Abstract
Perithecia at the base of dead flower stems of pyrethrum (Tanacetum cinerariifolium) plants in yield-decline affected fields of northern Tasmania were identified as Alternaria infectoria and Stemphylium herbarum. Identification was based on morphological description of cultures established from single ascospores; and conidiospores; ascospore shape and septation; and multigene phylogenetic analyses using the internal transcribed spacer (ITS) region, translation elongation factor 1-α (EF1), polymerase II second largest subunit (RPB2) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes. Stemphylium herbarum produced necrotic leaf lesions on both sides of the spray inoculated pyrethrum leaves which coalesced to encompass the entire leaf in in vivo and in vitro experiments. Alternaria infectoria produced small necrotic leaf lesions on both sides of the leaves two weeks after inoculation which did not expand and hence, was considered as a minor pathogen of pyrethrum. This is the first report of A. infectoria and S. herbarum as pathogens of pyrethrum in Australia. The role of A. infectoria and S. herbarum in pyrethrum yield-decline in northern Tasmania needs to be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrethrum, Tanacetum cinerariifolium is commercially used for production of natural pyrethrins (Pethybridge et al. 2008a). Pyrethrins are extracted from the achenes located within pyrethrum flower heads (Ambrizic et al. 2007). Approximately 3000 ha of pyrethrum, accounting for 70% of the world production of pyrethrins is grown in Australia each year (Hay et al. 2015). Pyrethrum seeds are sown in winter and the first harvest occurs in spring of the following year (15 to 17 months after planting). Pyrethrins yield is greatly affected by the foliar and soil-borne pathogens that cause reduced flower production. Ideally, pyrethrum plants can be harvested annually for three to four subsequent years, however, in fields severely affected by pathogens, growth is reduced after the first or the second harvesting season and the crops are then terminated (Hay et al. 2002).
During surveys conducted between April 2015 and September 2016 in northern Tasmania, necrotic leaf lesions, necrotic crown tissues and perithecia at the base of dead flower stems from the previous season’s harvest were consistently observed on pyrethrum plants growing in yield-decline affected sites. Many foliar pathogens of pyrethrum such as Stagonosporopsis tanaceti causing ray blight disease of pyrethrum (Vaghefi et al. 2012), Didymella tanaceti the causal agent of pyrethrum tan spot (Pearce et al. 2015); Colletotrichum tanaceti causing anthracnose disease of pyrethrum (Barimani et al. 2013); Sclerotinia sclerotiorum and S. minor causing sclerotinia flower blight (Scott et al. 2014); winter blight caused by Alternaria tenuissima and pink spot caused by Stemphylium botryosum (Pethybridge et al. 2004); and Botrytis flower blight caused by Botrytis cinerea (Pethybridge et al. 2008a) have been reported on the affected plants.
Alternaria tenuissima was first reported as a pathogen of pyrethrum by Srinath and Sarwar (1965) with necrotic lesions on the apical part of the pyrethrum leaves. Later, winter blight disease of pyrethrum caused by A. tenuissuma and pink spot disease caused by Stemphylium botryosum were reported as foliar pathogens with A. tenuissima producing necrotic leaf spots and S. botryosum brown water-soaked lesions with pink and brown margins on both sides of the leaves (Pethybridge et al. 2004; Pethybridge et al. 2008a). The teleomorph stage of S. botryosum was detected by Pethybridge et al. (2004) on dead plant material and the bases of old flower stems in pyrethrum fields, however, no further studies were undertaken to determine the contribution of this pathogen to pyrethrum yield reduction.
In a survey conducted in 2012 on foliar pathogens of pyrethrum causing necrotic leaf lesions, the isolation frequency of Alternaria spp. declined significantly from 92% of fields in winter to 40% in summer during three sampling times. Isolation frequency of S. botryosum was considerably lower (23% of fields in winter) (Hay et al. 2015). However, Hay et al. (2015) reported a flux in the population of Alternaria spp. and S. botryosum in different seasons of the year. Although these pathogens were mentioned as not being yield-limiting, the frequency of isolation from necrotic leaf lesions of pyrethrum was high (Hay et al. 2015). Pethybridge et al. (2003) frequently isolated A. tenuissima and S. botryosum from necrotic leaf spots of pyrethrum during October and November 2001, emphasizing that the population of these pathogens was higher in winter and spring compared to that in summer.
Alternaria are ubiquitous fungi with different lifestyles from saprophytic to phytopathogenic causing plant disease and post-harvest rots in wide variety of agricultural crops and ornamental plants. Alternaria species also cause human diseases resulting in problems in immunocompromised patients and are responsible for childhood asthma (Lawrence et al. 2013; Woudenberg et al. 2013). Taxonomy of Alternaria species has been changing since Nees first described it in 1816 based on production of multi-celled and melanised club-shaped spores in short or long chains (Woudenberg et al. 2013). Later, Wallroth in 1833 and Preuss in 1851 added two closely related genera of Stemphylium and Ulocladium to the Alternaria species complex based on production of phaeodictyospores = muriform spores (production of both longitudinal and transverse septa) and made the identification more complicated (Lawrence et al. 2013). However, Simmons in 2007 classified 275 species in the Alternaria complex according to morphological differences (Woudenberg et al. 2013). Currently, the Alternaria species complex possesses nine genera: Alternaria, Stemphylium, Chalastospora, Crivellia, Embellisia, Nimbya, Ulocladium, Undifilum and Sinomyces. The genus Alternaria includes eight sections with the isolates forming a sexual stage belonging to A. infectoria (syn. Lewia) species group clading distinctly from other Alternaria sections due to morphological differences in conidiospores and chemical differences in toxin production (Inderbitzin et al. 2009; Lawrence et al. 2013; Woudenberg et al. 2013). Alternaria and Stemphylium are among the most common asexual morphs of the order Pleosporales (Zhang et al. 2012).
Stemphylium species are mostly saprophytic growing on dead plant materials, however, S. botryosum, S. solani, S. vesicarium and S. loti have been known as pathogens of agriculturally important crops and stone fruits (Wang et al. 2009). Simmons (1967) established criteria for morphological identification of various Stemphylium spp. and introduced Pleospora herbarum as teleomorph of S. botryosum. However, Simmons in 1985 subsequently reclassified Stemphylium/Pleospora holomorphs and reported Pleaospora tarda as the sexual stage of S. botryosum and P. herbarum the sexual stage of S. herbarum (Inderbitzin et al. 2009).
The aims of this study were to i) identify the species associated with the perithecia isolated from the base of the pyrethrum dead flower stems using multigene phylogenetic analyses and morphological identification ii) determine the pathogenicity of these species on pyrethrum.
Methods and materials
Sample collection
In a field survey in September 2016 from fields located in Table Cape and Burnie, northern Tasmania, seven plants showing perithecia at the base of dead flower stems were collected and transferred to the laboratory for isolate identification. Pieces (5–10 cm in length) of necrotic flower stem bases containing perithecia were cut from each plant, placed on moisturised filter papers with sterile water in Petri dishes and incubated at room temperature (23–25 °C) for 2 days. Fertile perithecia were then lifted from the surface of the flower stems using a sterilised needle; vortexed in sterilised water and cultured on WA for single sporing. Perithecia were also mounted on microscopic slides with clear lactic acid and gently tapped to release asci for morphological identification.
Five additional Alternaria spp. strains isolated from necrotic leaf lesions of pyrethrum in northern Tasmania were also collected. All other strains used in the study were reference strains derived from the national centre of biotechnology information (NCBI) database at http://www.ncbi.nlm.nih.gov/. A culture of each isolate was sent to the Queensland Plant Pathology Herbarium (BRIP), Brisbane, Australia to obtain culture collection reference numbers.
Taxonomy
Morphological descriptions were made of monosporic isolates grown on synthetic poor nutrient agar (SNA) with pieces of sterilised filter papers (double autoclaved for 20 min at 121 °C) on the surface of the agar as carbon source to induce sporulation (Crous et al. 2009). Cultures were incubated at 21–22 °C under cool white fluorescent light in a 8/16 h regime for 7 days (Woudenberg et al. 2013). Morphological descriptions of ascospores for the species that did not form perithecia on SNA, were made directly from the perithecia that were isolated from flower stems. Slides were prepared with clear lactic acid. Microscopic images were captured using a Leica DM2900 compound microscope with both bright field and differential interference contrast illuminations. Colony growth rate was measured in 2-day intervals for 7 days and pigmentation was rated according to Rayner et al. (1970) color chart. The size of 30 conidiospores and ascospores; and 10 samples of all other structures including asci and conidiophores were measured (Table 1).
Molecular identification
DNA extraction and PCR amplification
Fungal mycelia were scraped directly from 7-day-old single spored cultures on oatmeal agar (OA), DNA extracted using the DNeasy Plant Mini Kit (Qiagen Pty. Ltd., Australia) following the manufacturer’s instruction, and quantified by comparing the band density using Lambda DNA/HindIII Marker (Thermo Ficsher Scientific, Australia) after electrophoresis in 1.5% agarose gel. The ITS region (700 bp) was amplified using primer pairs V9G (de Hoog and Gerrits van den Ende 1998) and ITS4 (White et al. 1990). Approximately 420 bp of the EF1 was amplified by PCR primers EF-728f (Carbone and Kohn 1999) and EF2 (O’Donnell et al. 1998); and 890 bp of the partial RPB2 genes were amplified by PCR primers RPB2-5F2 (Sung et al. 2007) and fRPB2-7cR (Liu et al. 1999). The GAPDH region was amplified using primer pairs gpd1 and gpd2 designed by Berbee et al. (1999). PCR thermal cycler for ITS was performed as described by White et al. (1990), for RPB2 touchdown PCR, for EF1 gradient PCR and for GAPDH were performed according to PCR conditions described in Woudenberg et al. (2013). DNA amplification was carried out using an Eppendorf thermal cycler (Pty. Ltd. Australia). PCR products were then purified using QIAquick PCR purification kit (Qiagen Pty. Ltd. Australia) and sequenced.
Multigene phylogenetic analysis
Purified amplicons were sequenced and sequence intensity was assessed using the Chromas Lite MFS computer package. Isolate sequences were tentatively identified after conducting BLAST searches of the database. Sequences were aligned manually using the sequence alignment editing program MEGA6 (Tamura et al. 2013). Consensus sequences were obtained from both forward and reverse sequences using DeNovo assembly in Geneious v 7.06 (Kearse et al. 2012). Consensus sequences were then pairwise aligned and annotated with the closely related reference sequences derived from the database within Geneious (Table 2). Maximum likelihood (ML) phylogenetic trees were constructed for each gene individually and in combination using MEGA6 software and PhyML (Guindon and Gascuel 2003) within Geneious.
Due to the lack of RPB2 gene sequences for most Stemphylium species, two separate multigene phylogenetic trees including ML combined tree of ITS-EF1-GAPDH-RPB2 for Alternaria and ITS-EF1-GAPDH for Stemphylium were generated. However, Stemphylium species were included in the combined phylogenetic tree constructed for Alternaria, where RPB2 gene sequences were available. In the tree for Alternaria, species of closely related genera to A. infectoria group including species of sections Chalastospora, Ulocladium and Stemphylium were included. Species of section Alternata were also included to determine whether recovered strains isolated from the dead flower stem bases were associated with the sexual stages of A. tenuissima or A. alternata. Moreover, an individual EF1 phylogenetic tree was constructed with all the aforementioned isolates plus the five isolates recovered from necrotic leaf lesions in 2016 to determine if they were anamorphs of A. infectoria. However, no further sequencing was carried out for these isolates.
In the combined tree for Stemphylium spp., species of different groups (Inderbitzin et al. 2009) were included to differentiate various groups in the Stemphylium species complex. The best substitution model was selected using MEGA6 with ML statistical analysis and complete deletion of the gaps. In PhyML analysis, the GTR substitution model was selected. To assess the relative stability of branches, bootstrap analysis with 1000 pseudoreplicates for ML analyses in MEGA6 and 500 pseudoreplicates in PhyML analyses was performed. Gaps were treated as missing data. Consensus sequences were deposited in the database and trees in TreeBASE at www.treebase.org. GenBank accession numbers for the reference markers are shown in Table 2.
Pathogenicity tests
Pathogenicity of A. infectoria and S. herbarum isolated from the dead pyrethrum flower stems was assessed in two trials. For each trial, the pyrethrum seedlings of cultivar Pyrate were germinated from steam sterilised seeds and raised in seedling mix in Tasmania (BRA Pty. Ltd.). Seedlings were transferred to 10 cm diam pots with potting mix, fertilised with 5 g of Osmocote Plus (Scotts Australia Pty. Ltd.) per pot and grown in the glasshouse for two months.
Inoculum was prepared from 5-day-old single spored cultures on SNA by adding 10 mL of sterile water to each plate, gently scraping the colony surface with a glass spreader and then filtering the spore suspension using cheese cloth. The concentration of each spore suspension was quantified to 106 spore/mL using a haemocytometer. Two drops of 0.1% Tween-20 solution was added to each spore suspension (Pethybridge et al. 2008b).
In vivo experiment
To assess pathogenicity of each isolate to produce leaf lesions, three replicates of healthy Pyrate pyrethrum plants were each spray inoculated with 20 mL of 106 spore/mL spore suspension of each isolate of A. infectoria and S. herbarum. A hand sprayer was used to spray the leaves and inoculation was continued until just before runoff. Three control plants were sprayed with sterilised water. Each plant was then covered with a plastic bag to increase humidity after inoculation and induce germination of the spores. The plastic bags were removed after 24 h and the plants were then maintained in the glasshouse until necrotic lesions appeared on the leaves. Necrotic lesions were cultured on potato dextrose agar (PDA) for pathogen identification. Tissue surface sterilisation and culture were carried out by dipping the leaf sections into 80% ethanol for 30 s, 1% active ingredient (ai) sodium hypochlorite for 1 min and then rinsed two times in sterilised water for 1 min. They were then blotted on a sterilised paper towel and small 5 mm2 sections of tissue were cultured onto WA and incubated according to the condition described above. Mycelium was subsequently subcultured onto PDA and incubated for a further 5 days at 23 °C.
In vitro experiment
An in vitro bioassay was undertaken with detached leaves of healthy pyrethrum plants inoculated by placing 106 spore/mL of spore suspension of each species onto the surface sterilised leaves. Controls were treated with sterilised water. Detached leaves were incubated in plastic containers, four leaves per container, with moistened filter papers at 21 °C for eight days and symptoms were assessed after eight days. A Leica DM205 FA stereomicroscope was used to assess the symptoms.
Results
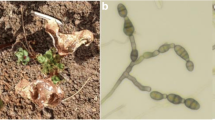
Perithecia isolated from dead flower stems of all the seven plants in 2016 were associated with both A. infectoria and S. herbarum (Fig. 1).
Taxonomy
Detailed taxonomic description of A. infectoria and S. herbarum are shown in Table 1.
Alternaria infectoria: Conidia obclavate in long or short chains predominantly without longitudinal septa and with short branched conidiophores growing in between the conidia. Ascospores muriform, 6–7 transverse septa with 3–4 longitudinal septa in the central segments, end cells without septa. Cylindrical asci with 8 ascospores, straight or somewhat curved (Fig. 2). No perithecia formed on SNA with pieces of filter papers and WA with sterilised flower stems.
Stemphylium herbarum: Oblong-ovoid shaped conidia, light to dark brown with meristemic growth occasionally, multicelled with transverse and longitudinal septa. Conidiophores short and long, branched. Ascospores yellow, 7–9 transverse and many longitudinal septa in central and lateral segments. Cylindrical asci with 8 ascospores, curved or straight (Fig. 3). Perithecia produced on SNA and WA with pieces of sterilised flower stems (Fig. 1b).
Phylogeny
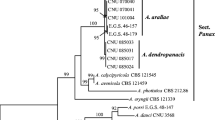
Multilocus combined phylogenetic analyses of ITS-EF1-RPB2-GAPDH; and ITS and RPB2 individual trees (individual trees were not shown) confirmed that A. infectoria (BRIP 65180) claded with the holotype strain A. infectoria (CBS 210.86) within the A. infectoria species group with bootstrap value of 100% (Fig. 4). However, in individual trees of EF1 and GAPDH, the isolate claded with A. conjuncta.
Maximum likelihood PhyML combined phylogenetic tree of ITS-EF1-RPB2-GAPDH for Alternaria and Stemphylium spp. Highest log likelihood −8961.6128. The analysis involved 22 nucleotide sequences. The tree was rooted to Exerohilum pedicellatum BMP0384. Bootstrap values less than 80% were deleted. * refers to strains recovered from the base of the pyrethrum flower stems. Scale bar indicates expected changes per site
Stemphylium herbarum (BRIP 65181) claded with the type strain of S. herbarum (CBS 191.86) in the ITS-EF1-RPB2-GAPDH phylogenetic tree (Fig. 4) with 100% bootstrap value and in ITS-EF1-GAPDH phylogenetic tree (Fig. 5) with 88% bootstrap support confirming that this was a different species to S. botryosum that had been previously reported on pyrethrum. Individual trees showed similar results to the combined phylogenetic trees (individual trees were not shown). Isolate BRIP 65181 clustered closely with related species to Stemphylium spp. group C (S. herbarum group; Inderbitzin et al. 2009) and next to the type strain of S. herbarum (CBS 191.86).
Maximum likelihood PhyML combined phylogenetic tree of ITS-EF1-GAPDH for Stemphylium spp. Highest log likelihood −5364.0913. The analysis involved 19 nucleotide sequences. The tree was rooted to Curvularia australis BRIP 12521. Bootstrap values less than 75% were deleted. * refers to the S. herbarum strain recovered from the base of the pyrethrum flower stems. Group C created according to Inderbitzin et al. (2009). Scale bar indicates expected changes per site
All the five isolates cultured from leaf lesions were identified as A. alternata and clustered in the Alternata species complex in the individual EF1 tree with 98% bootstrap support (tree was not shown). ML bootstrap supports obtained from MEGA6 multigene analysis were similar to those obtained in PhyML analysis.
In the combined maximum likelihood tree of ITS-EF1-RPB2-GAPDH constructed for the species of Alternaria, a total of 2035 (ITS: 510, EF1: 248, RPB2: 829, GAPDH: 448) positions in the final data set were obtained with 1201 constant sites (ITS: 386, EF1: 133, RPB2: 372, GAPDH: 310) and 613 (ITS: 97, EF1: 83, RPB2: 353, GAPDH: 80) parsimony informative sites and 221 uninformative variable characters.
In the combined maximum likelihood tree of ITS-EF1- GAPDH constructed for the species of Stemphylium, a total of 1342 (ITS: 394, EF1: 437, GAPDH: 511) positions in the final data set were obtained with 851 constant sites (ITS: 342, EF1: 150, GAPDH: 359); 376 (ITS: 133, EF1: 116, GAPDH: 127) parsimony informative sites and 115 uninformative variable characters.
Pathogenicity tests
Alternaria infectoria and S. herbarum were isolated from necrotic leaf lesions produced in both in vivo and in vitro experiments. Alternaria infectoria was able to produce small black spots on the surface of the leaves 4 days after inoculation with 106 spore/mL spore suspension in detached leaf bioassay and 2 weeks after inoculation in in vivo glasshouse experiments. Lesions were mostly small with regular margins and did not expand further beyond 14 days after inoculation in both experiments (Fig. 6A).
A Symptoms caused by Alternaria infectoria on pyrethrum in in vivo and in vitro trials. a-c necrotic and black spots produced 4 days after inoculation in vitro on both sides of the leaves. d necrotic lesions produced two weeks after inoculation in vivo. B Symptoms caused by Stemphylium herbarum on pyrethrum in in vivo and in vitro trials. a necrotic spots produced 3 days after inoculation in vitro bioassay b-c necrotic and reddish brown irregular lesions produced 5 days after inoculation in in vivo experiment on both sides of the leaves
Stemphylium herbarum also caused reddish-brown necrotic leaf lesions on both sides of the leaves after 3 days in in vitro and 5 days after inoculation in in vivo bioassays. Lesions expanded to encompass the entire leaf surface (Fig. 6B). No controls were found infected in in vivo and in vitro experiments for both species.
Discussion
Alternaria infectoria and Stemphylium herbarum have been identified as new pathogens of pyrethrum in Australia. Both pathogens were isolated from the perithecia collected from dead pyrethrum flower stems in plants affected by yield-decline syndrome in northern Tasmania.
Alternaria infectoria caused black necrotic lesions on both sides of the leaves in the glasshouse and detached leaf pathogenicity bioassays. However, leaf symptoms on plants in the glasshouse were not severe and developed slowly after 2 weeks. Low isolation frequency of this pathogen from leaf lesions of field plants and morphological similarities of conidia shape and size to other Alternaria species isolated from pyrethrum might have been the main reasons that A. infectoria was not identified in previous field surveys.
Pyrethrum winter blight disease has been reported to be caused by A. tenuissima (Pethybridge et al. 2003; Pethybridge et al. 2008a). Pethybridge et al. (2004) reported that A. tenuissima was a minor pathogen but had the potential to reduce the leaf area and hence affect photosynthesis and flower production.
Perithecia of plant pathogens on dead plant material are important sources of inoculum with ascospores dispersed by wind or water splash (Thomma 2003). However, the sexual morphs for most species of Alternaria have not been identified (Thomma 2003). Isolates of A. alternata from leaf lesions were in the Alternata species complex in which no sexual stage has been recorded. More extensive surveys of dead pyrethrum flower stems and trash need to be undertaken to assess the incidence of A. infectoria. In the study by Hay et al. (2015), different species of Alternaria were reported to be associated with pyrethrum leaf spots but the species were not identified. More investigations need to be carried out on Alternaria spp. as foliar pathogens of pyrethrum in Australia and the role they have in yield-decline of pyrethrum. Isolation of A. alternata was also reported by Pethybridge et al. (2004), however, this was shown to be a saprophyte colonizing the dead pyrethrum plant material and was unable to infect pyrethrum leaves in glasshouse bioassays. Nevertheless, A. alternata has been reported to be pathogenic to chrysanthemum (Pethybridge et al. 2008a).
The A. infectoria species group comprises many taxa which are morphologically differentiated from taxa in other Alternaria sections by the production of shorter and branched conidia (Andersen et al. 2009). Alternaria infectoria is taxonomically distinct from other closely related genera and clustered with other members of the sexual Alternaria lineage (Andersen et al. 2009; Woudenberg et al. 2013). In individual trees of EF1 and GAPDH the isolate from the base of the old flower stems claded with A. conjuncta, however, A. infectoria was morphologically different to A. conjuncta as it produced longer conidiospores and short conidiophores which grow between conidia (Andersen et al. 2009). Nevertheless, morphological characters of taxa within the A. infectoria species group are similar; so molecular phylogenetic studies are required to differentiate many members of the group.
Pathogenicity of S. herbarum on pyrethrum was also confirmed with symptoms very similar to those of S. botryosum (Pethybridge et al. 2004). Stemphylium herbarum is morphologically different to S. botryosum as it produces longer, narrower conidia. However, no information exists about the teleomorph of S. botryosum (syn. P. tarda). Pathogenic Stemphylium spp. survive on the debris of the host plants where perithecia are produced. Perithecia discharge ascospores under suitable environmental conditions. Penetration occurs through the epidermis or preferably stomata of the host plant and then hyphae enter the cells resulting in production of necrotic, brown lesions on the surface of the leaves (Kohl et al. 2009; Ahmed 2014). BLAST searches of the gene sequences of S. herbarum (BRIP 65181) also showed high similarity to S. vesicarium, however, the sexual stage of S. vesicarium is morphologically different to the sexual stage of S. herbarum where the width of asci is smaller and ascospore arrangement within asci is different. Other taxa within group C such as S. alfalfae showed genetic similarity to S. herbarum (Inderbitzin et al. 2009) but were morphologically different to S. herbarum as they produce long conidia and different ascospores with unpredictable longitudinal septa. Species grouped in S. herbarum clade (group C) were closely related and multilocus phylogenetic analyses have not been able to distinguish intra-species relationships, although they resolved new lineages. Hence, Camara et al. (2002) divided Stemphylium species into different groups according to differences in their nucleotide sequences, bootstrap value obtained by Bayesian analyses and phenotypic differences based on asexual spores.
Diseased pyrethrum plants frequently showed leaf lesions on both sides of the leaves and perithecia at the bases of the dead flower stems at different times of the year. This raises the question as to whether S. botryosum that was reported previously as a pathogen of pyrethrum does actually exist in pyrethrum fields of northern Tasmania. Hence, more field surveys and sample collections are needed to identify all Stemphylium spp. and Alternaria spp. existing in the fields and associated with pyrethrum leaf diseases; and to determine their effect on pyrethrum yield reduction.
The symptoms caused by Alternaria spp. and Stemphylium spp. have been reported to be very similar (Pethybridge et al. 2008a). Care must be taken in isolation and identification of the causal agent as many foliar pathogens in pyrethrum fields cause similar symptoms and can be easily confused.
References
Ahmed A (2014) Stemphylium grey leaf spot disease of lupins in Western Australia. PhD, The University of Western Australia
Ambrizic DJ, Kovac M, Zel J, Camloh M (2007) Pyrethrum (Tanacetum cinerariifolium) from the northern Adriatic as a potential source of natural insecticides. ANNALES Ser Hist Nat 39–46
Andersen B, Sorensen JL, Nielsen KF, van den Ende BG, de Hoog S (2009) A polyphasic approach to the taxonomy of the Alternaria infectoria species group. Fungal Genet Biol 46:642–656
Barimani M, Pethybridge SJ, Vaghefi N, Hay FS, Taylor PWJ (2013) A new anthracnose disease of pyrethrum caused by Colletotrichum tanaceti sp. nov. Plant Pathol 62:1248–1257
Berbee ML, Pirseyedi M, Hubbard S (1999) Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964–977
Camara MPS, O'Neill NR, van Berkum P (2002) Phylogeny of Stemphylium spp. based on ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 94:660–672
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Crous PW, Verkley GJM, Groenewald JZ et al (2009) Fungal biodiversity. CBS Laboratory manual series 1. CBS-KNAW Fungal Biodiversity Centre Utrecht, The Netherlands
de Hoog GS, Gerrits van den Ende GH (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41:183–189
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hay F, Stirling GR, Chung B, Groom T (2002) Investigation into the cause of pyrethrum regrowth decline (RPD) with emphasis on the role of plant-parasitic nematodes. Horticulture Australia, Sydney, NSW
Hay F, Gent DH, Pilkington S, Pearce TL, Scott JB, Pethybridge SJ (2015) Changes in distribution and frequency of fungi associated with foliar diseases complex of pyrethrum in Australia. Plant Dis 9:1227–1235
Inderbitzin P, Mehta YR, Berbee ML (2009) Pleospora species with Stemphylium anamorphs: a four locus phylogeny resolves new lineages yet does not distinguish among species in the Pleospora herbarum clade. Mycologia 101:329–339
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinform 28:1647–1649
Kohl J, Groenenboom-de Haas B, Goossen-van de Geijn H, Speksnijder A, Kastelein P, de Hoog S, van den Ende BG (2009) Pathogenicity of Stemphylium vesicarium from different hosts causing brown spot in pear. Eur J Plant Pathol 124:151–162
Lawrence DP, Gannibal PB, Peever TL, Pryor BM (2013) The sections of Alternaria: formalizing species-group concepts. Mycologia 105:530–546
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16:1799–1808
O’Donnell K, Kistler HC, Cigelnik E, Ploetz R (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Natl Acad Sci 97:2044–2049
Pearce TL, Scott JB, Crous PW, Pethybridge SJ, Hay FS (2015) Tan spot of pyrethrum is caused by a Didymella species complex. Plant Pathol 65:1170–1184
Pethybridge SJ, Hay FS, Groom T (2003) Seasonal fluctuations in fungi associated with pyrethrum foliage in Tasmania. Austral Plant Pathol 32:223–230
Pethybridge SJ, Hay FS, Wilson CR (2004) Pathogenicity of fungi commonly isolated from foliar disease in Tasmanian pyrethrum crops. Austral Plant Pathol 33:441–444
Pethybridge SJ, Hay FS, Esker PD, Gent DH, Wilson CR, Groom T, Nutter FW (2008a) Diseases of pyrethrum in Tasmania: challenges and prospects for management. Plant Dis 92:1260–1272
Pethybridge SJ, Jones SJ, Shivas RG, Hay FS, Wilson CR, Groom T (2008b) Tan spot: a new disease of pyrethrum caused by Microsphaeropsis tanaceti sp. nov. Plant Pathol 57:1058–1065
Rayner RW, Commonwealth Mycological Institute (Great Britain), British Mycological Society (1970) A mycological colour chart. Kew, Commonwealth Mycological Institute
Scott JB, Gent DH, Pethybridge SJ, Groom T, Hay FS (2014) Crop damage from sclerotinia crown rot and risk factors in pyrethrum. Plant Dis 98:103–111
Simmons EG (1967) Typification of Alternaria, Stemphylium and Ulocladium. Mycologia 59:67–92
Simmons SG (2001) Perfect states of Stemphylium-VI. Harvard University Herbaria 6(1):199–208
Srinath KV, Sarwar M (1965) Alternaria blight of pyrethrum. Curr Sci 34:295
Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW (2007) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol 44:1204–1223
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2727
Thomma BPHJ (2003) Alternaria spp.: from general saprophyte to specific parasite. Mol Plant Pathol 4:225–236
Vaghefi N, Pethybridge SJ, Ford R, Nicolas ME, Corus PW, Taylor PWJ (2012) Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Austral Plant Pathol 41:675–686
Wang Y, Fu HB, O'Neill NR, Zhang XG (2009) Two new species of Stemphylium from Northwest China. Mycol Prog 8:301–304
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Chapter 38. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, Orlando, pp 315–322
Woudenberg JH, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012) Pleosporales. Fungal Divers 53:1–221
Acknowledgements
Thanks to Botanical Resources Australia- Agricultural Services Pty. Ltd. for providing pyrethrum seedlings and supplementary funding for this project; and to Ruvini Lelwala for isolates of Alternaria sp. Azin Moslemi was supported by a University of Melbourne International Research Scholarship (MIRS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Moslemi, A., Ades, P.K., Groom, T. et al. Alternaria infectoria and Stemphylium herbarum, two new pathogens of pyrethrum (Tanacetum cinerariifolium) in Australia. Australasian Plant Pathol. 46, 91–101 (2017). https://doi.org/10.1007/s13313-016-0463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0463-y