Abstract
Dieback causes a progressive reduction in plant population health, resulting in the death of plant parts and often plant death. It is prevalent in many invasive woody weeds in Australia and has been suggested as a potential mechanism for biocontrol of these species. Parkinsonia aculeata one such invasive tree in northern Australia. It has naturalised across a wide range of climatic zones and some populations have been heavily reduced by dieback occurrence. The cause(s) of dieback in parkinsonia remain elusive, although fungal endophytes have been previously implicated. In this study, we characterised the culturable fungal endophyte community of healthy and dieback-affected parkinsonia using culture-based techniques, and identified cultured isolates via amplicon sequencing of the internal transcribed spacer (ITS) of the rDNA operon. Eight isolates, identified as pathogens, were selected for a 10-week pathogenicity trial, including water stress treatments, on parkinsonia seedlings. We isolated a taxonomically diverse fungal community from parkinsonia, representing 54 unique species from 25 families. Communities were similar across healthy and dieback-affected plants, but differed by plant tissue. Of the eight putative pathogenic isolates tested in the pathogenicity trial, inoculation with Lasiodiplodia pseudotheobromae, Botryosphaeria dothidea and Pestalotiopsis mangiferae resulted in the largest lesions, but systemic infection or dieback-like symptoms were not observed in any treatment despite plant stress being induced by drought or inundation. We concluded that inoculation of parkinsonia with the tested putative fungal pathogens is unlikely to result in dieback, which has implications for future work in biocontrol of parkinsonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinsonia aculeata (parkinsonia, family: Fabaceae) is a spiny, leguminous, thicket-forming tree, native to the Americas, but a serious invader in northern Australia (Thorp and Lynch 2000). The management of parkinsonia is expensive and labour-intensive and usually involves the use of herbicides followed by manual removal of dead trees (Deveze et al. 2004). Since the most recent estimates of population extent exceeds 3 million ha (van Klinken et al. 2009), more efficient and autonomous control methods are sought. The most promising mechanism for parkinsonia control has been the occurrence of dieback in some populations (van Klinken et al. 2009). We define dieback as a progressive reduction in plant health, resulting in the death of plant parts, often followed by outright tree death that may result in local population decline, either as a gradual or sudden occurrence (Mueller-Dombois 1987). Parkinsonia dieback begins with defoliation, followed by browning of the stems starting at the stem tips, and usually resulting in whole tree mortality (Diplock 2016). Dieback has been observed in a number of Australian Weeds of National Significance (WONS), but has not been observed in locally-occurring native species (Raghavendra et al. 2016; van Klinken et al. 2009; Wilson and Pitkethley 1992) and there is no evidence that dieback occurs in these WONS’ native ranges. If the cause of parkinsonia dieback is identified there is potential for its use as a self-sustaining biological control agent to be used alongside other control methods.
Plants host a diverse community of fungal species, the vast majority of which are mutualistic or benign endophytes but some may be pathogenic or saprophytic (Hawksworth 2001). In previous work, endophyte communities (archaea, bacteria and fungi) were analysed for correlation with dieback occurrence in parkinsonia using terminal restriction fragment length polymorphism (T-RFLP) analysis (Steinrucken et al. 2016). Bacterial community composition was not significantly correlated to parkinsonia dieback, and although significant correlations with archaeal OTUs and dieback were observed, little is known about archaeal endophytes and few archaea have ever been cultured (Schleper et al. 2005). With regard to endophytic fungal communities, in their previous work Steinrucken et al. (2016) also found a significant correlation between fungal community composition and dieback occurrence, suggesting the involvement of multiple fungal endophytic species which differ in composition across plant parts. Although this work demonstrated the potential involvement of fungal endophytes in parkinsonia dieback, the method of community fingerprinting with T-RFLP did not allow assignment of taxonomy or ecological roles.
Diplock (2016) and Toh (2009) isolated, identified and tested a number of endophytic fungal pathogens reported to be involved in parkinsonia dieback. In their studies one species stood out as a potential causal agent: Lasiodiplodia pseudotheobromae. This species has been implicated in dieback of other non-native tree species globally including multiple Australian leguminous and woody WONS (Diplock 2016; Haque 2015; Sacdalan 2015; Toh 2009), Prunus spp. in South Africa (Damm et al. 2007), mango in Egypt (Ismail et al. 2012) and Acacia spp. in Australia (Adair et al. 2009). In testing the pathogenicity of L. pseudotheobromae on parkinsonia, Toh (2009) inoculated sterile vermiculite substrate with colonised millet seed before transplanting parkinsonia seedlings into the mixture one week post-emergence. This study showed that L. pseudotheobromae (isolate NT039; Genbank Accession no. KX893409) was the most virulent of 83 tested, including other Botryosphaeriaceae. A concurrent four-year field trial on adult parkinsonia trees involved inserting colonised millet seed into holes drilled into the base of the trees (Diplock 2016). On some sites, the treatment resulted in lesion formation by L. pseudotheobromae, but was unable to recreate dieback symptoms or tree mortality. The study was further complicated by wounding reactions, bacterial contamination and adverse environmental conditions including a flood and fire (Diplock 2016).
Although it has been implicated in disease and dieback of woody hosts, L. pseudotheobromae has also been associated with healthy hosts as a non-pathogenic endophyte (Jami et al. 2013; Slippers and Wingfield 2007). A number of other Botryosphaeriaceae species have both pathogenic and endophytic associations with their host and many can be triggered to become pathogenic in the presence of abiotic factors such as water stress (Mehl et al. 2013; Schulz et al. 1998). These species are termed ‘latent pathogens’: microorganisms that remain benign or mutualistic until triggered to be pathogenic by an external factor such as environmental stress to the host, or co-infection by a more virulent pathogen (Slippers and Wingfield 2007). It is therefore difficult to predict whether endophytic fungi could be pathogenic under certain circumstances or if they are simply opportunistic, becoming pathogenic or saprophytic when the plant is stressed.
The interaction between the host, its environment and pathogens plays an integral part in the occurrence of disease (Agrios 2005). Conceptual models described by Houston (1992); Manion (1991) and Whyte et al. (2016) attempt to characterise the interactions between these inciting and contributing factors and how they relate to dieback occurrence. This complexity means it is unclear whether symptoms of dieback in parkinsonia are the primary cause of dieback or are the results of secondary infections by opportunistic pathogens, triggered by other biotic or abiotic factors. Parkinsonia and many other dieback-affected WONS are spread across regions of northern Australia that are subject to long-term drought and intermittent flooding, so it is possible that dieback is partly triggered by water availability. This has been observed in the decline of black alder (Alnus glutinosa) by the pathogen Phytophthora alni, whose virulence is associated with flooding episodes (Webber et al. 2004). Similarly, in drought-stressed oak trees, a number of ascomycete pathogens such as Biscogniauxia mediterranea take advantage of weakened host tissues and become more virulent, causing decline in several species (La Porta et al. 2008).
In this study we describe the culturable fungal endophyte community in healthy and dieback-affected parkinsonia from regions previously shown to have dieback/endophyte community correlations, and we identify putative pathogens to test against parkinsonia seedlings exposed to excessive, limiting, or optimal water treatments in a glasshouse inoculation study. We consequently address the following question: Can we induce systemic infection and dieback-like symptoms in parkinsonia, by inoculating plants with the selected putative fungal pathogens, and will water stress enhance this effect?
Materials and methods
Sampling, identification and analysis of the fungal endophyte community
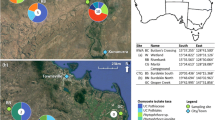
We sampled sub-dermal tissue from three roots, three secondary stems and three stem tips and seeds (when available) of five trees in each of three healthy and three dieback-affected parkinsonia populations near Charters Towers, Queensland. Endophyte communities have been previously shown to be structured by plant part (Steinrucken et al. 2016; Rudgers and Orr 2009). These plant parts were chosen to ensure any stratification of endophyte communities across an individual plant was accounted for, and since leaves and seeds were not always available, stem tips were collected. Sampling was conducted in March 2013 and repeated on the same trees in May 2013 in order to ensure sampled healthy trees did not develop dieback-like symptoms between sampling periods (they did not) and to avoid isolating a community of endophytes representative of only one point in time. Between sampling of different trees and plant parts, all tools were sterilized using 50% NaClO and then rinsed with sterile water. Samples from different plant parts and trees were stored in separate paper bags at 5 °C for up to 48 h until processing. Plant parts were vigorously pre-washed in distilled H2O for 20 s. An ethanol (70%) and UV-sterilized laminar flow cabinet was used for subsequent steps. For stems, stem tips and seeds a three-stage ethanol-bleach-ethanol surface sterilization method was used as recommended by Bills (1996). Seeds were then imbibed in 95 °C sterile, distilled H2O for 12 h. Roots were washed for 30 s in sterile, distilled H2O containing 0.1% Tween-20™ (Sigma-Aldrich, St Louis, MO, USA) since harsher sterilization techniques are not recommended for roots (Thorn et al. 2007). All samples were blotted dry with sterile filter paper and surface sterilization was checked by sliding tissue over the surface of 50% Potato Dextrose Agar amended with streptomycin (sPDA; 35 mg L−1) and incubating at 30 °C for seven days (Bacon and Hinton 2007). The bark of stem tips and stems, the seed coat of seeds and a small portion of root cortex was then removed using a sterile scalpel. Three tissue plugs (3–5 mm2) from each sample were placed on sPDA media and were maintained at room temperature in the dark for seven days. Isolates were subcultured daily, or when mycelial growth was observed.
Once pure fungal isolates were obtained, genomic DNA was isolated using a MO BIO Powersoil® DNA Isolation kit (MO BIO Laboratories Inc., Carlsbad, CA, USA), and identified via sequencing of amplified ITS rDNA amplicons. PCR reactions were undertaken in a total volume of 20 μl and consisted of 0.2 U BIOTAQ™ DNA polymerase (Bioline, London, UK), 10× NH4 Buffer (2 uL per reaction), MgCl2 (60 mM per reaction), dNTPs (50 mM each per reaction), ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers (4 mM each per reaction; Gardes and Bruns 1993), and 2 uL extracted DNA per reaction. PCR reactions were run at 94 °C for 3 min; 34 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min; and a final extension step of 72 °C for 10 min. PCR products were purified using the Wizard®SV Gel and PCR Clean-Up System (Promega Madison, WI, USA), and sequenced by Sanger sequencing using the same forward primer (ITS1), in one direction, at the Hawkesbury Institute for the Environment using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) as per the manufacturer’s instructions. Sequence chromatograms were analysed in Geneious® V6.1.6 (Biomatters Ltd., Auckland, New Zealand) and underwent BLASTn searches on the National Center for Biotechnology Information (NCBI) nucleotide database on 6th May 2016. Closest match was determined by comparing maximum sequence length and lowest e-values. The cut-off point for assigning species names to closest match on the database was 97–100% identity; genus names were 94–97% identity; family name 90–94% identity; and sequences with lower identity with members of several families were identified only at the ordinal level (Vega et al. 2010). Sequences sharing less than 85% identity with closest match sequences or sharing higher identity to unidentified sequences in GenBank, were identified only to class or phylum (Vega et al. 2010). All taxonomic classifications required >95% query coverage and sequences with the same % ID for different organisms were identified to the closest common taxonomic level. We aligned unique sequences using MUSCLE Alignment (Edgar 2004) with eight iterations over 456 bases as implemented in Geneious® v8.1 and constructed a neighbour-joining tree based on the UPGMA model with a Phytophthora ramorum voucher sequence as the outgroup (Fig. 1).
Neighbour-joining tree based (TreeBASE submission no. 20057) on the UPGMA Model constructed using Geneious® v8.1 on a 458 bp length MUSCLE alignment of ITS1-ITS4 sequences from representative endophytic fungal taxa (Table 2) including the number of those isolates isolated from each plant tissue type. Bootstrap values (n = 1000 replicates) are shown on the intercepts. Outgroup is a Phytophthora ramorum (HQ643339.1). Isolates used in the pathogenicity trial are in bold and indicated with *. Ordinal groups indicated on right. All isolates are Ascomycetes apart from those in orders marked with (B) Basidiomycete, (Z) Zygomycete and (O) Oomycete
Glass house pathogenicity trial
One month old parkinsonia seedlings grown from seed and collected from healthy populations in Charters Towers (QLD) were re-potted in 0.8 L free-draining square plastic pots in media consisting of 8 parts fine/medium pit sand, 1 part Mikskaar White Peat and 1 part Mikskaar Professional® substrate 250 (pH 5.2–6; Mikskaar AS, Tallinn, Estonia) and amended with 2.8 g/L Basacote® Plus Prilled slow release fertilizer, 1.5 g/L Osmoform® slow release fertiliser (Everris International B.V., Geldermalsen, The Netherlands) and 0.2 g/L SierraForm GT® Anti Stress (Everris International B.V). Plants were grown in an evaporatively-cooled glasshouse (21–27 °C) watered every second day, fertilised monthly with All Purpose Soluble Fertilizer (Hortico®, Padstow NSW, Australia), and treated for mites, thrips, scale and powdery mildew with Crown® SureGrow (Everris International B.V) at 2.5 mL/L at 3 and 6 months, and weekly with predatory mites. After ten months, 258 healthy plants were selected for this trial, and randomly arranged in a temperature-controlled glasshouse. Plants were not fertilized after this point and, during the trial, glasshouse pests were controlled only using predatory mites (Neoseiulus californicus; Bugs for Bugs, Mundubbera, QLD, Australia).
Eighty-six plants (the control group) were watered as before with 100 mL water every second day; 86 were placed in white plastic trays with the water level maintained at 5 cm depth, inundating the roots and the third group of 86 plants were drip-fed 8–10 mL water twice a week to simulate drought conditions. Glasshouse conditions were set at 28 °C during the day, 21 °C at night and 60% constant humidity for one week before inoculation and then a further ten weeks from January to March 2015 at the Ecoscience Precinct, Brisbane, Australia.
Eight fungal isolates were chosen from the identified endophytic species, identified via sequencing as species previously reported to be pathogenic and cause dieback in their host (Table 1). Representing five families (Table 1), all but one (CTQ089 L. pseudotheobromae) were isolated from dieback-affected plants. For a positive control we also included a L. pseudotheobromae isolate (NT039), obtained from the University of Queensland culture collection, which had been isolated and tested in previous dieback studies, and shown to be pathogenic on parkinsonia (Diplock 2016; Toh 2009). All nine isolates tested were ascomycetes. We tested the effect of three water stress treatments (drought, inundation and ‘normal’) on the pathogenicity of the selected fungal isolates and the growth of twelve month-old parkinsonia seedlings.
The nine isolates were passaged through Granny Smith apples to ensure they had not lost their pathogenicity due to prolonged subculturing (Erwin and Ribeiro 1996), and then re-isolated on PDA without streptomycin for use in subsequent inoculations. After seven days, underbark inoculation was carried out on surface-sterilised stems at approximately 7 cm above soil surface. Incisions of 8–10 mm long were made with a sterile scalpel blade. A 5 mm2 mycelial plug was fully inserted into the wound and the stem was bound with Parafilm® (Bemis, Oshkosh, WI, USA) to facilitate healing. The negative control (five of the 86 plants in each water treatment) consisted of a sterile PDA plug. Plants were arranged randomly within a split-plot design, with each fungal inoculant (subplot) occurring once nested within each water treatment (main plot), each with nine replicates (therefore, 9 isolates × 9 replicates =81, + 5 negative controls =86 plants × 3 water treatments =258 plants in total).
Immediately prior to inoculation and at the end of the trial plant growth by height (cm) from the soil surface and stem girth (mm) at the site of inoculation was measured. We also monitored any damage by mites (% foliage damage). At the conclusion of the trial (10 weeks following inoculation) plants were harvested at the root collar. After harvest, lesions were bisected with a sterile blade. Underbark lesion size, identified by discolouration from the site of inoculation, and any scarring was measured. To confirm that lesions were associated with the inoculated pathogen and to look for any systemic infection by the inoculated pathogen, a small amount of tissue was sampled from the lesion or cut site of three plants in each replicate group; 1 cm above and below the lesion; and 10 cm above the lesion. Tissue samples were plated on sPDA and incubated for 1 week at room temperature in the dark, and isolates were identified via ITS sequencing as above. Roots were freed from soil by carefully running them under water, being careful not to wash away fine roots and as with the above-ground parts, were placed in paper bags and dried in an oven at 60 °C for 14 days. We recorded the dry weight of above and below-ground parts.
We tested the effects of water treatment and inoculated isolate on lesion length and three measures of plant health: the change in height and stem circumference over the ten-week inoculation trial and post-harvest dry mass at the conclusion of the trial. Data were treated as a split-plot design (Schwarz 2015) in R (R Core Team 2016) using the ‘lme4’ (Bates et al. 2015) and ‘lmerTest’ (Kuznetsova et al. 2016) packages for analysis of variance (ANOVA) with Kenward-Roger approximation for degrees of freedom. ANOVAs were followed by post-hoc testing using Tukey HSD (Tukey 1949) using the ‘multcomp’ (Hothorn et al. 2008) package.
Results
Fungal endophytes in healthy and dieback-affected Parkinsonia aculeata
We cultured a total of 213 fungal isolates from multiple plant parts in healthy and dieback-affected parkinsonia and identified 54 unique operational taxonomic units (OTUs) through DNA sequencing of the ITS rDNA region (Table 2). The identified isolates (GenBank Accessions KT699870-KT699873; KX893353-KX893409) represented 16 fungal orders and 25 families. The majority (90%) were Ascomycetes while seven Basiodiomycetes and one Zygomycete were also isolated. Fungi from the order Pleosporales had the greatest number of representative isolates (Fig. 2) with a total of 85 isolates, but only 5 unique OTUs. The Xylariales were well represented (12 isolates, 9 unique species), as were the Hypocreales (10 isolates, 8 unique species) and Eurotiales (9 isolates, 5 unique species). We recovered 31 isolates from samples collected in March 2013 and 58 isolates from samples collected in May 2013. We isolated the greatest number of endophytes from parkinsonia stems with 28% of isolates from dieback trees and 11% from healthy trees (Fig. 2). This was followed by the branch tips (17% from dieback, 12% from healthy), the seeds (9% dieback, 4% healthy) and roots (4% dieback, 5% healthy). We isolated four species of Botryosphaeriaceae (8 isolates), and although four of these isolates were L. pseudotheobromae, this species was only isolated from healthy parkinsonia (Table 2). Overall, we isolated more endophytes from dieback-affected parkinsonia (61%) compared to healthy parkinsonia (39%) trees. Of the isolated taxa, 32 were previously shown to be pathogenic according to the literature, 27 had history as dieback pathogens, but only nine were involved in dieback of trees. Three of these were Pestalotiopsis (P. clavispora, P. mangiferae and P. visimae). Due to the number of available plants and our desire to maximise statistical power and therefore the number of replicates, we decided to exclude P. visimae from this trial.
Fungal community overview showing the proportion of taxonomic orders represented where (B) are Basidiomycetes, (Z) is a Zygomycete and all others are Ascomycetes; the proportion of isolates isolated by tissue type and host disease status; and the percentage of isolates found in healthy, dieback-affected parkinsonia, or both
Pathogenicity testing of isolates with a water stress interaction
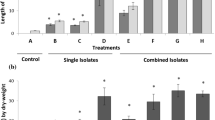
There was a statistically significant difference in lesion length, explained by inoculated isolate (F = 2.347, p = 0.01), but no effect by water treatment or the interaction between these factors (p > 0.05; Table 3). Universally, lesion size was greater underbark than on the surface. The length of incision in the negative controls (sterile ½ PDA plug) was consistent with the length of the underbark “lesion”, which we assume was a result of scarring, so we concluded that no lesion was formed for the negative control treatment. The positive control (L. pseudotheobromae, NT039) consistently formed larger lesions than any other pathogen tested (40.57 ± 0.51 mm; Fig. 3) and contributed significantly to variation in lesion length in post-hoc testing (Table 4). Pestalotiopsis mangiferae (CTQE067), L. pseudotheobromae (CTQE089), Botryosphaeria dothidea (CTQE031) and Pestalotiopsis clavispora (CTQE056) caused similar sized underbark lesions (23.94 ± 0.47 mm; Fig. 3). Diplodia pinea (CTQE005) and Phomopsis azadirachtae (CTQE007) resulted in the smallest lesions, with P. azadirachtae (12.83 ± 0.15 mm) only just exceeding the inoculation site scar length (10.67 ± 0.02 mm) but was greater than the negative control (11.77 ± 0.33 mm).
There was a statistically significant difference in plant health measurements by water treatment. Water treatment affected plant growth by height (F = 14.08, p < 0.001) stem circumference (F = 10.82, p < 0.001), and post-harvest biomass (F = 8.38, p < 0.001) but we found no significant effect of inoculated isolate or the interaction between isolate and water treatment on any recorded plant heath measurements (p > 0.05). Drought-affected plants showed the greatest levels of water stress (Table 5), which was reflected in their relatively slower growth rate over 10 weeks during the trial with smaller changes in stem circumference and height (Fig. 4a, b); lower post-harvest dry-mass (Fig. 4c); and their increased susceptibility to pests (Fig. 5). They also had a lower above-ground: below-ground plant dry mass ratio (1.43 ± 0.09) than inundated plants (2.08 ± 0.08) or control plants (2.13 ± 0.10). Plants that were inundated had reduced height compared to the control treatment (Fig. 4a); however, stem girth and post-harvest dry mass were similar between inundated plants and those in the control water regime (Table 5).
Despite confirming local infection by the inoculated pathogen via re-isolation from lesions, we were unable to re-isolate the pathogen more than 2 cm away from the lesion in any of the plants post-harvest. Isolates from post-harvest, healthy, plant tissue were identified as Myrothecium verrucaria, a ubiquitous contaminant and plant pathogen; Phoma sp. and Chaetomium globosum – both endophytes; and Fusarium oxysporum and Penicillium verruculosum, which are common saprotrophs and endophytes (Nguyen et al. 2016). We did not observe any dieback-like symptoms in the plants such as loss of foliage, internal staining or death of plant parts, other than lesions at the inoculation site. Any loss of foliage in drought-affected plants was consistent across inoculated pathogens, so was presumed to be due to water availability or mite damage, not infection by a pathogen. We therefore found no evidence of systemic infection by any inoculated pathogen, regardless of lesion size or level of stress.
Discussion
Despite significant levels of water stress, and a decrease in the health of stressed plants, underbark inoculation by any of the chosen fungal isolates did not cause systemic infection or dieback-like symptoms in parkinsonia. Four of the isolates tested in this trial were members of the Botryosphaeriaceae, many of which are known to persist as latent pathogens within their host (Jami et al. 2013; Mehl et al. 2013; Slippers and Wingfield 2007). This family of pathogens grow both intracellularly and intercellularly and after infection, are known to move via the mesophyll and vascular bundle (Mehl et al. 2013). Host response involving the formation of a new periderm can also lead to infection of the xylem and phloem tissue (Rayachhetry et al. 1996) leading to systemic infection within eight weeks. Lasiodiplodia pseudotheobromae (CTQ089) was only isolated from healthy, symptomless parkinsonia during field sampling, yet formed a significantly larger lesion in the pathogenicity trial than some of the other isolates that were isolated from dieback-affected parkinsonia. This supports the idea that at least this strain of L. pseudotheobromae is a latent pathogen (Jami et al. 2013). Lesions formed by Diplodia pinea were relatively small, and this may be because it is potentially more pathogenic to other tree species such as Pinus sp. (de Wet et al. 2000). Botryosphaeria dothidea has been observed to cause girdling and death in defoliated downy birch (Betula pubescens) stems after just four weeks, suggesting that defoliation stress might be essential for increased B. dothidea virulence (Crist and Schoeneweiss 1974).
Pestalotiopsis spp. are responsible for a number of plant diseases, mostly in the tropics (Chen et al. 2013; Espinoza et al. 2008; Ismail et al. 2013; Keith et al. 2006) and are commonly isolated as saprobes, although some are likely to have both endophytic and pathogenic stages in their lifecycles (Maharachchikumbura et al. 2011). Endophytes from this group are ubiquitous and not associated with geographic limits, but their host colonisation rates are lower in monsoon seasons than in the dryer winter season (Tejesvi et al. 2005). This indicates that they may be limited by drought-like conditions, and take advantage of their host in sustained wet weather. In our study, we did not observe significant variation in lesion length between inundated and drought-affected plants by either P. clavispora or P. mangiferae. This might be because parkinsonia is relatively healthy in inundated conditions compared to drought-affected conditions (Fig. 4), thereby not presenting with the stress required by the two Pestalotiopsis spp. for increased colonisation or pathogenicity. There are no records of dieback occurrence in parkinsonia in relation to rainfall conditions in the field.
Out of the other three isolates used in this study, only Alternaria alternata and Rhizopycnis vagum caused underbark lesions that were significantly greater than the negative control. A. alternata is known to produce host-specific phytotoxins which may cause defoliation (Babu et al. 2003). This species may therefore require a susceptible host for it to be more virulent. Rhizopycnis vagum is most frequently a root-colonizing endophyte (Knapp et al. 2012), although some studies have shown it to be pathogenic to musk-melon roots (Armengol et al. 2003) and involved in mature watermelon vine decline (Westphal et al. 2011). We isolated it from the roots of dieback-affected parkinsonia, but it too, only resulted in small localised lesions when inoculated. Westphal et al. (2011) suggests Rhizopycnis vagum may require other factors to increase disease severity, such as soil inoculation.
We attempted to ensure that each inoculated isolate was triggered into pathogenicity by first passaging the isolate through an apple. Although we achieved local infection in the plant, we did not observe systemic infection, despite first ensuring that the host was under water stress. It is possible that extending the length of the trial past 10 weeks may have resulted in eventual mortality. Incubation times vary between studies (Ismail et al. 2013; Pitt et al. 2013) but many report significant results within 10 days of inoculation (e.g., Armengol et al. 2003; Stukely and Crane 1994). Additionally, any response observed in an inoculation trial may be different to that observed in the field, even under similar conditions. The age of the plant tissue may affect the plant’s response to inoculation, and the endophyte community hosted by plants in the field may be different to those hosted by glasshouse plants grown from seed. A latent pathogen may only be triggered into pathogenicity by a combination of these factors which may also explain the lack of dieback symptoms observed in this glasshouse trial. Plants are complex organisms, playing host to multiple taxonomic and trophic groups, with environmental responses ranging from inherent to symbiotically-assisted. It is therefore difficult to predict or monitor infection from inoculation with one organism, without distinctive symptoms. Future pathogenicity work in this system, as demonstrated in Toh (2009) using seedlings, should be assessed histologically during and after the trial.
We isolated a taxonomically diverse range of fungal endophytes from multiple plant parts of healthy and dieback parkinsonia, including some reportedly pathogenic species, with a total of 54 unique taxa from 204 isolates as identified by ITS amplicon sequencing. These species came mostly from dieback-affected plants, and the greatest number were isolated from stems. The fungal endophyte community of other invasive plants is similar in regards to culturable endophyte species found in this study. Diplock (2016) only isolated fungi from stems and identified 20 unique fungal endophyte species of 48 isolates associated with dieback-affected parkinsonia. We identified 31 taxa from dieback stems collected in the same region. Twenty-four fungal endophyte taxa (out of 1352 isolates) were recovered from healthy and dieback-affected Mimosa pigra stems by Sacdalan (2015), and 23 taxa from 166 isolates from healthy and dieback-affected Vachellia nilotica subsp. indica stems and roots (Haque 2015). Overall, the number of taxa recovered from dieback-affected plants in this study was greater than from healthy plants, which is expected if additional dieback-causing pathogens are present, or as the host is colonised by incoming saprophytes during cell death brought on by dieback. The composition of endophyte communities between individual hosts and host species may also differ due to local environmental conditions, distance decay (i.e. increasing dissimilarity between communities with increasing spatial distance; Peršoh 2015) and mode of endophyte transmission (i.e. vertical vs. horizontal). However, there is a high chance that the isolates recovered in this study are dominant and/or fast growing members of the parkinsonia endophyte community, since these species are more likely to be isolated. Conversely, this also implies that slower-growing or more benign species may not have been recovered and that unculturable taxa were missed. Steinrucken et al. (2016) recovered over 150 unique OTUs from dieback-affected plant parts and over 70 from healthy plant parts, which is more than double those isolated in this study. The availability of molecular techniques and the decreasing price of high-throughput sequencing technology has consistently demonstrated that the diversity of fungi is grossly underestimated by culture-based studies (Peay et al. 2016).
Our observations support the idea that some of the fungal endophytes isolated from parkinsonia, particularly the Botryosphaeriaceae, exist commonly as endophytes and may act as latent pathogens but in order to cause disease in their host some external environmental trigger is required. Despite the formation of localised lesions, no dieback-like symptoms were observed via underbark inoculation of parkinsonia with the eight chosen isolates in this study. Under the right conditions (e.g., a specific environmental stress or the presence of other microorganisms) however, underbark inoculation may still be an appropriate method for testing other potential putative pathogens. In the future, other factors such as salinity, heat, and defoliation stress could be used during pathogenicity screening, and may provide insight into host susceptibility to dieback-associated pathogens. More thorough reporting of dieback occurrence in the field, and any associated environmental conditions, would also aid greatly in determining which stress factors are important for disease expression. Any potential dieback-causing agent(s) identified should be systematically tested for host-specificity (see Wapshere 1974; Evans 2000) – particularly against locally occurring native plant species – prior to release and widespread use as a biocontrol agent(s). Dieback syndromes adversely affect many desired tree species globally, but with the right combination of effective and specific dieback-causing pathogens, efficient inoculation techniques and conducive conditions, dieback may become an alternative tool for use in large scale weed management.
References
Adair RJ, Burgess T, Serdani M, Barber P (2009) Fungal associations in Asphondylia (Diptera: Cecidomyiidae) galls from Australia and South Africa: implications for biological control of invasive acacias. Fungal Ecol 2:121–134. doi:10.1016/j.funeco.2009.02.003
Agrios GN (2005) Plant Pathology, Elsevier Academic Press, Burlington MARudgers JA & Orr S (2009) Non-native grass alters growth of native tree species via leaf and soil microbes. J. Ecol 97:2 247-255. doi: 10.1111/j.1365-2745.2008.01478.x
Armengol J, Vicent A, Martinez-Culebras P, Bruton BD, Garcia-Jimenez J (2003) Identification, occurrence and pathogenicity of Rhizopycnis vagum on muskmelon in Spain. Plant Pathol 52:68–73. doi:10.1046/j.1365-3059.2003.00796.x
Babu RM, Sajeena A, Seetharaman K (2003) Bioassay of the potentiality of Alternaria alternata (Fr.) keissler as a bioherbicide to control water hyacinth and other aquatic weeds. Crop Prot 22:1005–1013. doi:10.1016/S0261-2194(03)00115-7
Bacon CW, Hinton DM (2007) Isolation, in planta detection, and uses of endophytic bacteria. In: Hurst C, Crawford RL, Mills AL, Garland JL, Stetzenbach LD, Lipson DA (eds) Manual of environmental microbiology, 3rd edn. ASM Press, Washington, DC, pp 638–651
Bakys R, Vasaitis R, Barklund P, Thomsen I, Stenlid J (2009) Occurrence and pathogenicity of fungi in necrotic and non-symptomatic shoots of declining common ash (Fraxinus excelsior) in Sweden. Eur J Forest Pathol 128:51–60. doi:10.1007/s10342-008-0238-2
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:48. doi:10.18637/jss.v067.i01
Bills GF (1996) Isolation and analysis of endophytic fungal communities from woody plants. In: Redlin SC, Carris LM (eds) American Phytopathological society. St, Paul, pp 31–65
Chen F, Lu L, Wang D, Wang Y, Ni H, Du Z (2013) Biological characterization and genetic diversity analysis of two species of Pestalotiopsis causing twig dieback of Myrica rubra. Eur J Forest Pathol 136:737–747. doi:10.1007/s10658-013-0203-x
Crist CR, Schoeneweiss DF (1974) The influence of controlled stresses on susceptibility of European white birch stems to attack by Botryosphaeria dothidea. Phytopathology 65:369–373
Damm U, Crous PW, Fourie PH (2007) Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia 99:664–680. doi:10.2307/20444885
de Wet J, Wingfield MJ, Coutinho TA, Wingfield BD (2000) Characterization of Sphaeropsis sapinea isolates from South Africa, Mexico, and Indonesia. Plant Dis 84:151–156. doi:10.1094/pdis.2000.84.2.151
Deveze M et al (2004) Approaches to the management of parkinsonia (Parkinsonia aculeata) in Australia. Department of Natural Resources, Mines and Energy, Queensland
Diplock ND (2016) Parkinsonia dieback: investigations into its cause, ecology and potential for biological control. The University of Queensland, PhD
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. 32:1792–1797. doi:10.1093/nar/gkh340
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. APS Press, St Paul
Espinoza JG, Briceno EX, Keith LM, Latorre BA (2008) Canker and twig dieback of blueberry caused by Pestalotiopsis spp. and a Truncatella sp in Chile. Plant Dis 92:1407–1414. doi:10.1094/pdis-92-10-1407
Evans HC (2000) Evaluating plant pathogens for biological control of weeds: an alternative view of pest risk assessment. Australas. Plant Pathol 29:1–14. doi:10.1071/AP00001
Ferreira JHS, Matthee FN, Thomas AC (1989) Fungi associated with dieback and pruning wounds of grapevines in South Africa. S Afr J Enol Vitic 10:62–66
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes--application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
González P, Alaniz S, Montelongo MJ, Rauduviniche L, Rebellato J, Silvera-Perez E, Mondino P (2012) First report of Pestalotiopsis clavispora causing dieback on blueberry in Uruguay. Plant Dis 96:914–914. doi:10.1094/pdis-12-11-1070-pdn
Haque A (2015) Investigation of the fungi associated with dieback of prickly acacia (Vachellia nilotica subsp. indica) in Northern Australia. PhD, The University of Queensland
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432. doi:10.1017/S0953756201004725
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. doi:10.1002/bimj.200810425
Houston DR (1992) A host-stress-saprogen model for forest dieback-decline diseases. In: Manion PD, Lachance D (eds) Forest decline concepts. The American Phytopathological Society, St. Paul, pp 3–25
Ismail AM, Cirvilleri G, Polizzi G, Crous PW, Groenewald JZ, Lombard L (2012) Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas Plant Path 41:649–660. doi:10.1007/s13313-012-0163-1
Ismail AM, Cirvilleri G, Polizzi G (2013) Characterisation and pathogenicity of Pestalotiopsis uvicola and Pestalotiopsis clavispora causing grey leaf spot of mango (Mangifera indica L.) in Italy. Eur J Forest Pathol. 135:619–625. doi:10.1007/s10658-012-0117-z
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2013) Greater Botryosphaeriaceae diversity in healthy than associated diseased Acacia karroo tree tissues. Australas Plant Path 42:421–430. doi:10.1007/s13313-013-0209-z
Johnson GI, Mead AJ, Cooke AW, Dean JR (1992) Mango stem end rot pathogens - fruit infections by endophytic colonization of the inflorescence and pedicel. Ann Appl Biol 120:225–234. doi:10.1111/j.1744-7348.1992.tb03420.x
Keith LM, Velasquez ME, Zee FT (2006) Identification and characterization of Pestalotiopsis spp. causing scab disease of guava, Psidium guajava, in Hawaii. Plant Dis 90:16–23. doi:10.1094/pd-90-0016
Knapp DG, Pintye A, Kovacs GM (2012) The dark side is not fastidious - dark septate endophytic fungi of native and invasive plants of semiarid Sandy areas. PLoS One 7:e32570. doi:10.1371/journal.pone.0032570
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) Tests in Linear Mixed Effects Models: Package 'lmerTest' V3.3
La Porta N, Capretti P, Thomsen IM, Kasanen AM, Hietala AM, Von Weissenberg K (2008) Forest pathogens with higher damage potential due to climate change in Europe. Can J Plant Pathol 30(2):177–195. doi:10.1080/07060661.2008.10540534
Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyde KD (2011) Pestalotiopsis-morphology, phylogeny, biochemistry and diversity. Fungal Divers 50:167–187. doi:10.1007/s13225-011-0125-x
Manion PD (1991) Tree disease concepts. Prentice-Hall Inc., Englewood Cliffs
Mehl JWM, Slippers B, Roux J, Wingfield MJ (2013) Cankers and other diseases cause by the Botryosphaeriaceae. CABI, Wallingford
Mueller-Dombois D (1987) Natural dieback in forests. Bioscience 37:575–583. doi:10.2307/1310668
Nguyen NH et al (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. doi:10.1016/j.funeco.2015.06.006
Peay KG, Kennedy PG, Talbot JM (2016) Dimensions of biodiversity in the earth mycobiome. Nat Rev Microbiol 14:434–447. doi:10.1038/nrmicro.2016.59
Peršoh D (2015) Plant-associated fungal communities in the light of meta'omics. Fungal Divers 75:1–25. doi:10.1007/s13225-015-0334-9
Pitt WM, Trouillas FP, Gubler WD, Savocchia S, Sosnowski MR (2013) Pathogenicity of Diatrypaceous fungi on grapevines in Australia. Plant Dis 97:749–756. doi:10.1094/pdis-10-12-0954-re
R Core Team (2016) R: A language and environment for statistical computing, 3.1.1 ed. R foundation for statistical computing, Vienna
Raghavendra AK et al. (2017) Characterization of above-ground endophytic and soil fungal communities associated with dieback-affected and healthy plants in five exotic invasive species. Fungal Ecol Accepted 10 Jan 2017
Rayachhetry MB, Blakeslee GM, Miller T (1996) Histopathology of Botryosphaeria ribis in Melaleuca quinquenervia: pathogen invasion and host response. Int J Plant Sci 157:219–227. doi:10.1086/297340
Sacdalan A (2015) Mimosa pigra dieback in the northern territory. Investigation into possible causes. PhD, The University of Queensland, Australia
Schleper C, Jurgens G, Jonuscheit M (2005) Genomic studies of uncultivated archaea. Nat Rev Microbiol 3:479–488. doi:10.1038/nrmicro1159
Schulz B, Guske S, Dammann U, Boyle C (1998) Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction. Paper presented at the 2nd International Congress on Symbiosis, Woods Hole Massachusetts, 13–18 April 1997
Schwarz CJ (2015) Two-factor split-plot designs. Course notes for beginning and intermediate statistics. Simon Fraser University, British Columbia
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106. doi:10.1016/j.fbr.2007.06.002
Steinrucken TV, Bissett A, Powell JR, Raghavendra AKH, van Klinken RD (2016) Endophyte community composition is associated with dieback occurrence in an invasive tree. Plant Soil 405:311–323. doi:10.1007/s11104-015-2529-y
Stukely MJC, Crane CE (1994) Genetically based resistance of Eucalyptus marginata to Phytophthora cinnamomi. Phytopathology 84:650. doi:10.1094/Phyto-84-650
Tejesvi MV, Mahesh B, Nalini MS, Prakash HS, Kini KR, Subbiah V, Shetty HS (2005) Endophytic fungal assemblages from inner bark and twig of Terminalia arjuna W. & a. (Combretaceae). World J Microbiol Biotechnol 21:1535–1540. doi:10.1007/s11274-005-7579-5
Thorn RG, Scott J, Lachance MA (2007) Methods for studying terrestrial fungal ecology and diversity. In: Reddy CA (ed) Methods for general and molecular microbiology, 3rd edn. ASM Press, Washington, pp 929–950
Thorp JR, Lynch R (2000) The determination of the weeds of National Significance. National Weeds Strategy Executive Committee, Launceston
Toh R (2009) Investigation of fungi pathogenic towards seedlings of Parkinsonia aculeata – their potential for use as mycoherbicides. The University of Queensland
Tsahouridou PC, Thanassoulopoulos CC (2000) First report of Alternaria alternata as a dieback pathogen of kiwifruit. Plant Dis 84:371–371. doi:10.1094/PDIS.2000.84.3.371C
Tukey JW (1949) Comparing individual means in the analysis of variance biometrics 5:99 doi:10.2307/3001913
van Klinken RD, Campbell SD, Heard TA, McKenzie J, March N (2009) The biology of Australian weeds: Parkinsonia aculeata L. Plant Prot Q 24:100–117
Vega FE et al (2010) Fungal endophyte diversity in coffee plants from Colombia, Hawai'i. Mexico Puerto Rico Fungal Ecol 3:122–138. doi:10.1016/j.funeco.2009.07.002
Wapshere AJ (1974) A strategy for evaluating the safety of organisms for biological weed control. Ann Appl Biol 77:201–211. doi:10.1111/j.1744-7348.1974.tb06886.x
Webber JF, Gibbs J, Hendry S (2004) Phytophthora disease of Alder. Forestry Commission, Edinburgh
Westphal A, Xing L, Goodwin SB (2011) Mature watermelon vine decline: suppression with fumigants of a soil-borne problem and association with Rhizopycnis vagum. Crop Prot 30:111–117. doi:10.1016/j.cropro.2010.11.014
Whyte G, Howard K, Hardy GEJ, Burgess T (2016) The tree decline recovery seesaw; a conceptual model of the decline and recovery of drought stressed plantation trees. Forest Ecol Manag 370:102–113. doi:10.1016/j.foreco.2016.03.041
Wilson CG, Pitkethley RN (1992) Botryodiplodia dieback of Mimosa pigra, a noxious weed in northern Australia. Plant Pathol 41:777–779. doi:10.1111/j.1365-3059.1992.tb02563.x
Zwolinski JB, Swart WJ, Wingfield MJ (1990) Economic impact of a post-hail outbreak of dieback induced by Sphaeropsis sapinea. Eur J Forest Pathol 20:405–411. doi:10.1111/j.1439-0329.1990.tb01155.x
Acknowledgements
This research is supported by Meat and Livestock Australia via a technical assistance grant (B.STU.0271), the Australian Government via an Australian Post Graduate Award and the Hawkesbury Institute for the Environment at Western Sydney University. The authors thank Kelli Pukallus (Biosecurity Queensland) for fieldwork assistance, Eva Pôtet (Agro Campus Oest, Paris) and Gio Fichera (CSIRO) for help with the glasshouse trial, Dr. Gavin Hunter, Dr. Luke Barrett (CSIRO), and two anonymous reviewers for helpful comments and suggestions which greatly improved this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinrucken, T.V., Raghavendra, A.K.H., Powell, J.R. et al. Triggering dieback in an invasive plant: endophyte diversity and pathogenicity. Australasian Plant Pathol. 46, 157–170 (2017). https://doi.org/10.1007/s13313-017-0472-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-017-0472-5