, Nigeria in March 2012. They were authenticated and voucher specimens deposited with reference numbers: IFE 16537, 16534, 16535 and 16536, respectively. The leaves were oven-dried (ADVANTEC FP-612, Tokyo, Japan) at 40 °C and separately powdered using a laboratory mill (Griffin, London, UK). The mixture (ratio 1:1:1:1) was extracted using the decoction method of boiling in distilled water (powder-water ratio, 1:10) for 1 h.
2.3 Experimental Animals
Swiss albino mice of both sexes (12–16 weeks in age) were obtained from the Central Animal House, University of Ibadan, Nigeria. The animals were housed at 24–26 °C under a 12 h light/dark cycle for 2 weeks with unrestricted access to feed and water in accordance with the “Guide for the care and use of laboratory animals” [21]. Approval for the study was obtained from the Health Research Ethics Committee of the Obafemi Awolowo University, Ile-Ife, Nigeria (IPHOAU/12/90).
2.4 Pilot Study
Three groups of healthy (uninfected) mice (3 mice per group) were each given combinations of AQ (10 mg/kg body weight) plus MD (240 mg/kg body weight), and AQ (10 mg/kg body weight) plus MD (120 mg/kg body weight), respectively, as single oral doses while the control group was given distilled water. In addition, the third group of uninfected mice was pre-treated over 3 days with oral doses of MD (120 mg/kg body weight/day) with a single dose of AQ (10 mg/kg) co-administered with the last dose of MD (on day 3). The animals were observed for signs of morbidity and mortality for 14 days.
2.5 Herb–Drug Interaction Study
The effect of MD on the pharmacokinetics of AQ in mice was studied in three phases. Each phase utilized a total of 72 animals (body weight, 21–36 g) assigned to 12 groups of 6 animals to which were administered AQ and/or MD orally, after an overnight fast, with feeding and access to water resuming 2 h after drug administration.
In the first phase, single oral doses of AQ (10 mg/kg) prepared in distilled water at a final volume of 200 µL were administered. Thereafter, each animal was anesthetized with ether for 3–5 min and blood (0.6–1.0 mL) drawn by cardiac puncture at 0, 0.25, 0.5, 1, 2, 4, 6, 12, 24, 48, 72 and 96 h from all animals (n = 6) for each time point. All blood samples were immediately transferred into heparinized tubes and centrifuged at 3000 × g for 10 min for plasma collection.
For the second phase, all study animals were administered single, concurrent, oral doses of AQ (10 mg/kg) and MD (120 mg/kg). Blood was collected and processed as earlier described for the first phase. In the final phase, all study mice were pre-treated orally with MD (120 mg/kg), once daily, for 3 days. This was followed by the administration of single oral doses of AQ (10 mg/kg) along with the last dose of MD on day 3. Blood samples were similarly collected and processed as described for the earlier phases. All biological samples were stored at − 20 °C until analysis.
2.6 Plasma Analysis
The concentrations of AQ and its major metabolite, desethylamodiaquine, in rodent plasma were determined as earlier described, with some modifications [22]. Liquid chromatography analysis was performed on an Agilent 1100 series system (Palo Alto, CA, USA). Separation of study analytes was achieved at 27 °C with a C18 column (5 µm, 150 × 4.6 mm i.d., Sigma-Aldrich (Supelco), St Louis, MO, USA). The mobile phase, pumped through the column at a flow rate of 1.5 mL/min, comprised triethylamine (2%) in distilled water and methanol (81:19), and a final pH of 2.2. Column effluent was monitored at 340 nm.
Plasma (100 µL), extracted with 3 replicates of 1 mL of diethyl ether with vortex-mixing for 2 min, was centrifuged for 10 min at 3000 × g. The organic phases were aspirated, bulked, and dried under a gentle stream of nitrogen gas at 27 °C. Extracted analytes were reconstituted in 100 µL of liquid chromatography mobile phase, and spiked with 10 µL of quinidine (5 ng/µL), the internal standard. Thereafter, 30 µL was injected for analysis.
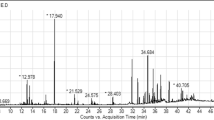
Linear calibration curves were generated by spiking drug-free mouse plasma in the range of 10–150 ng/mL for both analytes. The assay was validated following standard protocols [23]. Assay imprecision was studied at 20, 80 and 150 ng/mL concentrations and expressed as coefficients of variation (%). Retention times of the internal standard, desethylamodiaquine and AQ were 3.1, 5.4 and 6.2 min, respectively (Fig. 1).
The assay limits of detection and quantification were 3.43 ng/mL and 10.39 ng/mL for AQ while corresponding values for desethylamodiaquine were 4.23 ng/mL and 12.82 ng/mL. Intra-assay imprecision varied between 1.72 and 8.02% for AQ, with inter-assay imprecision assuming values between 1.57 and 8.11%. For desethylamodiaquine, intra-assay imprecision varied between 3.41 and 6.50%, while inter-assay imprecision ranged between 6.24 and 10.73%. The relative recovery values (± standard deviation) at 20, 80 and 150 ng/mL were 81–90% for AQ, and 73–88% for desethylamodiaquine.
2.7 Pharmacokinetic Data Analysis
The sampling period of 0–96 h in the present study was based on an earlier human study [24]. Concentration–time data across the three phases of the study were fitted by a non-compartmental model, using WinNonlin (version 5.3, Pharsight Corp, Mountain View, CA, USA), for the determination of pharmacokinetic end points. Mean ratios alongside their 90% confidence intervals were computed for the maximum concentrations of AQ and desethylamodiaquine to assess the significance of MD intervention. An absence of clinical significance was inferred when the 90% CI fell within the 80–125% range [25].
3 Results
A pilot study, carried out prior to the main study, showed that the solutions of AQ and MD, at the doses used in this study, either singly or in combination, were well tolerated by the animals. The weights and temperature values of the test mice were stable throughout the period of observation and no adverse effects were observed during and after drug administration. The pilot evaluation, however, observed reduced physical activity in study animals administered the combination of AQ and the decoction at 240 mg/kg body weight, thus informing the study dose implemented.
The pharmacokinetic endpoints for AQ and its metabolite, desethylamodiaquine, are presented in Tables 1 and 2. Plasma concentration–time profiles of AQ and desethylamodiaquine in mice following a single oral administration of AQ alone, its concurrent administration with MD, and dosing after pre-treatment of mice with MD are presented in Figs. 2 and 3.
Concentration-time profile of amodiaquine following oral administration of a single dose of amodiaquine (AQ) alone (10 mg/kg), concurrent single oral administration of AQ (10 mg/kg) and MAMA decoction (120 mg/kg/day), and after a single oral administration of amodiaquine (10 mg/kg) to mice pre-treated with MAMA decoction (120 mg/kg/day) for three days
Concentration-time profile of desethylamodiaquine following oral administration of a single dose of amodiaquine (10 mg/kg), concurrent single oral administration of AQ (10 mg/kg) and MAMA decoction (120 mg/kg/day), and after single oral administration of amodiaquine (10 mg/kg) to mice pre-treated with MAMA decoction (120 mg/kg/day) for 3 days
Systemic concentration of AQ in mice reached a peak in 0.5 h with and without MD interventions. The maximum systemic concentration, Cmax, increased by 12.17% with concurrent administration of MD, whereas pre-treatment of mice with the decoction resulted in a much higher increase (84.70%) in the Cmax (Table 1). MD interventions led to longer mean residence time of AQ in mice with increases of 195.15% and 340.78% following concurrent administration and pre-treatment, respectively (Table 1). The overall systemic exposure of AQ, represented by the AUC, increased by 51.69% when MD was concurrently administered and by 295.46% when the mice were pre-treated with the decoction (Table 1).
Comparable maximum systemic concentrations of the metabolite, desethylamodiaquine, were derived following the administration of AQ alone and after pre-treatment with MD. The Cmax of desethylamodiaquine increased by 40.71% and its systemic exposure was highest (33.54% ↑) in the mice given the herb–drug combination (Table 2). The Cmax of AQ was significantly altered by the interventions of MD while significant changes in the Cmax of desethylamodiaquine were only observed during concurrent administration of MD (Table 3).
4 Discussion
This study demonstrated that the pharmacokinetics of AQ were altered by MD in mice. The herb–drug combination produced an increase in the half-life of AQ. Similar changes were also observed in the disposition of desethylamodiaquine, where the half-life increased by > 10%, and systemic exposure by > 19%. Derived pharmacokinetic data also suggest that MD increased the absorption rate of AQ as reflected by a significant rise of approximately 12% in the Cmax, despite a consistent Tmax of 0.5 h after MD intervention.
In humans, AQ undergoes rapid and extensive biotransformation to desethylamodiaquine and a minimal level of AQ synergizes the efficacy of desethylamodiaquine especially in the first few days after AQ administration. This property results in the effectiveness of AQ in current malaria therapy [26]. Thus, the prolonged systemic presence of both AQ and desethylamodiaquine following the co-administration of MD and AQ, as observed in this study, may be responsible for the increased Plasmodium parasite clearance and longer survival of the experimental animals earlier reported [13].
A limitation to this study may be noted in the employment of composite blood sampling which allows one blood draw through cardiac puncture. Therefore, blood was pooled from a group of animals per time point which may obliterate individual variation. The choice of mice in the present study was to exclude species-related bias in the interpretation and comparison of pharmacokinetic data, generated herein, with the previously reported pharmacodynamic outcome observed when AQ was co-administered with MD. It is, however, worth noting that while the present study used healthy mice, the previous report which documented the increased efficacy of AQ in the presence of MD utilized malaria-infected animals. It is interesting to note that a second concentration peak for AQ was observed (at 2 h) after the Cmax (at ½ h). This phenomenon is in agreement with previous observations in humans and in the fecal excretion of AQ in the rat [24, 27].
Some plant components of MD have previously been studied for their interaction with drug transport and metabolism processes. Mangifera indica extract inhibited CYP1A2, 2A6, 2C9, 2D6 and 3A4 in a concentration-dependent manner [28]. In addition, M. indica and Alstonia boonei, significantly inhibited CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4 [29] which are CYP enzymes that are largely responsible for the metabolism of several drugs in humans. Furthermore, mild inhibition of p-glycoprotein, a major class of transport proteins for xenobiotics, has also been reported with mangiferin (chemical constituent of M. indica) and the ethanol extract of Azadirachta indica [28, 30]. Moreover, an earlier study demonstrated that the concurrent oral administration of an aqueous leaf extract of A. indica, one of the plant components of MD, with chloroquine in rabbits, resulted in decreased serum clearance, volume of distribution, AUC and Cmax of chloroquine, while the Tmax of chloroquine was unchanged, and the half-life increased from 26.7 to 60.2 h [18].
As reported in this study, concurrent administration of AQ with MD or after pre-treatment of mice with MD, led to an increase in the half-life and systemic exposure of the drug. This observation suggests an inhibition of the metabolism of AQ and that of its major metabolite, desethylamodiaquine, as reflected by increased systemic exposure of desethylamodiaquine in both administration arms of the study. The metabolism of AQ to desethylamodiaquine in humans, is principally mediated by CYP2C8 [15]. Although the metabolic pathway that results in the production of desethylamodiaquine in mice is not fully characterized, it is likely that this biotransformation proceeds through the mice isoenzyme of CYP2C8. Hence, the increased half-life of AQ may have resulted, in part, from the inhibitory effect of M. indica in MD [28], amongst its other plant components. A longer half-life for AQ following pre-treatment with MD, compared with concurrent herb–drug administration, might be due to a steady accumulation of the inhibitory component(s) of the herbal preparation over time. This would be in agreement with results from an earlier report which noted a concentration-dependent inhibition of CYP enzymes by M. indica, recording approximately 50% reduction in enzyme activity at 250 µg/mL of extract [30].
The enhancement of the absorption of AQ by MD, as reflected by an increased Cmax despite the unchanged Tmax, typifies the complex interaction that can occur between herbs and orthodox drugs. Inhibitory effects of the components of MD on p-glycoproteins [31], which serve as active efflux transporters in the hepatobiliary, direct intestinal and urinary excretion of drugs and their metabolites [32], would be expected to alter the absorption process of co-administered xenobiotics. Hence, the increased Cmax of AQ might have resulted from reduced intestinal efflux activity of p-glycoproteins with MD interventions, or from competition between AQ and MD components for p-glycoprotein binding sites.
5 Conclusion
MD influenced the pharmacokinetics of AQ and desethylamodiaquine, its major metabolite. Concurrent administration and pre-treatment with MD prolonged the half-life of AQ, and increased the systemic exposure of both AQ and desethylamodiaquine, suggesting an inhibitory effect on the metabolism of AQ, which is more pronounced with pre-treatment with MD. The present findings may provide a basis for the enhanced pharmacological effect of the combination in an earlier study in Plasmodium-infected mice. However, this beneficial interaction of MD with AQ may have to be validated in human volunteers.
References
Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. J Clin Pharmacol. 2000;40(5):451–6.
Kinung’hi SM, Mashauri F, Mwanga JR, Nnko SE, Kaatano GM, Malima R, et al. Knowledge, attitudes and practices about malaria among communities: comparing epidemic and non-epidemic prone communities of Muleba district, North-western Tanzania. BMC Public Health. 2010;10(1):395–406.
Idowu OA, Mafiana CF, Luwoye IJ, Adehanloye O. Perceptions and home management practices of malaria in some rural communities in Abeokuta, Nigeria. Travel Med Infect Dis. 2008;6(4):210–4.
Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63(6):965–81.
WHO: World Health Organisation. World Malaria Report. 2018. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed on 15 May 2019.
Willcox ML, Bodeker G. Traditional herbal medicines for malaria. BMJ. 2004;329(7475):1156–9.
Amoah LE, Kakaney C, Kwansa-Bentum B, Kusi KA. Activity of herbal medicines on Plasmodium falciparum gametocytes: implications for malaria transmission in Ghana. PLoS One. 2015;10(11):e0142587.
Odugbemi TO, Akinsulire OR, Aibinu IE, Fabeku PO. Medicinal plants useful for malaria therapy in Okeigbo, Ondo State, Southwest Nigeria. Afr J Tradit Complement Altern Med. 2006;4(2):191–8.
Brantley SJ, Argikar AA, Lin YS, Nagar S, Paine MF. Herb-drug interactions: challenges and opportunities for improved predictions. Drug Metab Dispos. 2014;2(3):301–17.
Onyeji CO, Igbinoba SI, Olayiwola G, Adehin A. Insight into clinically effective herbal antimalarial products: effects on drug metabolizing enzymes and p-glycoprotein. Afr J Pharm Pharmacol. 2017;11(48):591–613.
Shi S, Klotz U. Drug interactions with herbal medicines. Clin Pharmacokinet. 2012;51(2):77–104.
Adepiti AO, Elujoba AA, Bolaji OO. In vivo antimalarial evaluation of MAMA decoction on Plasmodium berghei in mice. Parasitol Res. 2014;113(5):505–11.
Odediran SA, Elujoba AA, Adebajo AC. Influence of formulation ratio of the plant components on the antimalarial properties of MAMA decoction. Parasitol Res. 2014;113(5):1977–84.
Adepiti AO, Elujoba AA, Bolaji OO. Evaluation of herbal antimalarial MAMA decoction-amodiaquine combination in murine malaria model. Pharm Biol. 2016;54(10):2298–303.
Li XQ, Björkman A, Andersson TB, Ridderström M, Masimirembwa CM. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther. 2002;300(2):399–407.
Churchill FC, Patchen LC, Campbell CC, Schwartz IK, Nguyen-Dinh P, Dickinson CM. Amodiaquine as a prodrug: importance of metabolite (s) in the antimalarial effect of amodiaquine in humans. Life Sci. 1985;36(1):53–62.
Wang R, Zhang H, Sun S, Wang Y, Chai Y, Yuan Y. Effect of Ginkgo leaf tablets on the pharmacokinetics of amlodipine in rats. Eur J Drug Met Pharmacokinet. 2016;41(6):825–33.
Nwafor SV, Akah PA, Okoli CO, Oyirioha AC, Nworu CS. Interaction between chloroquine sulphate and aqueous extract of Azadirachta indica A. Juss (Meliaceae) in rabbits. Acta Pharm. 2003;53:305–11.
Mustapha KB, Bakare-Odunola MT, Garba M, Obodozie OO, Enemali IS, Inyan US. Effect of phytomedicines, AM-1, niprisan® and nifadin on the pharmacokinetics of chloroquine in rats. Eur J Drug Met Pharmacokinet. 2009;34(3–4):151–5.
Olawoye OS, Adeagbo BA, Bolaji OO. Effect of Moringa oleifera leaf powder suspension on the pharmacokinetics of amodiaquine in rats. J Complement Alt Med Res. 2017;3(4):1–8.
National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington DC: National Academy Press; 1996.
Adedeji ON, Bolaji OO, Falade CO, Osonuga OA, Ademowo OG. Validation and pharmacokinetic application of a high-performance liquid chromatographic technique for determining the concentrations of amodiaquine and its metabolite in plasma of patients treated with oral fixed-dose amodiaquine-artesunate combination in areas of malaria endemicity. Antimicrob Agents Chemother. 2015;59(9):5114–22.
United States Food and Drug Administration: Bioanalytical Method Validation: Guidance for Industry. 2018.
Winstanley P, Edwards G, Orme M, Brekenridge A. The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol. 1987;23(1):1–7.
United States Food and Drug Administration. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products – general considerations. Rockville, MD: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; 2003.
Mariga ST, Gil JP, Sisowath C, Wernsdorfer WH, Björkman A. Synergism between amodiaquine and its major metabolite, desethylamodiaquine, against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 2004;48(11):4089–96.
Winstanley PA, Edwards G, Curtis CG, Orme M, Powel GM, Breckenridge AM. Tissue distribution and excretion of amodiaquine in the rat. J Pharm Pharmacol. 1988;40(5):343–9.
Rodeiro I, José Gómez-Lechón M, Perez G, Hernandez I, Herrera JA, Delgado R, et al. Mangifera indica L. extract and mangiferin modulate cytochrome P450 and UDP-glucuronosyltransferase enzymes in primary cultures of human hepatocytes. Phytother Res. 2013;27(5):745–52.
Showande SJ, Fakeye TO, Kajula M, Hokkanen J, Tolonen A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal herbs—implication for herb–drug interactions. Food Sci Nutr. 2019;7(1):44–55.
Chieli E, Romiti N, Rodeiro I, Garrido G. In vitro effects of Mangifera indica and polyphenols derived on ABCB1/P-glycoprotein activity. Food Chem Toxicol. 2009;47(11):2703–10.
Kawami M, Yamada Y, Issarachot O, Junyaprasert VB, Yumoto R, Takano M. P-gp modulating effect of Azadirachta indica extract in multidrug-resistant cancer cell lines. Pharmazie. 2018;73(2):104–9.
Szakács G, Váradi A, Özvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today. 2008;13(9–10):379–93.
Acknowledgements
AOA is grateful to the Obafemi Awolowo University (OAU), Nigeria, for support under the Staff Development Programme. The OAU Research Committee is acknowledged for Grant 11813AFL. The authors are grateful to the Carnegie Foundation/Therapeutic Drug Monitoring Laboratory, OAU Teaching Hospitals Complex, Ile-Ife, Nigeria for the high-performance liquid chromatography facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Support for the study was provided by the Obafemi Awolowo University, Ile-Ife, Nigeria under the Staff Development Programme and the University Research Committee Grant 11813AFL.
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Approval for the study was obtained from the Health Research Ethics Committee of the Obafemi Awolowo University, Ile-Ife, Nigeria (IPHOAU/12/90). “The Guide for the care and use of laboratory animals” was strictly followed.
Rights and permissions
About this article
Cite this article
Adepiti, A.O., Adeagbo, B.A., Adehin, A. et al. Influence of MAMA decoction, an Herbal Antimalarial, on the Pharmacokinetics of Amodiaquine in Mice. Eur J Drug Metab Pharmacokinet 45, 81–88 (2020). https://doi.org/10.1007/s13318-019-00583-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-019-00583-7