Abstract
This experiment aimed to test the combined effects of arbuscular mycorrhizal fungi (AMF) and rhizobium (Sinorhizobium medicae) on the alfalfa root rot (Microdochium tabacinum) disease. The results show a significant increase in alfalfa growth, induced by AMF, independent of inoculation with rhizobium. Inoculation with S. medicae increased alfalfa N concentration compared with un-inoculated plants. In the presence of both AMF and S. medicae, the N, P concentration of plant were significantly greater than in their absence. M. tabacinum caused plant branches wilt and significantly reduced plant total dry weight as well as N, P concentration. Inoculation of AMF increased nodule numbers, independent of the presence of other factors. AMF and rhizobium reduced alfalfa branches discoloration by 18%. Plant disease related enzyme, peroxidase (POD), and catalase (CAT) showed a positive response to both AMF and rhizobium, while the malondialdehyde (MDA) content responded negative.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alfalfa (Medicago sativa) is a widely grown crop in northern China, providing hay and silage for livestock throughout the year. For 2015, more than 2 million hectares of alfalfa was estimated to be cultivated in China. In the year of 2015, a root rot was detected in the alfalfa fields of Huanxian County of Gansu, China on the cultivar Longdong. Symptoms included discoloration of infected tissues and development of brown and rotted areas on both root and crown tissues. The average percentage of diseased plant reached to 30% in three years old alfalfa field, and the brown and rotted areas occupies 25% ~ 100% of plant roots. Infestation often leads to the death of the entire plant. We previously found the pathogen causing these symptoms as Microdochium tabacinum (Wen et al. 2015). This was the first report of alfalfa root rot caused by M. tabacinum in China. The only other report of M. tabacinum causing disease in alfalfa was a study of Pegg and Parry (1983), in which the authors isolated Fusarium tabacinum (a former name for M. tabacinum) from fields in Kent, UK.
Arbuscular mycorrhizal fungi (AMF) and rhizobia are two types of important microorganisms in the agro-ecosystem. AMF are known to associate with the majority of plant families (Simon et al. 1993), with estimates of up to 80% of all species potentially acting as hosts (Smith and Read 2008). AMF have been reported to have positive influence on plant tolerance to disease, e.g. Diseases caused by F. oxysporum on Cucumis sativus seedlings (Wang and Hao 2008), Phytophthora parasitica on Lycopersicon esculentum root (Vigo et al. 2000; Pozo et al. 2002), Verticillium sp. on Capsicum annuum (Garmendia et al. 2004). The positive effects was especially via the suppression of a broad range of root pathogens (Dehne 1982; Larsen and Bødker 2001), their major impact on plant health is through increased nutrient acquisition. AMF have specific to plant and pathogens, e.g. Glomus versiforme has stronger effect to disease caused by Fusarium oxysporum in Cucumis sativus than that Glomus intraradices (Wang et al. 2012). Moreover, each of AMF alone and mixes has functional biodiversity in plant disease protection (Wehner et al. 2010), the mix of AMF inoculation have different influence on plant growth and nutrition acquisition than that of individual of AMF inoculation as well (Duan et al. 2011).The suspected mechanism includes improved nutrient availability for the host plant, damage compensation, changed root growth and morphology, competition with pathogens for colonization sites and plant photosynthates, changed microbial activity in the mycorrhizosphere and activation of plant defense mechanisms (Perrin 1990).
It is well known that the rhizospheres of legumes contain large populations of rhizobia, which are specific to the legume species (Vigo et al. 2000). The rhizosphere of alfalfa was commonly colonized by Sinorhizobium medicae. The rhizobium are capable of protecting their hosts against fungal pathogens (Tu 1978; Chakraborty and Purkayastha 1984) by increasing phytoalexin production in cross protection (Rahe et al. 1969; Svoboda and Paxton 1972; Skipp and Deverall 1973; Al-Ani et al. 2012; Elkhateeb 2014; Siddiqui et al. 2013).
The effects of inoculations of AMF and rhizobium on growth and nutrition uptake of the host plant have been well documented for legume hosts such as Glycine max (Ding et al. 2012), and Astragalus adsurgens (Wu et al. 2013). Moreover, mycorrhiza formation tends to be a necessary precursor for nodulation which suggests that legumes must have adequate phosphorus to support the development of nodules and bacteroids (van Rhijn et al. 1997). However, despite these numerous reports, little work has been done to investigate the combined effects of AMF and rhizobium on legume plant diseases, in particular the new alfalfa root rot caused by M. tabacinum. Due to increased demand for alfalfa in China and enhanced risk of crop loss caused by M. tabacinum infestation, it is vitally important to explore possible methods of disease control.
The experiment was designed to test the combined effects of AMF and rhizobium on alfalfa root rot caused by M. tabacinum. We used two AMF (Funnelliformis mosseae and Glomus tortuosum separately and mixed). Alfalfa were inoculated with and without Sinorhizobium medicae, as well as with or without M. tabacinum. We hypothesized that: (1) F. mosseae, G. tortuosum and S. medicae’scontribution will influence plant disease occurrence and damage caused by M. tabacinum. (2) When the two AMF mixed inoculated, they will have stronger positive effect on plant disease resistance compared with individual inoculation. (3) Resistance of alfalfa to M. tabacinum will be enhanced when AMF and Sinorhizobium medicae are combined compared to individual inoculation.
Materials and methods
Plants and fungi
Seeds of alfalfa (Medicago sativa cv Longdong) were afforded by Entry–exit Quarantine Bureau of Gansu province, Lanzhou. The seeds were surface sterilized in 10% oxydol for 10 min, subsequently washed three rinses with sterilized distilled water and then leaving at 25 °C incubator for 48 h to germinate. Six germinated seeds were planted in each pot, and thinned to 4 seedlings after 1 week.
Funnelliformis mosseae and Glomus tortuosum were provided by College of Resource and Environment, China Agricultural University. It was prepared from pot cultures of AMF grown on Trifolium subterraneum in the same soil mix we used for the experiments. The inoculums consisted of substrate and Trifolium subterraneum root fragments (20 g/pot) were mixed with the soil, 10 g of each AM fungi for the mixture. Non-inoculated plants were supplied with 20 g of the sterilized soil that cultured the AMF inoculation.
Sinorhizobium medicae was provided by Center for Studies of Rhizobia, China Agricultural University. Cultures of S. medicae was grown on yeast extract mannitol (YEM) (pH 6.5) for 6 days at 25 °C on a shaker. The concentration (2.1 × 108 /mL) of inoculum was standardized using a colorimeter (Chakraborty and Purkayastha 1984). 5 mL of the suspension was then watered to each 7-day-old alfalfa plant, the same amount of sterilized distilled water were added to the non-inoculated treatments.
Microdochium tabacinum was obtained from Entry–exit Quarantine Bureau of Gansu province, Lanzhou. M. tabacinum grown on potato dextrose agar (PDA). Conidia were harvested in sterilized water from 26-day-old cultures incubated at 25 °C under fluorescent lights (40 ìE·m-2·s-1). The conidial suspension was sieved (45 ìm). The inoculum concentration was 3 × 106 conidia per milliliter and consisted of microconidia and macroconidia in a 5: 2 ratio (Caron et al. 1986). 2.5 mL spore suspension was added to each of 6-week-old alfalfa, the same amount of sterilized distilled water was added to the non-inoculated treatments.
Growth medium
The soil was collected from Xinglong Mountain, Lanzhou, China for our preparations of the soil mix used throughout the experiments. The mix consisted of a blend of 50% of soil and 50% of sand. Both components were sieved through a 2 mm sieve, and the soil and sands were sterilized via autoclaving at 121 °C for 1 h twice over a period of 3 days and then dried in an oven at 110 °C for 36 h. The size of the pot was 6 cm × 22 cm × 15 cm (bottom × height × top) contains 1.5 kg of the soil.
Experimental design
A fully-crossed three factor experiment was designed: AMF (4 levels) × rhizobium (2 levels) × Microdochium (2 levels) = 16 treatments, each with 4 replicate plants. The alfalfa were cultivated in soil that inoculated with the following AMF combinations: F. mosseae, G. tortuosum, a combination of F. mosseae and G. tortuosum, or clean soil non-inoculated with AMF (NM). S. medicae was inoculated for half of the AMF treatments one week after alfalfa emergence. M. tabacinum was inoculated for half of each AMF and S. medicae treatments at 6 weeks after plant emergence.
Plant growth and harvesting
The experiment was conducted in a glasshouse with photosynthetic photon flux density in the range of 180–850 mmol m−2 s−1 during the growth period. The average temperatures were 23–28 °C (day) and 20–25 °C (night). The plants were watered with tap water every other day to permanent weight of 10% of dry weight of soil. Twice a week, we applied a modified Hogland nutrient solution (without P) (Duan et al. 2011) to the pots during the experiment. The plants were harvested 3 weeks after inoculation with M. tabacinum.
At harvest, the discoloration percentage of alfalfa was determined by counting all the aboveground of the plants. The stem length and branch number of each plant was determined, then shoots were cut from the plants and divided into three subsamples, approximately 0.6 g fresh weight were used to determine enzymes with the published methods including peroxidase (POD) (Hammerschmidt et al. 1982), catalase (CAT) (Bailly et al. 1996), approximately 0.3 g fresh weight was used to determine malondialdehyde (MDA) (Heath and Packer 1968) and Chlorophyll content (Arnon 1949), the rest were used to determine dry weight (from the ratios of the subsamples), N and P concentration.
Roots were washed, floated in shallow trays of water and both tap and lateral roots were scored independently using a 6-step rating scheme where: score 0 = root healthy, no discoloration; 1 = <25% of root slightly brown, no significant lesions; 2 = 25- < 50% of root brown, lesions towards base of tap root. 3 = 50–75% root brown, lesions mid tap root; 4=75% root brown; 5 = plant dead. The number of plants in each disease severity category was recorded. The diseased plant rate was calculated by the diseased plant divided the total plant for each treatment. The disease index (DI) was calculated by the following formula,
where i is the disease severity scale (i = 0, 1, 2, 3, 4, 5) and LN and Ln are the total number of plants of each disease severity, respectively.
Then three subsamples were taken to determine total root dry weight (from the ratios of the subsamples) and N, P concentration, tap root length, M. tabacinum invading rate, nodule number and AM colonization. Each of the 4 plants root in a pot was subsampled for the pathogen re-isolation (Wen et al. 2015). Nodules were counted per pot respectively and the roots were preserved in formalin-aceto-alcohol to determine AMF colonization of roots by the slide technique. AM colonization of roots was determined by the method of Giovannetti and Mosse (1980). The root samples were cut into small segment (~1 cm) cleared in 10% KOH and stained with trypan blue lactophenol (Phillips and Hayman 1970).
Plant dry shoot and root samples were ground through a 2 mm sieve, 0.2 g samples were digested by 10 mL H2SO4, catalyst are 3 g K2SO4 and 0.3 g CuSO4. The extract was used to determine N, P concentrations. Plant N and P was determined with Flow injection Analyzer (FIAstar 5000 Analyzer, FOSS, Sweden) in State Key Laboratory of Grassland Agro-Ecosystems of Lanzhou University.
Statistical analysis
Data are presented as means and standard errors of means of four replicates (pot), homogeneity of variances was determined via Bartlett’s test. Data were analyzed by analysis of variance (ANOVA) using SPSS 19.0 statistical analysis software (SPSS Inc., Chicago, USA). Comparisons between means were based on the least significant differences at the 0.05 probability level. Data for percent AM colonization were ARCSIN-transformed to achieve normality.
Results
Disease occurrence, nodulation and AMF colonization
The inoculation of AMF average increased the nodule numbers from 19 to 52, however, we found no difference between individual or mixed conditions. Pathogen infection reduced alfalfa nodule number (P < 0.05) (Fig. 1a). No AM colonization was found in the NM treatment. For AMF treatment, the roots of alfalfa were well infected by the fungi, displaying colonization percentage numbers ranging from 70.14% to 86.74% (Fig. 2b). AM fungi displayed very similar root colonization regardless of treatment (individual or mixed), and infection with M. tabacinum reduced the root colonization of AMF significantly (P < 0.05), while the inoculation with S. medicae slightly increased AMF colonization (Fig. 2b, Table 1).
Nodule number (a), colonization percentage (b) and disease incidence (c) of alfalfa (Medicago sativa) inoculated without (R-) and with (R+) Sinorhizobium medicae and colonized by Funnelliformis mosseae and Glomus tortuosum, the mix of the two fungi or non-mycorrhizal (NM) at harvest. Mean ± SEM of four replicates. P = Microdochium tabacinum, NP=No M. tabacinum. The same capital letters on the bars show there is no significant differences between R- or R+ at the level of P < 0.05 (b); Bars with same lower case letters show there is no significant differences between mycorrhial × pathogen (a), or there is no significant differences between P and NP within R- or R+ at P < 0.05 level (b). See Tables 1 for ANOVA results
Shoot dry weight (dw) (a) and root dry weight (b) of alfalfa (Medicago sativa) inoculated without (R-) and with (R+) Sinorhizobium medicae and colonized by Funnelliformis mosseae and Glomus tortuosum, the mix of the two fungi or non-mycorrhizal (NM) at harvest. Mean ± SEM of four replicates. P = Microdochium tabacinum, NP=No M. tabacinum. The same capital letters on the bars show there is no significant differences between mycorrhizal treatments within R- or R+ at the level of P < 0.05 (a, b); Bars with same lower case letters show there is no significant differences between mycorrhizal × M. tabacinum in R- or R+ at P < 0.05 level (b). See Tables 1 for ANOVA results
The infection of the pathogen caused alfalfa discoloration by 8~15%, of which AMF alone and the co-inoculation of AMF and S. medicae reduced plant discoloration by 47% while S. medicae had no effect on plant disease occurrence (Fig. 2c). No typical root rot symptoms was found across the treatments, and no M. tabacinum was re-isolated from the un-inoculated treatment, while M. tabacinum was re-isolated from the roots of all the inoculated treatments. The inoculation of M. tabacinum caused very lightly brown of 6 alfalfa roots. The disease index was between 0 and 0.75. AMF and S. medicae had similar affect on pathogen invading, the co-inoculation slightly reduced the root rot disease severity (P > 0.05).
Plant growth
Alfalfa responded positively to AMF colonization(Fig. 2a, b; Table 1). The effects of AMF colonization was first clearly apparent at 14 d when all inoculated plants featured a larger number of leaves compared to NM plants (data not shown). The two AMF resulted the same effect on alfalfa biomass accumulation independent of whether they were inoculated individually or in combination. Alfalfa displayed a negative response to infection with M. tabacinum, especially in the growth of roots, which was significantly reduced by the pathogen (Fig. 2b). The infection of the pathogen affected tap root length across the treatments (data not shown). This was true for both the AMF treatments and the co-inoculation of AMF and rhizobium. Root/shoot (R/S) ratios were decreased by the M. tabacinum infection, and the co-inoculation of AMF and rhizobium increased tap root length of plants (data not shown).
Plant N and P concentrations
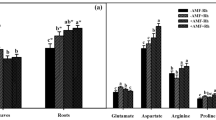
Alfalfa N and P concentrations responded positively to AMF colonization (Figs. 3 and 4) (P < 0.05). The inoculation of S. medicae only increased alfalfa N absorb (Fig. 4) while had no effect on plant P uptake (Fig. 4). The co-inoculation of AMF and S. medicae had combine interactions and enhanced alfalfa N uptake across the treatment (Fig. 3, Table 1) (P < 0.05), however had no similar influence to plant P uptake (Fig. 4). The infection of the pathogen significantly deceased shoot and root N concentration and also shoot P concentration across the experiment (P < 0.05) (Figs. 3, and 4, Table 1).
Shoot N content (a) and Root N content (b) of alfalfa (Medicago sativa) inoculated without (R-) and with (R+) Sinorhizobium medicae and colonized by Funnelliformis mosseae and Glomus tortuosum, the mix of the two fungi or non-mycorrhizal (NM) at harvest. Mean ± SEM of four replicates. P = Microdochium tabacinum, NP=No M. tabacinum. The same capital letters on the bars show there is no significant differences between R- or R+ at the level of P < 0.05 (a, b); Bars with same lower case letters show there is no significant differences between P and NP in R- or R+ at P < 0.05 level (a, b). See Tables 1 for ANOVA results
Shoot P content (a) and root P content (b) of alfalfa (Medicago sativa) inoculated without (R-) and with (R+) Sinorhizobium medicae and colonized by Funnelliformis mosseae and Glomus tortuosum, the mix of the two fungi or non-mycorrhizal (NM) at harvest. Mean ± SEM of four replicates. P = Microdochium tabacinum, NP=No M. tabacinum. The same capital letters on the bars show there is no significant differences between R- or R+ at the level of P < 0.05 (a, b); Bars with same lower case letters show there is no significant differences between P and NP in R- or R+ at P < 0.05 level (a). See Tables 1 for ANOVA results
Enzymes activities, MDA content
The effects of AMF and rhizobium on the physiological and biochemical of alfalfa show a very similar trend compared to the growth performance of alfalfa. All the physiological and biochemical indexes show positive response to AMF and rhizobium except for MDA, which responds negatively to colonization of AMF and rhizobium. M. tabacinum infected alfalfa had relatively higher values for POD and CAT activities (Fig. 5a, b) compared to the control, while also displaying slightly higher values for MDA content in un-inoculated treatments and treatments that were inoculated with the rhizobium, respectively (Fig. 5c). The inoculation of AMF improved Chlorophyll concentration across the treatment, while the inoculation of pathogen and rhizobium had no effect on plant Chlorophyll concentration (Fig. 6).
POD activity (a), CAT activity (b) and MDA content (c) of alfalfa (Medicago sativa) inoculated without (R-) and with (R+) Sinorhizobium medicae and colonized by Funnelliformis mosseae and Glomus tortuosum, the mix of the two fungi or non-mycorrhizal (NM) at harvest. Mean ± SEM of four replicates. P = Microdochium tabacinum, NP=No M. tabacinum. The same capital letters on the bars show there is no significant differences between R- or R+ at the level of P < 0.05 (a, b, c); Bars with same lower case letters show there is no significant differences between P and NP in R- or R+ at P < 0.05 level (a, b, c). See Tables 1 for ANOVA results
Chlorophyll content of alfalfa (Medicago sativa) inoculated without (R-) and with (R+) Sinorhizobium medicae and colonized by Funnelliformis mosseae and Glomus tortuosum, the mix of the two fungi or non-mycorrhizal (NM) at harvest. Mean ± SEM of four replicates. P = Microdochium tabacinum, NP=No M. tabacinum. The same capital letters on the bars show significant differences between R- and R+ at P < 0.05 level. Bars with same lower case letters show there is no significant differences between AM and NM in R- and R+ at P < 0.05 level. See Tables 1 for ANOVA results
Discussion
In this research, we firstly studied the effect of AMF and rhizobium on a novel alfalfa root rot disease in China. M. tabacinum significantly reduced plant N, P concentration and total dry weight and caused discoloration of plant without typical root rot in this study. However, the inoculation still caused plant discoloration by 8~15% and slightly root brown. The M. tabacinum usually has long latent before it caused typical symptom, the disease mostly occurred at the late growth stage and in the alfalfa field established more than 2 years. We found that M. tabacinum did not reduce branch numbers nor shoot length (data not shown), but pathogen infected plants had fine roots and lower root dry weight compared to uninfected plants, which indicated that the pathogen reduced plant root dry weight by changing the root morphology even without the typical root rot.We re-isolated M. tabacinum from the roots of all inoculated plants. This suggests that the disease may occur under suitable environmental conditions without long time latent period such as in the field conditions. The present study afforded valuable information of the occurrence of Microdochium tabacinum on alfalfa.
Mycorrhizal colonization significantly increased the growth, including shoot length, root length, and number of branches as well as shoot and root dry weight of alfalfa independent of both rhizobium and pathogen presence (Fig. 2, Table 1). Regardless of model of inoculation (individual or mix), the two AMF had similar effect on plant P and N uptake biomass accumulation and shoot discoloration. Rhizobia are able to establish symbiotic associations with their specific host plant and absorb atmospheric N (Scheublin et al. 2004). Our results showed that inoculation of rhizobium increased plant N concentration leading a slight increase in plant growth, while had no effect on plant disease resistance. The infection with the pathogen M. tabacinum significantly reduced nodule numbers across the treatment, leading the less N concentration in plants. This is inconsistent with the findings of Sawada (1982), who reported inoculation of Rhizobium meliloti decreased root rot of F. oxysporum, the nodulation was reduced by the pathogen in alfalfa. The differences of our results with others indicated the functional diversity of plant-microorganism (Wehner et al. 2010).
Nodules can fix atmospheric nitrogen, the efficiency of the process is mostly determined by the phosphorous nutrient content of the host plant since appropriate phosphorous nutrient support is indispensable for the growth of host plant (Sánchez-Díaz et al. 1990). Only when organic phosphorus under the action of soil phosphatase hydrolysis to inorganic phosphate, could it be absorbed and utilized by roots. This experiment shown that AMF could significantly improve alfalfa P concentrations, which indicates inoculation with AMF may increase rhizosphere soil phosphatase activity. Tarafdar and Jungk (1987) had observed a considerable increase in both acid and alkaline phosphatase activity in all the four soil-root interfaces after inoculation with AMF. Previous researches on legume species such as alfalfa (Nielsen and Jensen 1983; Azcon and El-Atrash 1997) and soybean (Ross 1971; Xie et al. 1995) showed that rhizobium or AM can significantly affect plant growth, nutrient uptake, and each other through competitive root colonization (Bethlenfalvay et al. 1985; Hodge 2000). In our study, we found rhizobium and AMF are inter-dependent and mutually-promoting. AMF inoculation promotes the formation of root nodules and rhizobium inoculation increased the percentage of AMF infection (Fig. 1). This result is consistent with Barea et al. (1987) and Erman et al. (2011). Moreover, in the presence of rhizobium, frequency of root colonization increased in pathogen infection plants compared to the treatment absent of rhizobium. These findings also align with others (Goicoechea et al. 1997; Castagno et al. 2014). Despite successful nodulation of S. medicae, rhizobium appeared to have little or no effect on biomass accumulation of alfalfa, however the inoculation increased plant N concentration. This may due to the sufficient N in soils for normal plant growth (Shockley et al. 2004).
Enzyme activity of plants responded negatively to the infection of M. tabacinum when co-inoculated with AMF and rhizobium. The POD and CAT are plant protection enzymes that clean toxic substances from cells (such as phenols, formaldehyde, etc.), and could alleviate or avoid plant from poisoning. MDA is the product of membrane lipid peroxidation of plants in adversity. MDA levels could represent the degree of membrane lipid peroxidation, and thus are closely related to plant disease resistance (Liu et al. 2014). Our results show that AM colonization increases the activity of POD and CAT, but decreases the content of MDA. M. tabacinum infection increased POD and CAT (Fig. 5a, b) and plants produced more disease related enzymes (such as POD and CAT) to alleviate the stress caused by the pathogen (Liu et al. 2014). However, other studies reported that the infection of pathogen decreased plant disease protection enzyme activity. Maya and Matsubara (2013) reported that the infection of Fusarium oxysporum and Colletotrichum gloeosporioides decreased antioxidative activity of cyclamen plants. The lower values of MDA content display lower cell membrane damage or higher disease resistance.
The colonization of AMF responded positively to enhance plant resistance to the pathogen. The rhizobium treatment decreased CAT content which indicates that rhizobium could increase alfalfa resistance to root rot disease. Chakraborty and Chakraborty (1989) found Rhizobium leguminosarum can produce a phytoalexines (pisatine et 4-hydroxy-2, 3, 9-trimethoxyptcarpan) and enhenced pea roots against the infection by Fusarium solani. Chlorophyll content was reduced by the infection of M. tabacinum treatment due to suppression of specific enzymes (e.g. protochlorophyllide reductase). These enzymes are responsible for the synthesis of photosynthetic pigments). A higher chlorophyll content in leaves of mycorrhizal plants under pathogen stress has been observed by Li et al. (2004) who found chlorophyll content to be significantly increased by the inoculation of AMF or combined inoculation with AMF and rhizobium. In the presence of AMF, the antagonistic effects of pathogen on Mg uptake is counterbalanced and suppressed. Our hypothesis that co-inoculation of AMF and S. medicae enhances plant resistance to the pathogen was partly upheld judging from growth, discoloration rate of plant and disease protection enzyme activity.
In conclusion, our findings suggest that although root rot caused by M. tabacinum usually show typical root rot symptoms in 2 years old alfalfa in field, however, with suitable environmental conditions in greenhouse, the pathogen can successfully invading plant roots and cause slightly root rot symptoms. Furthermore, AMF colonization can increase tolerance against M. tabacinum infection. Rhizobium either individually inoculated or co-inoculated with AMF had a slightly positive effect on plant growth and antioxidative enzyme activity, still it increased alfalfa plant N concentration. Considering that alfalfa is a perennial crop, we assume an important role of rhizobium in fixation of N and an overall improved plant growth. Co-inoculation bears the potential to be used as bio-control agent to control root rot disease of alfalfa caused by M. tabacinum. Further studies are required to evaluate the long time effects of increased levels of AMF and rhizobium on the root rot in the field, as well as the interaction mechanism of the microorganisms with alfalfa.
References
Al-Ani RA, Adhab MA, Mahdi MH, Abood HM (2012) Rhizobium japonicum as a biocontrol agent of soybean root rot disease caused by Fusarium solani and Macrophomina phaseolina. Plant Prot Sci 48:149–155
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Azcon R, El-Atrash F (1997) Influence of arbuscular mycorrhizae and phosphorus fertilization on growth, nodulation and N2 fixation (15N) in Medicago sativa at four salinity levels. Biol Fertil Soils 24(1):81–86. https://doi.org/10.1007/BF01420225
Bailly C, Benamar A, Corbineau F, Come D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol Plant 97(1):104–110. https://doi.org/10.1111/j.1399-3054.1996.tb00485.x
Barea JM, Azcon-Aguilar C, Azcón R (1987) Vesicular-arbuscular mycorrhiza improve both symbiotic N2 fixation and N uptake from soil as assessed with a 15N technique under field conditions. New Phytol 106(4):717–725. https://doi.org/10.1111/j.1469-8137.1987.tb00172.x
Bethlenfalvay GJ, Brown MS, Stafford AE (1985) Glycine-Glomus-Rhizobium symbiosis II. Antagonistic effects between mycorrhizal colonization and nodulation. Plant Physiol 79(4):1054–1058. https://doi.org/10.1104/pp.79.4.1054
Caron M, Fortin JA, Richard C (1986) Effect of phosphorus concentration and Glomus intraradices on Fusarium crown and root rot of tomatoes. Phytopathology 76(9):942–946. https://doi.org/10.1094/Phyto-76-942
Castagno LN, García IV, Sannazzaro AI, Bailleres M, Ruiz OA, Mendoza RE, Estrella MJ (2014) Growth, nutrient uptake and symbiosis with rhizobia and arbuscular mycorrhizal fungi in Lotus tenuis plants fertilized with different phosphate sources and inoculated with the phosphate-solubilizing bacterium Pantoea eucalypti M91. Plant Soil 385(1-2):357–371. https://doi.org/10.1007/s11104-014-2237-z
Chakraborty U, Chakraborty BN (1989) Interaction of rhizobium leguminosarum and Fusarium solani f. sp. pisi on pea affecting disease development and phytoalexin production. Can J Bot 67(6):1698–1701. https://doi.org/10.1139/b89-214
Chakraborty U, Purkayastha RP (1984) Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina infection. Can J Microbiol 30(3):285–289. https://doi.org/10.1139/m84-043
Dehne HW (1982) Interaction between vesicular-arbuscular mycorrhizal fungi and plant pathogens. Phytopathology 72:1115–1119
Ding XD, Zhang L, Li SY, Feng G (2012) Effects of inoculations of Glomus mosseae and/or Bradyrhizobium japonicum on formation and distribution of nodules and phosphorus uptake of soybean. Plant Nutrition and Fertilizeer Science 18:662–669
Duan T, Facelli E, Smith SE, Smith FA, Nan Z (2011) Differential effects of soil disturbance and plant residue retention on function of arbuscular mycorrhizal (AM) symbiosis are not reflected in colonization of roots or hyphal development in soil. Soil Biol Biochem 43(3):571–578. https://doi.org/10.1016/j.soilbio.2010.11.024
Elkhateeb N.M.M., (2014). Influence of Rhizobium sp. combined with Trichoderma spp. on damping-off disease and growth parameters of faba bean plants. Egyptian J Pest Control 24:139-149.
Erman M, Demir S, Ocak E, Tüfenkçi Ş, Oğuz F, Akköprü A (2011) Effects of rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rainfed conditions 1—yield, yield components, nodulation and AMF colonization. Field Crop Res 122(1):14–24. https://doi.org/10.1016/j.fcr.2011.02.002
Garmendia I, Goicoechea N, Aguirreolea J (2004) Effectiveness of three glomus species in protecting pepper (Capsicum annuum L.) against Verticillium wilt. Biol Control 31(3):296–305. https://doi.org/10.1016/j.biocontrol.2004.04.015
Giovannetti M, Mosse B (1980) Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Goicoechea N, Antolin MC, Sánchez-Díaz M (1997) Influence of arbuscular mycorrhizae and Rhizobium on nutrient content and water relations in drought stressed alfalfa. Plant Soil 192(2):261–268. https://doi.org/10.1023/A:1004216225159
Hammerschmidt R, Nuckles EM, Kuć J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20(1):73–82. https://doi.org/10.1016/0048-4059(82)90025-X
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hodge A (2000) Microbial ecology of the arbuscular mycorrhiza. FEMS Microbiol Ecol 32(2):91–96. https://doi.org/10.1111/j.1574-6941.2000.tb00702.x
Larsen J, Bødker L (2001) Interactions between pea root-inhabiting fungi examined using signature fatty acids. New Phytol 149:487–493
Li S M, Li L, Zhang F S. (2004). Enhancing phosphorus and nitrogen uptake of faba bean by inoculating arbuscular mycorrhizal fungus and Rhizobium leguminosarum. J China Agric Univ 9:11-15.(In Chinese)
Liu XL, Xi XY, Shen H, Liu B, Guo Y (2014) Influences of arbuscular mycorrhizal(AM) fungi inoculation on resistance of tobacco to bacterial wilt. Tobacco Agronomy 94–98. https://doi.org/10.3969/j.issn.1002-0861.2014.05.020
Maya MA, Matsubara YI (2013) Tolerance to fusarium wilt and anthracnose diseases and changes of antioxidative activity in mycorrhizal cyclamen. Crop Prot 47:41–48. https://doi.org/10.1016/j.cropro.2013.01.007
Nielsen JD, Jensen A (1983) Influence of vesicular-arbuscular mycorrhiza fungi on growth and uptake of various nutrients as well as uptake ratio of fertilizer P for lucerne (Medicago sativa). Plant Soil 70(2):165–172. https://doi.org/10.1007/BF02374777
Pegg GF, Parry DW (1983) Infection of lucerne (Medicago sativa) by Fusarium species. Ann Appl Biol 103(1):45–55. https://doi.org/10.1111/j.1744-7348.1983.tb02739.x
Perrin R (1990) Interactions between mycorrhizae and diseases caused by soil-borne fungi. Soil Use Manag 6(4):189–194. https://doi.org/10.1111/j.1475-2743.1990.tb00834.x
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55(1):158–163. https://doi.org/10.1016/S0007-1536(70)80110-3
Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, Azcón-Aguilar C (2002) Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J Exp Bot 53(368):525–534. https://doi.org/10.1093/jexbot/53.368.525
Rahe JE, Kuc J, Chuang CM, Williams EB (1969) Induced resistance in Phaseolus vulgaris to bean anthracnose. Phytopathology 59:1641–1645
Ross JP (1971) Effect of phosphate fertilization on yield of mycorrhizal and nonmycorrhizal soybeans. Phytopathology 61:12
Sánchez-Díaz M, Pardo M, Antolin M, Pena J, Aguirreolea J (1990) Effect of water stress on photosynthetic activity in the Medicago-Rhizobium-Glomus symbiosis. Plant Sci 71(2):215–221. https://doi.org/10.1016/0168-9452(90)90011-C
Sawada Y (1982) Interaction of rhizobial nodulation of alfalfa and root rot by Fusarium oxysporum. Bulletin Nation Grassland Res Institute 6:19–26
Scheublin TR, Ridgway KP, Young JPW, Van Der Heijden MGA (2004) Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70(10):6240–6246. https://doi.org/10.1128/AEM.70.10.6240-6246.2004
Shockley FW, Mcgraw RL, Garrett HE (2004) Growth and nutrient concentration of two native forage legumes inoculated with Rhizobium and Mycorrhiza in Missouri, USA. Agrofor Syst 60(2):137–142. https://doi.org/10.1023/B:AGFO.0000013269.19284.53
Siddiqui ZA, Fatima M, Alam S (2013) Interactions of Meloidogyne Incognita, Xanthomonas campestris and Rhizobium sp. on the disease complex of chickpea. Turk J Agric For 37:173–178
Simon L, Bousquet J, Eacute RC, Amp V, Lalonde M (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363(6424):67–69. https://doi.org/10.1038/363067a0
Skipp RA, Deverall BJ (1973) Studies on cross-protection in the anthracnose disease of bean. Physiol Plant Pathol 3(3):299–313. https://doi.org/10.1016/0048-4059(73)90002-7
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic press, Longdon
Svoboda WE, Paxton JD (1972) Phytoalexin production in locally cross-protected Harosoy and Harosoy-63 soybeans. Phytopathology 62(12):1457–1460. https://doi.org/10.1094/Phyto-62-1457
Tarafdar JC, Jungk A (1987) Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biol Fertil Soils 3(4):199–204. https://doi.org/10.1007/BF00640630
Tu JC (1978) Protection of soybean from severe Phytophthora root rot by Rhizobium. Physiol Plant Pathol 12:233–240
van Rhijn P, Fang Y, Galili S, Shaul O, Atzmon N, Wininger S, Eshed Y, Lum M, Li Y, To V, Fujishige N, Kapulnik Y, Hirsch AM (1997) Expression of early nodulin genes in alfalfa mycorrhizae indicates that signal transduction pathways used in forming arbuscular mycorrhizae and rhizobium-induced nodules may be conserved. Proc Natl Acad Sci U S A 94(10):5467–5472. https://doi.org/10.1073/pnas.94.10.5467
Vigo C, Norman JR, Hooker JE (2000) Biocontrol of the pathogen Phytophthora parasitica by arbuscular mycorrhizal fungi is a consequence of effects on infection loci. Plant Pathol 49(4):509–514. https://doi.org/10.1046/j.1365-3059.2000.00473.x
Wang CX, Hao ZP (2008) Effects of arbuscular mycorrhizal fungi on Fusarium wilt of cucumber seedlings. Mycosystema 27:395–404
Wang CX, Li XL, Song FQ, Wang GQ, Li BQ (2012) Effects of arbuscular mycorrhizal fungi on fusarium wilt and disease resistance-related enzyme activity in cucumber seedling root. Chin J Eco-Agric 20(1):53–57. https://doi.org/10.3724/SP.J.1011.2012.00053
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53(3):197–201. https://doi.org/10.1016/j.pedobi.2009.10.002
Wen ZH, Duan TY, Christensen MJ, Nan ZB (2015) Microdochium tabacinum, confirmed as a pathogen of alfalfa in Gansu Province, China. Plant Dis 99(1):87–92. https://doi.org/10.1094/PDIS-10-13-1048-RE
Wu FY, Kun WY, Bi YL, Qi LS, Li LJ (2013) Inoculation of arbuscular mycorrhizal fungi and Rhizobium on the growth and nutrition uptake of Astragalus adsurgens pall. Under water stress. Agric Res Arid Areas 31:161–166
Xie ZP, Staehelin C, Vierheilig H, Wiemken A, Jabbouri S, Broughton WJ, Vogeli-Lange R, Boller T (1995) Rhizobial nodulation factors stimulate mycorrhizal colonization of nodulating and nonnodulating soybeans. Plant Physiol 108(4):1519–1525. https://doi.org/10.1104/pp.108.4.1519
Acknowledgements
This research was financially supported by China Agriculture Research System-Green manure (CARS-22) and The National Natural Science Foundation (31100368).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, P., Guo, Y., Li, Y. et al. Effects of dual inoculation of AMF and rhizobium on alfalfa (Medicago sativa) root rot caused by Microdochium tabacinum. Australasian Plant Pathol. 47, 195–203 (2018). https://doi.org/10.1007/s13313-018-0543-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-018-0543-2