Abstract
Glioblastoma is the most common primary malignant brain tumor in adults and outcomes remain poor despite the current standard of care multimodal therapy. Oncolytic virotherapy utilizes engineered viruses to exert an anti-tumor effect via both direct oncolysis and stimulation of an immune response within the tumor microenvironment, turning tumors from “cold” to “hot.” This has shown promise as a novel therapeutic modality and attempts to circumvent the challenges associated with traditional treatments. Many oncolytic viruses have been investigated in completed and ongoing clinical trials and while safety has been demonstrated, clinical outcomes have been variable, often with only a subgroup of patients showing a significant response. This review summarizes these studies, addresses relevant technical aspects of oncolytic virus administration, and highlights practical considerations to assist providers in appropriately caring for patients treated with oncolytic virotherapy. Additionally, future directions within the field that may help to maximize efficacy of this modality are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overview
Glioblastoma, WHO grade 4, is the most common primary malignant brain tumor in adults, accounting for 48.3% of malignant central nervous system tumors and affecting approximately 13,000 people per year [1]. Despite the current standard of care treatment for newly diagnosed glioblastoma consisting of maximal safe resection, radiation, and temozolomide, outcomes remain poor with a median overall survival (mOS) of approximately 15–21 months [2,3,4,5]. There are very few long-term survivors, and virtually all glioblastomas recur. Following recurrence, there is no standard therapy and mOS is only 8–12 months with current salvage treatment options [6, 7].
Glioblastoma has remained challenging to successfully treat for several reasons. First, the tumor is highly invasive and complete resection at the cellular level is unachievable [8]. Second, glioblastoma cell survival is promoted by an immunosuppressive, or “cold,” tumor microenvironment. The generation of both cell-mediated and humoral immune responses is blunted through the upregulation of immunosuppressive cytokines, myeloid-derived suppressor cells, and regulatory T cells, while concurrent induced T cell exhaustion and the paucity of effective tumor antigens further enhances the ability of the tumor to escape the immune system [9]. In addition, tumorigenic self-renewing glioma stem cells within the tumor microenvironment help to promote tumor initiation, heterogeneity, and recurrence [10]. Importantly, this heterogeneity exists both intratumorally and intertumorally due to the variability in and evolution of the driving molecular features of glioblastoma, which limits the effectiveness of targeted agents and leads to therapeutic resistance as the tumor can utilize other genomic pathways for growth and survival. Lastly, adequate penetration of systemic therapies into the brain is impeded by the blood–brain barrier, which only allows the passage of small, lipophilic molecules across tight junctions [8]. Although there is some breakdown of the blood–brain barrier in parts of the tumor, many of the cells, particularly the invading cells, reside behind an intact blood–brain barrier. Tactics to overcome this include direct delivery of drugs into the region of the tumor through methods such as intratumoral injection or convection-enhanced delivery (CED), and attempts to alter the blood–brain barrier to allow drug passage, such as with osmotic agents, focused ultrasound, or electromagnetic radiation [11]. However, maintaining therapeutic drug levels can be difficult even with such methods due to the upregulation of drug efflux pumps within glioblastoma cells [8].
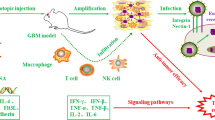
Undoubtedly, novel approaches are needed to improve outcomes in glioblastoma and one such focus is oncolytic virotherapy, which utilizes viruses to exert an anti-tumor effect. Oncolytic viruses can be divided into two broad categories: replication-competent and selectively replication-competent. Selectively replication-competent viruses serve as a platform for gene therapy by acting as viral vectors to introduce specific genes, such as suicide genes, tumor suppressor genes, or immunostimulatory genes, into tumor cells that result in an anti-tumor response when expressed [12]. In contrast, replication-competent viruses infect tumor cells and replicate until the cell lyses, at which time the virus is then able to infect and lyse neighboring cells. The anti-tumor effect in this setting occurs through two mechanisms: (1) direct oncolysis of malignant cells, and (2) subsequent stimulation of an immune response within the tumor microenvironment, thereby turning tumors from immunologically “cold” to “hot” [13]. Following cell lysis, dendritic cells are attracted into the tumor microenvironment and recognize both pathogen-associated molecular patterns and damage-associated molecular patterns on the oncolytic virus and tumor cell, respectively. These tumor-associated antigens (TAAs) are then presented to CD4 + T cells in the lymph nodes, resulting in the maturation of TAA-specific cytotoxic CD8 + T cells, which then migrate to the tumor site and exert cytotoxic activity against malignant cells.
Since its introduction, oncolytic virotherapy has garnered significant interest as a novel therapeutic modality for the treatment of glioblastoma. This review will briefly address relevant technical aspects of oncolytic virus administration, summarize completed clinical trials, introduce ongoing studies and future directions within the field, and discuss practical considerations to assist providers in appropriately caring for patients treated with oncolytic virotherapy.
Technical Considerations
Delivery Methods
Similar to other immunotherapeutic agents under investigation for treatment of high-grade glioma, oncolytic viruses can be delivered via various routes of administration, including systemic administration, direct intratumoral injection, and CED. Intravenous administration negates the need for a neurosurgical procedure, but the efficacy is often impacted due to poor passage of the agent across the blood–brain barrier and the incidence of systemic toxicities is increased. Additionally, there can be increased risk of systemic viral neutralization and off-target sequestration, thereby reducing viral load [14]. In contrast, direct intratumoral injection and CED are local therapies involving drug administration directly into the tumor itself, thereby bypassing the blood–brain barrier and reducing the likelihood of systemic side effects. While direct intratumoral injection depends on concentration gradient-based diffusion of the administered agent, a process which can be impaired by the drug’s molecular size and contribute to neurotoxicity, CED utilizes a “bulk flow” positive pressure gradient which allows for larger distribution volumes and more homogenous drug concentrations irrespective of an agent’s molecular weight [15, 16]. For these reasons, CED has become a promising treatment modality that is being increasingly utilized in oncolytic virotherapy trials. However, these invasive approaches can be limited by tumor location, make repeated doses challenging, and may lead to early viral clearance in the setting of surgical-related inflammation. Other less commonly utilized drug delivery methods also being investigated include endovascular selective intra-arterial administration, a technique that disrupts the blood–brain barrier using hyperosmotic solution to enhance localized delivery of systemically administered agents [17], and stem cell-based carriers which possess a tropism for tumor cells allowing for targeted delivery of agents [18].
Tumor Size and Location
Given the distribution dynamics of intratumorally administered agents, risk of treatment-related edema, and risk of neurologic morbidity, tumor size and location must be considered prior to treating a patient with oncolytic virotherapy. With regard to drug distribution, it has been demonstrated that agents administered by CED will preferentially flow toward areas of least resistance, including white matter tracts, CSF spaces, and regions of pre-existing peritumoral edema, thereby potentially affecting the efficacy of the infused agent [15, 16]. Furthermore, the neurosurgical procedure and inserted catheter can result in cerebral injury, which is then amplified when adding additional volume with CED infusate to the pre-existing mass effect of larger tumors. Edema in regions with little room for the brain to compensate, such as the brainstem or posterior fossa, can lead to neurologic debility, impaired consciousness, or life-threatening herniation, while edema involving eloquent cortex can result in focal neurologic deficits such as aphasia, weakness, or sensory loss. Additionally, cortical edema can increase the risk of new or worsening seizures. For these reasons, most clinical trials will exclude patients with a tumor size greater than 4 × 4 cm in bi-directional dimensions, tumors located in the posterior fossa, or multifocal disease. Tumor location must also be amenable to recommended placement of CED catheters at least 1–2 cm from the subarachnoid spaces and at least 0.5 cm from the ependymal space [15].
Completed Clinical Trials Using Oncolytic Viruses
Numerous oncolytic viruses have been employed in completed and ongoing clinical trials to treat patients with high-grade glioma (Table 1). These studies have been reviewed previously [12, 19,20,21,22] and are additionally summarized in the following sections.
Herpes Simplex Virus-1-Based
Herpes simplex virus-1 (HSV-1) belongs to the Herpesviridae family, which are double-stranded DNA viruses. Several modified strains have been studied in clinical trials for treatment of high-grade glioma as detailed below. Notably, one of the most studied oncolytic viruses is talimogene laherarepvec (TVEC, OncovexGM−CSF, or IMLYGIC), which was the first, and to date only, FDA-approved oncolytic virus with an indication for advanced melanoma [23]. While it has not been investigated in glioblastoma to date, there are many ongoing trials utilizing TVEC in other cancer types.
HSV1716
HSV1716 is a first-generation oncolytic virus modified with deletions of both γ134.5 loci to ensure selective replication within target cells [24]. In the UK, three phase I clinical trials demonstrated overall safety of intratumoral injection either alone or following resection in newly diagnosed or recurrent high-grade glioma [25,26,27]. Of the 33 total patients enrolled, reported adverse events included one patient with transient fever, one patient with worsening focal neurologic symptoms secondary to edema that improved with dexamethasone, and one patient with intracerebral hemorrhage following resection.
G207
A second generation oncolytic virus, HSV G207 contains deletions of both γ134.5 loci and an E. coli lacZ insertion disabling the UL39 gene in order to prevent replication in non-dividing cells [28]. This strain was investigated in an initial phase I study (NCT00036699) via intratumoral injection, followed by a subsequent phase Ib study (NCT00028158) exploring intratumoral injection both before and after resection for recurrent glioblastoma, both of which demonstrated safety without attributable serious adverse events, such as the development of HSV encephalitis [28, 29]. In the latter study, three patients experienced transient fever and focal weakness that either resolved spontaneously or with dexamethasone; headache and nausea were also common side effects. Furthermore, one patient experienced inadvertent oncolytic virus inoculation into the adjacent lateral ventricle. Post-resection tissue demonstrated evidence supporting G207 replication within tumor tissue, as well as an induced immune response. In addition, RNA sequencing of tissue obtained pre-injection and 2 or 5 days after G207 injection demonstrated that patients with a survival benefit had an increased oncolytic HSV-induced type 1 interferon response and a subsequent recruitment of an adaptive immune response [30]. In a third phase I trial (NCT00157703), intratumoral injection of HSV G207 was combined with a single radiation dose and demonstrated tolerability; notably, two patients safely underwent retreatment several months later, supporting the feasibility of multiple treatments [31]. Adverse events included one patient with fever and seizure that resolved spontaneously, one patient with a new CSF leak and subsequent meningitis, and one patient with persistently worsened weakness and neglect following treatment. Most recently, a phase I clinical trial (NCT02457845) investigating HSV G207 with or without a single radiation fraction (5 Gy) in twelve children and adolescents with recurrent supratentorial brain tumors reported the absence of dose-limiting toxicity or serious adverse events attributed to G207 [32]. All adverse events attributed to G207 were grade 1 and were most frequently fever, headache, nausea, vomiting, fatigue, seizure, and hemorrhage due to the surgical catheter implantation. Median OS was 12.2 months [95% confidence interval (CI): 8.0–16.4). An increase in the number of tumor-infiltrating lymphocytes (TILs) post-treatment with G207 comparatively to the pre-infusion sample was observed in four patients who underwent a surgical procedure due to concerns for disease progression.
G47Δ
G47Δ is a third-generation HSV-1 created from G207 by additionally deleting the α47 gene, thereby enhancing viral replication and generation of an immune response through upregulated MHC class I expression [33]. In Japan, a phase I/II trial (UMIN000002661) demonstrated safety of intratumoral injection in patients with recurrent high-grade glioma [34]; in a subsequent phase II study (UMIN000015995), thirteen patients were treated with up to six serial injections and demonstrated a 1-year survival of 92.3% according to interim analysis [35]. Two patients experienced severe adverse events consisting of grade 2 fever. Given these promising results, teserpaturev (DELYTACT®) has received conditional and time-limited approval from the Japan Ministry of Health, Labour, and Welfare and is the first oncolytic virus to receive approval for use in any primary brain cancer [36].
Adenovirus-Based
Belonging to the double-stranded DNA Adenoviridae family, adenovirus has been utilized both as a conventional oncolytic virus and as a vector for gene therapy. Numerous modified strains have been investigated and are reviewed below.
ONYX-015
ONYX-015 is a first-generation chimeric adenovirus type 2/type 5 with an E1B gene deletion to restrict replication to p53-deficient tumor cells [37]. In patients with recurrent high-grade glioma, intratumoral injection was deemed safe in a phase I study without related serious adverse events, although no definite antitumor activity was observed with a median time to progression (mTTP) of 46 days (range 13–452 days) and mOS of 6.2 months (range 1.2–28 months) [38].
DNX-2401 (Formerly Delta-24-RGD)
A second-generation adenovirus type 5, DNX-2401, contains a partial deletion of the E1A gene and insertion of a Arg-Gly-Asp (RGD) sequence in the fiber knob receptor known to interact with integrins present in glioma cells; these modifications restrict replication to Rb-deficient tumor cells and enhance infectivity, respectively [39]. In a phase I study (NCT00805376), patients with recurrent high-grade glioma were treated with either a single intratumoral injection or administration via CED, resection, then injection into the resection cavity. In the latter group, there was evidence of increased cytotoxic T cell infiltration into the tumor; mOS was 13 months and 12% of patients showed a durable complete response [40]. Two patients experienced related adverse events, which included grade 1–2 headache, nausea/vomiting, confusion, and/or fever. A second phase I study (NCT01582516) conducted in the Netherlands also utilized CED in patients with recurrent glioblastoma, but results are not published. In addition, DNX-2401 has been investigated with other agents in combinatory regimens to assess for a synergistic benefit. Administration via intratumoral injection followed by four cycles of temozolomide showed no safety concerns in a phase I study (NCT01956734), with serious adverse events attributed to temozolomide or the underlying tumor; three patients showed durable responses and were still alive at 19, 27, and 30 months post-treatment [41]. In a phase Ib trial (NCT02197169, TARGET-1), patients received intratumoral injection with or without interferon-γ; however, the combinatory regimen was poorly tolerated and showed no clinical benefit over DNX-2401 alone [42]. Lastly, a phase II study (NCT02798406, CAPTIVE) investigated a single intratumoral injection followed by recurrent doses of pembrolizumab; according to an interim report, mOS was 12.5 months and four patients were alive for > 21 months [43]. The most common toxicities included headache, edema, and fatigue.
Adenoviral Vectors for Gene Therapy
AdV-tk is a non-replicating adenoviral vector modified to contain the herpes simplex thymidine kinase gene [44]. Following infection of malignant cells, a combinatory prodrug, valacyclovir or ganciclovir, becomes phosphorylated and incorporates into the glioma cell genome resulting in inhibition of DNA synthesis and repair and subsequent cell death; notably, this effect is enhanced by chemotherapy and radiation. AdV-tk has been investigated in several clinical trials to date. In an initial phase I trial (NCT00002824), AdV-tk was administered by single intratumoral injection in patients with recurrent high-grade glioma and resulted in variable responses with mOS of 4.0 months (range 1.1–29.2 months) [45]. Reported adverse events included three patients with worsening hemiparesis and one patient with perioperative focal seizures secondary to new small intratumoral hemorrhage. Additionally, two patients treated at the highest dose level developed confusion, fever, and hyponatremia; of these, one patient was noted to have intratumoral hemorrhage, while the second patient also developed severe headache with air noted in the ventricular system raising the possibility of inadvertent vector entry into the CSF. AdV-tk was then administered via intra-arterial infusion in a subsequent phase II study (NCT00870181) resulting in mOS and mPFS of 45.4 weeks (95% CI: 0.096–0.444, p = < 0.001) and 29.6 weeks (95% CI: 0.044–0.356, p = < 0.001), respectively [46]. No related serious adverse events were observed; reported toxicities included one patient with mild headache and fever that spontaneously resolved and one patient with grade 1 vasospasm 10 days after treatment. Furthermore, there have been two separate trials (phase Ib, NCT00751270 and phase IIa, NCT00589875) in which AdV-tk was administered at the time of resection followed by standard of care chemoradiation in patients with newly diagnosed high-grade glioma; mOS was 12.4 months [47] and 17.1 months (95% CI: 0.52–0.99) [44], respectively. In the former trial, one patient was reported to experience confusion, hyponatremia, and several weeks of intermittent fever of unclear etiology starting 1 week after treatment that spontaneously resolved. In the latter trial, the most common possibly related adverse events included grade 1–2 fever, fatigue, and headache; additionally, one patient experienced new hemiparesis and progressive dysphasia immediately after surgery that spontaneously resolved. AdV-tk has also been combined with AdV-Flt3L, an immunostimulatory cytokine which results in the proliferation of dendritic cells, and administered at the time of resection in a phase I study (NCT01811992) for patients with newly diagnosed high-grade glioma or ependymoma followed by standard of care chemoradiation. Results are not yet published, although an initial report suggested safety and tolerability of this combination and noted increased inflammatory infiltrate following resection at first recurrence [48]. Lastly, AdV-tk was investigated in a phase I trial (NCT00634231) in children with recurrent high-grade glioma or ependymoma via intratumoral injection at resection, followed by radiation and if indicated, temozolomide. This combination was safely tolerated and resulted in mOS of 25.3 months and mPFS of 8.9 months [49]. Grade 1–2 fever, fatigue, and nausea/vomiting were the most commonly observed adverse events. Another adenoviral vector, AdV-p53, has been engineered to express the tumor suppressor gene p53 and also demonstrated successful cell death and radiosensitization of tumor cells in preclinical models [50]. AdV-p53 has been investigated in two similar phase I trials (NCT00004041 and NCT00004080), although results for the latter are not published. In the former study, patients with recurrent high-grade glioma were treated with intratumoral injection of AdV-p53, followed by resection, then repeat injection into the resection cavity [51]. The treatment was safe and tolerable, and p53 was histopathologically detected in tumor cell nuclei within 5 mm of the injected site. Lastly, Ad-RTS-IL-12 is an adenoviral vector modified to express pro-inflammatory interleukin-12 under the regulation of the gene switch, RheoSwitch Therapeutic System®, which is controlled by the oral activator ligand, veledimex [52]. Ad-RTS-IL-12, administered by post-resection injection into the cavity, with dose-escalated veledimex was investigated in a phase I trial (NCT02026271) in patients with recurrent high-grade glioma; for patients treated at the optimal veledimex dose, mOS was 12.7 months and increased to 17.8 months when steroid dosing was minimized. Additionally, mostly inflammatory infiltrate with increased IFN-γ-producing TILs and PD-1 expression (i.e., pseudoprogression) was confirmed in all patients who underwent re-resection due to suspected recurrence [53]. For the 31 patients enrolled, related grade ≥ 3 adverse events included headache (3 patients), cerebral edema (1 patient), confusional state (1 patient), and aseptic meningitis (1 patient). Symptoms of cytokine release syndrome and lab abnormalities were also observed with veledimex, all of which were reversible with holding or discontinuing the drug.
Reovirus-Based
Reovirus (respiratory enteric orphan virus) is a naturally occurring double-stranded RNA virus that can be isolated from the respiratory and gastrointestinal tracts of humans. REOLYSIN, a wild-type serotype 3 strain that is non-pathogenic to humans, targets the Ras pathway which is commonly upregulated in tumor cells [54]. It was first investigated using single intratumoral injection in a phase I trial in patients with recurrent high-grade glioma; no significant safety concerns were observed, while efficacy outcomes were variable with mOS of 21 weeks (range 6–234 weeks) and mPFS of 4.3 weeks (2.6–39 weeks) [55]. While there were no serious adverse events definitely or probably related to treatment, one patient experienced fever, headache, and nausea/vomiting of unclear etiology 8 days after treatment and another patient experienced fever and nausea/vomiting 4 days after treatment followed by focal seizure and grade 3 weakness attributed to cerebral edema. A second phase I study (NCT00528684) was the first to administer an oncolytic virus via CED in the USA; again, efficacies were variable with mOS of 140 days (range 97–989 days) and mPFS of 61 days (range 29–150 days) [56]. The most common serious adverse event was grade 3 seizure, which was observed in three of five patients and deemed possibly related to treatment in one case. In a phase Ib study assessing the immune effects of intravenously administered REOLYSIN in patients with recurrent high-grade glioma or brain metastases, there was confirmed infection of tumor cells, upregulation of interferon-regulated gene expression and the PD-1/PD-L1 axis, and increased T cell tumor infiltration [54]. Preclinical models demonstrated that REOLYSIN administration followed by PD-1 immune checkpoint inhibition improved survival as compared to monotherapy, suggesting a potential synergistic benefit of such combinatory regimens.
Poliovirus-Based
Belonging to the Piconaviridae family, poliovirus is a positive sense, single-stranded RNA virus. Lerapolturev, formally PVSRIPO, is a live attenuated virus which has been modified with human rhinovirus type 2 regulatory sequences in order to eliminate neurovirulence in non-malignant cells [57, 58]; furthermore, tropism for CD155, an immune checkpoint molecule aberrantly expressed on solid tumor cells, promotes selective infectivity. In a completed phase I trial (NCT01491893) for patients with recurrent glioblastoma, lerapolturev was administered via CED and demonstrated mOS of 12.5 months (95% CI: 9.9–15.2) with OS-24 and OS-36 of 21% [59]. There was one dose-limiting toxicity in which one patient experienced a grade 4 intracerebral hemorrhage immediately after catheter removal that required surgical evacuation; of note, histopathological analysis of tissue obtained at that time did not show evidence of vascular abnormalities, viral activity, or inflammatory events related to lerapolturev infusion. In the dose-expansion phase, the most common adverse events of any grade were headache (52%), hemiparesis (50%), seizure (45%), dysphasia (28%), and cognitive disturbance (25%). Grade 3 or higher adverse events were observed in 19% of these cases with the most common being hemiparesis (8%) and seizure (4%). Subsequently, five patients who demonstrated benefit from the first infusion underwent retreatment with lerapolturev following tumor recurrence and no grade ≥3 adverse events were observed [60]. Four patients received two total infusions and were retreated 72 months, 43 months, 34 months, and 6 months after the first infusion, while one patient was retreated twice 60 months and 78 months after the first infusion. Three patients remained alive for more than 81, 80, and 52 months from the time of first infusion, and two patients died 63 months and 20 months after the first infusion.
Retrovirus/Murine Leukemia Virus-Based
Retroviruses, including the murine leukemia virus, are positive sense, single-stranded RNA viruses whose RNA genome is reverse transcribed into DNA and integrated into the genome of the host cell. Vocimagene amiretrorepvec (Toca 511) is a non-lytic murine leukemia viral vector that has been modified to include the yeast cytosine deaminase gene, which is inserted into the genome of malignant cells once selectively infected [61]. When given in combination, the prodrug, 5-fluorocytosine (Toca FC, an investigational extended-release version), is converted to the chemotherapeutic 5-FU within these cells resulting in both a cytotoxic effect and pro-inflammatory state through the concurrent depletion of myeloid-derived suppressor cells within the tumor microenvironment [61, 62]. Three phase I studies have been completed in recurrent high-grade glioma patients in which Toca 511 alone was administered by intratumoral injection (NCT01156584), intratumoral injection following resection (NCT01470794), or intravenous administration (NCT01985256), all of which demonstrated safety and tolerability [63]. Subgroup analysis was conducted for the phase III-eligible patients (i.e., meeting enrollment criteria and dosing) in NCT01470794 and demonstrated an objective response rate of 21.7% and mOS 14.4 months (95% CI: 11.3–28.1) with complete responders still alive at 33.9–52.2 months [64]. Two dose-limiting toxicities were observed in this study: one patient with grade 3 asthenia possibly related to Toca 511 and one patient with grade 3 normal pressure hydrocephalus deemed unrelated. In the following phase III study (NCT02414165, Toca 5), Toca 511 injection into the resection cavity followed by Toca FC demonstrated no clinical benefit with mOS of 11.1 months, compared to 12.2 months (95% CI: 0.83–1.35; p = 0.62) for the control arm [62]. The adverse event profile was similar between the investigational and standard of care groups; the most common related grade 3–4 adverse events included aphasia (8%), hemiparesis (7.5%), headache (6.5%), and seizure (4%).
Measles-Based
Measles virus, belonging to the Paramixoviridae family, is a negative sense, single-stranded RNA virus. In vivo and in vitro efficacy of measles virus against glioma stem cells has been demonstrated [65]. MV-CEA is derived from the Edmonston lineage, an attenuated strain used in the human measles vaccination, and expresses carcinoembryonic antigen (CEA) as a means of detecting viral gene expression [12, 20]. MV-CEA, which exerts anti-tumor activity through the interaction of fusion and hemagglutinin proteins that have a high affinity for overexpressed CD46 receptors on glioblastoma cells, was investigated in a single phase I trial (NCT00390299) in which two subgroups were safety treated with intratumoral injection either pre- and post-resection or post-resection only; mOS for the two groups was 11.8 (95% CI: 4.3–NA) vs. 11.4 months (95% CI: 4.4–NA), respectively [66].
Newcastle Disease Virus-Based
Newcastle disease virus is a negative sense, single-stranded RNA virus belonging to the Paramyxoviridae family. NDV-HUJ, an attenuated lentogenic strain, was investigated in a phase I trial which demonstrated safety in patients with recurrent glioblastoma when administered intravenously [67]. The only adverse event deemed possibly or probably related to treatment was fever, which was observed in 45% of patients. Seizures were also common, affecting 73% of patients, but these were viewed unrelated or unlikely related to treatment given the absence of change from patients’ baseline seizure frequencies. A different virulent mesogenic strain, MTH-68/H, was safely administered to recurrent glioblastoma patients in a phase I/II study in which a subset of patients survived 5–9 years with repeated intravenous treatment [68].
Parvovirus-Based
Parvovirus is a single-stranded DNA virus belonging to the Parvoviridae family. H-1PV is a rat protoparvovirus which is non-pathogenic in humans and wields a cytotoxic effect by inducing DNA damage and cell-cycle arrest [21]. In a single phase I/IIa trial (NCT01301430, ParvOryx01), no safety concerns were observed when recurrent glioblastoma patients underwent H-1PV administration either by intravenous or intratumoral injection, followed by tumor resection and reinjection into the resection cavity; additionally, there was evidence of viral spread throughout the tumor and related immune activation [69]. Of note, one patient experienced progressively deteriorating consciousness 2 days after treatment with imaging suggestive of hydrocephalus; surgical intervention did not reveal elevated intracranial pressure and no clear etiology or direct treatment-related cause could be determined. The patient never regained consciousness after 6 months and died after removal of life support.
Ongoing Clinical Trials Using Oncolytic Viruses (Table 2)
Herpes Simplex Virus-1-Based
G207
A phase I clinical trial investigating HSV G207 combined with a single radiation dose in children with recurrent cerebellar (NCT03911388) tumors is currently enrolling, while a phase II trial (NCT04482933) investigating a similar treatment strategy in pediatric recurrent high-grade glioma is planned.
rQNestin
This HSV-1 strain has been modified with deletion of both γ134.5 loci, followed by reinsertion of a copy of γ134.5 under the control of a synthetic nestin promoter, which is highly expressed in glioma cells, in order to enhance selective viral replication and propagation [70]. It is being investigated via intratumoral injection during biopsy with or without pre-operative cyclophosphamide in patients with recurrent high-grade glioma in an ongoing phase I trial (NCT03152318).
M032
M032 was engineered to exert a “double-barrel” effect. While it has direct oncolytic activity, it also acts as a gene therapy vector, inducing tumor cells to synthesize and secrete interleukin-12 (IL-12), which in turn enhances the immune response against surrounding tumor cells and provides an anti-angiogenic effect [71]. There is one active phase I trial (NCT02062827) investigating single intratumoral injection in patients with recurrent high-grade glioma.
C134
Developed as a chimeric virus, C134 was modified by deletion of both γ134.5 loci and expression of human cytomegalovirus IRS1 gene in order to enhance viral replication through the restoration of late viral protein synthesis [72]. It is in a phase I trial (NCT03657576) for patients with recurrent high-grade glioma when administered by intratumoral injection.
Adenovirus-Based
DNX-2401
There is an ongoing phase I trial (NCT03896568) investigating allogenic bone-marrow derived mesenchymal stem cells loaded with DNX-2401 in patients with recurrent glioblastoma, while a second phase I study (NCT03178032) is evaluating DNX-2401 in children with newly diagnosed diffuse intrinsic pontine glioma.
DNX-2440 (Formerly Delta-24-RGDOX)
DNX-2440 is an engineered version of DNX-2401 that expresses the immunostimulatory OX40 ligand, which enhances antigen presentation in tumor cells, thereby increasing tumor-specific immunity [73]. A phase I trial (NCT03714334) is ongoing to investigate this modified strain via intratumoral injection in recurrent glioblastoma.
CRAd-S-pk7
CRAd-S-pk7 is an adenovirus type 5 in which the surviving (S) promoter is incorporated to drive E1A gene expression and the fiber knob protein modified to contain a poly-lysine sequence (pk7) in order to improve selectively for and replication within tumor cells [74]. A phase I trial (NCT03072134) is investigating the safety of CRAd-S-pk7 loaded onto neural stem cells administered by intratumoral injection in combination with standard of care chemoradiation in patients with newly diagnosed high-grade glioma.
Adenoviral Vectors for Gene Therapy
AdV-tk is under active investigation in two clinical trials: a phase I/II (NCT03603405) combining AdV-tk and standard of care chemoradiation for newly diagnosed high-grade glioma, and a phase I/II (NCT03596086) combining AdV-tk and radiation with or without standard of care chemotherapy for recurrent high-grade glioma.
Reovirus-Based
REOLYSIN is being investigated in a combinatory regimen with sargramostim (GM-CSF) in pediatric patients with recurrent high-grade brain tumors in an ongoing phase I trial (NCT02444546). It is expected that this combination should stimulate tumor antigen presentation and dendritic cell maturation, thereby enhancing the immune response [75].
Poliovirus-Based
Lerapolturev is actively being investigated in a multicenter, phase II trial (NCT02986178) for patients with recurrent glioblastoma, as well as in a phase Ib trial (NCT03043391) evaluating a single intratumoral injection for children with recurrent malignant glioma. Preliminary results for 120 patients treated in the former study demonstrate mOS 11.5 months (95% CI: 10.4–13.0) and OS-12 and OS-24 of 48% and 17%, respectively, which replicate the results from the initial single-center, phase I study (NCT01491893) [76].
Vaccinia
Belonging to the Poxviridae family, vaccinia is an enveloped double-stranded DNA virus. TG6002 has been modified to express the yeast suicide gene, FCU1, which encodes a fusion protein combining cytosine deaminase and uracil phosphoribosyl transferase that results in the production of chemotherapeutic 5-FU when administered with the prodrug, flucytosine (5-FC) [77]. An active phase I trial (NCT03294486) is investigating this TG6002/flucytosine combination in patients with recurrent glioblastoma.
Practical Guide to Management
Once patients are enrolled on a trial utilizing oncolytic viruses, investigators should be knowledgeable regarding common side effects and approaches to management, expected radiographic responses, and appropriate indications for taking a patient off study.
Clinical Complications and Management
Peritumoral Edema
Peritumoral edema is an expected side effect of oncolytic virotherapy, in part related directly to the intracerebral modes of administration, as well as the secondary immune response generated by the treatment. As a consequence, patients may experience localized neurologic deficits, such as weakness or aphasia; additionally, the risk of new or worsening seizures is increased. New or worsening symptoms should prompt brain imaging to evaluate for edema. Management strategies include both symptom-directed treatment, such as for seizures, and control of the edema itself. While steroids are a commonly utilized treatment for edema and have been shown preclinically to improve CED efficiency when administered pre-infusion [78], high doses of steroids can potentially suppress the immune response generated by oncolytic virotherapy, thereby reducing treatment efficacy, as demonstrated in other immunotherapy trials in glioblastoma [79]. A recommended maximum dexamethasone dose is 4 mg/day for such trials [59]. For persistent, symptomatic edema, bevacizumab, a humanized anti-vascular endothelial growth factor (VEFG) antibody that is FDA-approved for the treatment of recurrent GBM, can be utilized as a steroid-sparing agent. Bevacizumab decreases edema by normalizing decreased vascular permeability and in the post-CED infusion setting, has been used safely at least 2 weeks after completion of oncolytic virotherapy infusion [59].
Cerebral Edema
In cases where cerebral edema is more extensive, symptoms such as headache, altered mental status, and nausea/vomiting may be experienced. Similar evaluation and management strategies as for peritumoral edema should be utilized in cases of cerebral edema as well. In addition, for refractory, life-threatening cases of cerebral edema treatment with osmotic agents or surgical tumor debulking can be considered.
Intracerebral Hemorrhage
Although the risk is low, intracerebral hemorrhage can be observed after tumor resection, direct intratumoral injection, or catheter placement and removal for CED. Patients are often asymptomatic and can be managed with observation only. To limit the risks of bleeding along catheter tracts, some clinical trials utilizing CED now require a platelet count of at least 125,000 prior to catheter insertion [59].
Hydrocephalus
Hydrocephalus has been reported as an adverse event in oncolytic virus clinical trials and may or may not be associated with elevated intracranial pressure. Obstructive hydrocephalus can arise in the setting of cerebral edema secondary to immunotherapeutic treatment effects, while non-obstructive hydrocephalus may occur secondary to proteinorachia following tumor breakdown. Brain imaging can diagnose hydrocephalus, and it can be managed non-operatively or operatively depending on the severity and degree of associated symptoms. Non-operative management options include carbonic anhydrase inhibitors, such as acetazolamide, or low-dose bevacizumab for control of cerebral edema. Operatively, surgical procedures including ventriculoperitoneal shunt placement or endoscopic third ventriculostomy can be performed.
Aseptic Meningitis
Although uncommon, aseptic meningitis can be observed following invasive agent delivery methods including direct intratumoral injection or CED. The risk is further increased with any breach into the cerebrospinal fluid space; therefore, tumor location must be carefully considered prior to treating a patient with oncolytic virotherapy to reduce this risk. If there is clinical or radiographic concern for meningitis, cerebrospinal fluid evaluation should be performed to rule out infectious etiologies as management strategies differ. Aseptic meningitis is typically self-limited and often resolves without specific intervention.
Fever
While intratumoral agent administration reduces the risk of systemic side effects, fever has been observed in numerous clinical trials utilizing oncolytic virotherapy. Typically, fever is transient and resolves spontaneously, and the mechanism may be related to the viral replication and associated immune activation. Before attributing fever to the oncolytic viral therapy, however, other etiologies typically seen with surgical procedures should be eliminated, for example, infectious processes (aspiration pneumonia, urinary tract infection), atelectasis, or thromboembolic event.
Determination of Disease Progression Versus Treatment Effect
One particular challenge frequently encountered following treatment with oncolytic virotherapy is the difficulty in establishing whether changes seen on imaging are due to the immunotherapeutic effect or due to true tumor progression from lack of efficacy. Post-treatment MRIs often demonstrate an increase in fluid-attenuated inversion recovery (FLAIR) signal abnormalities suggestive of an increase in peritumoral edema, while post-contrast images show an increase in lesion size with polycystic degeneration, also known as a “soap bubble” appearance [32, 40, 59]. These findings may persist for several months before a contraction of the area, consistent with treatment response, is observed; in addition, the observation of an increase in number of TILs or other markers of treatment effect in areas sampled due to suspicion for tumor progression suggests that imaging criteria originally proposed to define progressive disease following immunotherapy [80] will need to be adjusted to take into consideration the delayed pattern of immune response following oncolytic viral therapy. Currently, the neuro-oncology community is working to refine these criteria for oncolytic virotherapy specifically. Due to this difficulty, investigators evaluating viral oncolytic therapies have cautioned against initiating new therapies which could ablate the immune response too rapidly and instead recommend proceeding first with mitigating strategies like bevacizumab or surgical debulking of the area of concern.
Future Directions
Oncolytic virotherapy has shown promise as a novel therapeutic approach for the treatment of glioblastoma and attempts to circumvent the challenges associated with conventional treatments, including limited passage of the agent across the blood–brain barrier, a “cold” immunosuppressive tumor microenvironment, and tumor heterogeneity. However, scientific challenges remain, including balancing viral safety with virulence and replicative efficacy, sustaining an optimal anti-tumor inflammatory response without limiting viral spread, and determining the optimal route of delivery. Outcomes of clinical trials to date have been variable, often with only a subgroup of patients demonstrating a durable clinical benefit, suggesting there may be biologic or molecular differences between tumors and/or the tumor microenvironment to account for this, which if identified could be helpful in predicting responders to treatment. Interestingly, one such factor may be tumor mutation burden, as it was recently shown that low tumor mutational burden correlated with longer survival and enriched inflammatory gene signatures after treatment with the genetically modified poliovirus in recurrent glioblastoma patients [81]. Additionally, it is important to note that the majority of completed trials to date have been conducted in recurrent disease, at which time patients are often heavily pretreated with agents (i.e., chemotherapy, corticosteroids) further compounding their immunosuppressive state and limiting the possibility of response to viral oncolytic therapy; efficacy in the upfront setting before exposure to those agents has been only minimally explored and warrants further investigation.
Given the aforementioned challenges and outcomes associated with oncolytic viruses in monotherapy, there has been increasing interest in combinatory regimens that may provide a synergistic therapeutic benefit. First, concurrent oncolytic virus and chemotherapy administration allow for the targeting of separate pathways for cell death, as well as the opportunity to leverage chemotherapy-induced immunomodulation for increased viral replication and spread, such as has been shown with cyclophosphamide preconditioning [82, 83]. Additionally, combinatory regimens may be able to capitalize on the augmentation of traditional resistance mechanisms, such as DNX-2401-induced silencing of the MGMT promotor thereby reestablishing sensitivity to temozolomide [41, 84]. Second, concomitant radiation is of interest given evidence that it can enhance viral infectivity and replication, which was initially demonstrated in preclinical studies investigating oncolytic HSV-1 in lung cancer [85], and afterward in murine U-87 glioma xenografts [86], while the synergistic effect and potential benefit in malignant glioma of the combination of radiation and oncolytic HSV-1 were first suggested in human U-87 malignant glioma xenografts [87]. Oncolytic viruses can also be engineered as radiosensitizers, such as in the case of AdV-p53 and viruses expressing the sodium iodide symporter (NIS), thereby increasing the apoptotic effect of radiation [50, 88]. Third, there is significant interest in the potential synergistic effect of oncolytic viruses and various immunotherapies, such as checkpoint inhibitors, chimeric antigen receptor T (CAR-T) cells, and cancer vaccines, all of which serve to create a pro-inflammatory anti-tumor response. It is theorized that combinatory regimens may promote a sustained response as opposed to that often seen in oncolytic virus monotherapy due to diminished viral replicative capacity and systemic clearance [89]. Regarding immune checkpoint inhibitors, efficacy in glioblastoma has been subpar compared to other solid tumors, in part due to a low density of targetable TILs on which the efficacy of immune checkpoint inhibitors depends. However, the direct oncolysis triggered by oncolytic virotherapy can modulate the tumor microenvironment to attract cytotoxic TILs and upregulate the PD-1/PD-L1 axis, thus priming for immunological checkpoint blockade, as was shown in a window-of-opportunity trial investigating reovirus; additionally, correlative preclinical studies demonstrated improved survival following sequential treatment with reovirus and immune checkpoint inhibition [54]. While CAR-T cells have been approved for the treatment of multiple myeloma, successful targeting by CAR-T cells in brain tumors is hindered by a lack of effective tumor antigens, induced T cell exhaustion, and risks of off-target toxicity; combinatory regimens with CAR-T cells are therefore of interest given that oncolytic viruses can be engineered to selectively express in tumor cells the same antigen used in constructing CAR-T cells for targeting. This has been demonstrated preclinically using an engineered oncolytic vaccinia virus expressing a truncated CD19 protein (OV19t) in combination with CD19-CAR-T cells, which resulted in tumor control and a synergistic positive feedback immunostimulatory loop in various solid tumor mouse models [90]. Ongoing clinical trials are investigating some of these combinatory regimens, the results of which will help further identify promising treatments moving forward.
Finally, clinical challenges remain and emphasize the importance of best management practices in addressing both the symptomatic complications and radiographic changes related to oncolytic virotherapy in order to avoid negating, such as with steroids, or prematurely discontinuing a treatment that may otherwise be beneficial to the patient. Overall, oncolytic virotherapy appears to be a safe and promising therapeutic modality for patients with glioblastoma, and optimizing delivery of the agent, multimodal regimens, patient selection, and clinical management practices are all necessary for maximizing its potential.
References
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21:v1–100. https://doi.org/10.1093/neuonc/noz150.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. https://doi.org/10.1056/NEJMoa1308345.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. https://doi.org/10.1056/NEJMoa1308573.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. https://doi.org/10.1056/NEJMoa043330.
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–16. https://doi.org/10.1001/jama.2017.18718.
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–53. https://doi.org/10.1016/S1470-2045(14)70314-6.
van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AME, Vandertop WP, van de Ven PM, Wagemakers M, van der Weide HL, Enting RH, Walenkamp AME, Verheul HMW. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135:183–92. https://doi.org/10.1007/s11060-017-2564-z.
Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L, Wu Y, Daldrup-Link HE. Glioblastoma multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021;171:105780. https://doi.org/10.1016/j.phrs.2021.105780.
Humphries W, Wei J, Sampson JH, Heimberger AB. The role of tregs in glioma-mediated immunosuppression: potential target for intervention. Neurosurg Clin N Am. 2010;21:125–37. https://doi.org/10.1016/j.nec.2009.08.012.
Bernstock JD, Mooney JH, Ilyas A, Chagoya G, Estevez-Ordonez D, Ibrahim A, Nakano I. Molecular and cellular intratumoral heterogeneity in primary glioblastoma: clinical and translational implications. J Neurosurg. 2019. https://doi.org/10.3171/2019.5.JNS19364.
Mo F, Pellerino A, Soffietti R, Ruda R. Blood-brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222312654.
Estevez-Ordonez D, Chagoya G, Salehani A, Atchley TJ, Laskay NMB, Parr MS, Elsayed GA, Mahavadi AK, Rahm SP, Friedman GK, Markert JM. Immunovirotherapy for the treatment of glioblastoma and other malignant gliomas. Neurosurg Clin N Am. 2021;32:265–81. https://doi.org/10.1016/j.nec.2020.12.008.
Achard C, Surendran A, Wedge ME, Ungerechts G, Bell J, Ilkow CS. Lighting a fire in the tumor microenvironment using oncolytic immunotherapy. EBioMedicine. 2018;31:17–24. https://doi.org/10.1016/j.ebiom.2018.04.020.
Moaven O, Mangieri CW, Stauffer JA, Anastasiadis PZ, Borad MJ. Evolving role of oncolytic virotherapy: challenges and prospects in clinical practice. JCO Precis Oncol. 2021. https://doi.org/10.1200/PO.20.00395.
D’Amico RS, Aghi MK, Vogelbaum MA, Bruce JN. Convection-enhanced drug delivery for glioblastoma: a review. J Neurooncol. 2021;151:415–27. https://doi.org/10.1007/s11060-020-03408-9.
Healy AT, Vogelbaum MA. Convection-enhanced drug delivery for gliomas. Surg Neurol Int. 2015;6:S59-67. https://doi.org/10.4103/2152-7806.151337.
Srinivasan VM, Lang FF, Kan P. Intraarterial delivery of virotherapy for glioblastoma. Neurosurg Focus. 2021;50:E7. https://doi.org/10.3171/2020.11.FOCUS20845.
Zhang Q, Xiang W, Yi DY, Xue BZ, Wen WW, Abdelmaksoud A, Xiong NX, Jiang XB, Zhao HY, Fu P. Current status and potential challenges of mesenchymal stem cell-based therapy for malignant gliomas. Stem Cell Res Ther. 2018;9:228. https://doi.org/10.1186/s13287-018-0977-z.
Banerjee K, Nunez FJ, Haase S, McClellan BL, Faisal SM, Carney SV, Yu J, Alghamri MS, Asad AS, Candia AJN, Varela ML, Candolfi M, Lowenstein PR, Castro MG. Current approaches for glioma gene therapy and virotherapy. Front Mol Neurosci. 2021;14:621831. https://doi.org/10.3389/fnmol.2021.621831.
Immidisetti AV, Nwagwu CD, Adamson DC, Patel NV, Carbonell AM. Clinically explored virus-based therapies for the treatment of recurrent high-grade glioma in adults. Biomedicines. 2021. https://doi.org/10.3390/biomedicines9020138.
Rius-Rocabert S, Garcia-Romero N, Garcia A, Ayuso-Sacido A, Nistal-Villan E. Oncolytic virotherapy in glioma tumors. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21207604.
Shoaf ML, Peters KB. Clinical trials of oncolytic viruses in glioblastoma. Adv Oncol. 2022.
Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum Vaccin Immunother. 2018;14:839–46. https://doi.org/10.1080/21645515.2017.1412896.
McKie EA, MacLean AR, Lewis AD, Cruickshank G, Rampling R, Barnett SC, Kennedy PG, Brown SM. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours–evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74:745–52. https://doi.org/10.1038/bjc.1996.431.
Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, Hadley D, Patterson J, Brown SM, Rampling R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–58. https://doi.org/10.1038/sj.gt.3302289.
Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, Harland J, Mabbs R, Brown M. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. https://doi.org/10.1038/sj.gt.3301664.
Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E, Mabbs R, Brown M. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–66. https://doi.org/10.1038/sj.gt.3301184.
Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. https://doi.org/10.1038/sj.gt.3301205.
Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Gillespie GY. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. https://doi.org/10.1038/mt.2008.228.
Miller KE, Cassady KA, Roth JC, Clements J, Schieffer KM, Leraas K, Miller AR, Prasad N, Leavenworth JW, Aban IB, Whitley RJ, Gillespie GY, Mardis ER, Markert JM. Immune activity and response differences of oncolytic viral therapy in recurrent glioblastoma: gene expression analyses of a phase IB study. Clin Cancer Res. 2022;28:498–506. https://doi.org/10.1158/1078-0432.CCR-21-2636.
Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M, Nabors LB, Markiewicz M, Agee BS, Coleman JM, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Weichselbaum RR, Fiveash JB, Gillespie GY. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014;22:1048–55. https://doi.org/10.1038/mt.2014.22.
Friedman GK, Johnston JM, Bag AK, Bernstock JD, Li R, Aban I, Kachurak K, Nan L, Kang KD, Totsch S, Schlappi C, Martin AM, Pastakia D, McNall-Knapp R, Farouk Sait S, Khakoo Y, Karajannis MA, Woodling K, Palmer JD, Osorio DS, Leonard J, Abdelbaki MS, Madan-Swain A, Atkinson TP, Whitley RJ, Fiveash JB, Markert JM, Gillespie GY. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N Engl J Med. 2021;384:1613–22. https://doi.org/10.1056/NEJMoa2024947.
Todo T. Oncolytic virus therapy using genetically engineered herpes simplex viruses. Front Biosci. 2008;13:2060–4. https://doi.org/10.2741/2823.
Taguchi S, Fukuhara H, Todo T. Oncolytic virus therapy in Japan: progress in clinical trials and future perspectives. Jpn J Clin Oncol. 2019;49:201–9. https://doi.org/10.1093/jjco/hyy170.
Todo T. ATIM-14. Results of phase II clinical trial of oncolytic herpes virus G47Δ in patients with glioblastoma. Neuro Oncol. 2019;21:vi4. https://doi.org/10.1093/neuonc/noz175.014.
Daiichi-Sankyo. DELYTACT® oncolytic virus G47∆ approved in Japan for treatment of patients with malignant glioma. 2021. https://www.daiichisankyo.com/files/news/pressrelease/pdf/202106/20210611_E_47.pdf. Accessed 2021.
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6. https://doi.org/10.1126/science.274.5286.373.
Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K, Rosenblum M, Mikkelsen T, Olson J, Markert J, Rosenfeld S, Nabors LB, Brem S, Phuphanich S, Freeman S, Kaplan R, Zwiebel J. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–66. https://doi.org/10.1016/j.ymthe.2004.07.021.
Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–6.
Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, Gumin J, Vence LM, Wistuba I, Rodriguez-Canales J, Villalobos PA, Dirven CMF, Tejada S, Valle RD, Alonso MM, Ewald B, Peterkin JJ, Tufaro F, Fueyo J. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36:1419–27. https://doi.org/10.1200/JCO.2017.75.8219.
Alonso MM, García-Moure M, Gonzalez-Huarriz M, Marigil M, Hernandez-Alcoceba R, Buñales M, Hervás S, Gallego J, Gomez-Manzano C, Fueyo J, Lang F, Peterkin J, Diez-Valle R, Tejada S. Abstract CT027: Oncolytic virus DNX-2401 with a short course of temozolomide for glioblastoma at first recurrence: clinical data and prognostic biomarkers. Cancer Res. 2017;77:CT027. https://doi.org/10.1158/1538-7445.AM2017-CT027.
Lang FF, Tran ND, Puduvalli VK, Elder JB, Fink KL, Conrad CA, Yung WKA, Penas-Prado M, Gomez-Manzano C, Peterkin J, Fueyo J. Phase 1b open-label randomized study of the oncolytic adenovirus DNX-2401 administered with or without interferon gamma for recurrent glioblastoma. J Clin Oncol. 2017;35:2002–2002. https://doi.org/10.1200/JCO.2017.35.15_suppl.2002.
Zadeh G, Daras M, Cloughesy TF, Colman H, Kumthekar PU, Chen CC, Aiken R, Groves MD, Ong S, Ramakrishna R, Vogelbaum MA, Khagi S, Kaley T, Melear JM, Peereboom DM, Rodriguez A, Yankelevich M, Nair SG, Puduvalli VK, Nassiri F, Sonabend AM, Agensky L, Ewald B, Levisetti M, Lang FF. LTBK-04. Phase 2 multicenter study of the oncolytic adenovirus DNX-2401 (TASADENOTUREV) in combination with pembrolizumab for recurrent glioblastoma; captive study (KEYNOTE-192). Neuro Oncol. 2020;22:ii237. https://doi.org/10.1093/neuonc/noaa215.989.
Wheeler LA, Manzanera AG, Bell SD, Cavaliere R, McGregor JM, Grecula JC, Newton HB, Lo SS, Badie B, Portnow J, Teh BS, Trask TW, Baskin DS, New PZ, Aguilar LK, Aguilar-Cordova E, Chiocca EA. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18:1137–45. https://doi.org/10.1093/neuonc/now002.
Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, Hamilton WJ, Rojas-Martinez A, Chen SH, Woo SL, Grossman RG. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. https://doi.org/10.1006/mthe.2000.0030.
Ji N, Weng D, Liu C, Gu Z, Chen S, Guo Y, Fan Z, Wang X, Chen J, Zhao Y, Zhou J, Wang J, Ma D, Li N. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget. 2016;7:4369–78. https://doi.org/10.18632/oncotarget.6737.
Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R, McGregor J, Lo S, Ray-Chaudhuri A, Chakravarti A, Grecula J, Newton H, Harris KS, Grossman RG, Trask TW, Baskin DS, Monterroso C, Manzanera AG, Aguilar-Cordova E, New PZ. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–9. https://doi.org/10.1200/JCO.2011.35.5222.
Lowenstein PR, Orringer DA, Sagher O, Heth J, Hervey-Jumper SL, Mammoser AG, Junck L, Leung D, Umemura Y, Lawrence TS, Kim MM, Wahl DR, McKeever P, Camelo-Piragua SI, Lieberman A, Venneti S, Comba A, Altshuler D, Muraszko K, Castro M. First-in-human phase I trial of the combination of two adenoviral vectors expressing HSV1-TK and FLT3L for the treatment of newly diagnosed resectable malignant glioma: Initial results from the therapeutic reprogramming of the brain immune system. J Clin Oncol. 2019;37:2019–2019. https://doi.org/10.1200/JCO.2019.37.15_suppl.2019.
Kieran MW, Goumnerova L, Manley P, Chi SN, Marcus KJ, Manzanera AG, Polanco MLS, Guzik BW, Aguilar-Cordova E, Diaz-Montero CM, DiPatri AJ, Tomita T, Lulla R, Greenspan L, Aguilar LK, Goldman S. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol. 2019;21:537–46. https://doi.org/10.1093/neuonc/noy202.
Badie B, Kramar MH, Lau R, Boothman DA, Economou JS, Black KL. Adenovirus-mediated p53 gene delivery potentiates the radiation-induced growth inhibition of experimental brain tumors. J Neurooncol. 1998;37:217–22. https://doi.org/10.1023/a:1005924925149.
Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR, Chandler W, Zwiebel JA, Kaplan RS, Yung WK. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–18. https://doi.org/10.1200/JCO.2003.21.13.2508.
Barrett JA, Cai H, Miao J, Khare PD, Gonzalez P, Dalsing-Hernandez J, Sharma G, Chan T, Cooper LJN, Lebel F. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System((R)) (RTS((R))) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 2018;25:106–16. https://doi.org/10.1038/s41417-018-0019-0.
Chiocca EA, Yu JS, Lukas RV, Solomon IH, Ligon KL, Nakashima H, Triggs DA, Reardon DA, Wen P, Stopa BM, Naik A, Rudnick J, Hu JL, Kumthekar P, Yamini B, Buck JY, Demars N, Barrett JA, Gelb AB, Zhou J, Lebel F, Cooper LJN. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aaw5680.
Samson A, Scott KJ, Taggart D, West EJ, Wilson E, Nuovo GJ, Thomson S, Corns R, Mathew RK, Fuller MJ, Kottke TJ, Thompson JM, Ilett EJ, Cockle JV, van Hille P, Sivakumar G, Polson ES, Turnbull SJ, Appleton ES, Migneco G, Rose AS, Coffey MC, Beirne DA, Collinson FJ, Ralph C, Alan Anthoney D, Twelves CJ, Furness AJ, Quezada SA, Wurdak H, Errington-Mais F, Pandha H, Harrington KJ, Selby PJ, Vile RG, Griffin SD, Stead LF, Short SC, Melcher AA. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aam7577.
Forsyth P, Roldan G, George D, Wallace C, Palmer CA, Morris D, Cairncross G, Matthews MV, Markert J, Gillespie Y, Coffey M, Thompson B, Hamilton M. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–32. https://doi.org/10.1038/sj.mt.6300403.
Kicielinski KP, Chiocca EA, Yu JS, Gill GM, Coffey M, Markert JM. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther. 2014;22:1056–62. https://doi.org/10.1038/mt.2014.21.
Gromeier M, Nair SK. Recombinant poliovirus for cancer immunotherapy. Annu Rev Med. 2018;69:289–99. https://doi.org/10.1146/annurev-med-050715-104655.
Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, Gromeier M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol. 2004;6:208–17. https://doi.org/10.1215/S1152851703000577.
Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379:150–61. https://doi.org/10.1056/NEJMoa1716435.
Desjardins A, Gromeier M, Herndon JE, Randazzo D, Threatt S, Lipp ES, Miller ES, Jackman J, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Peters KB, Johnson MO, Sampson JH, Ashley DM, Bigner DD. Oncolytic polio/rhinovirus recombinant (PVSRIPO) against WHO grade IV malignant glioma (MG): experience with retreatment of survivors from the phase I trial. J Clin Oncol. 2019;37:2060–2060. https://doi.org/10.1200/JCO.2019.37.15_suppl.2060.
Perez OD, Logg CR, Hiraoka K, Diago O, Burnett R, Inagaki A, Jolson D, Amundson K, Buckley T, Lohse D, Lin A, Burrascano C, Ibanez C, Kasahara N, Gruber HE, Jolly DJ. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20:1689–98. https://doi.org/10.1038/mt.2012.83.
Cloughesy TF, Petrecca K, Walbert T, Butowski N, Salacz M, Perry J, Damek D, Bota D, Bettegowda C, Zhu JJ, Iwamoto F, Placantonakis D, Kim L, Elder B, Kaptain G, Cachia D, Moshel Y, Brem S, Piccioni D, Landolfi J, Chen CC, Gruber H, Rao AR, Hogan D, Accomando W, Ostertag D, Montellano TT, Kheoh T, Kabbinavar F, Vogelbaum MA. Effect of vocimagene amiretrorepvec in combination with flucytosine vs standard of care on survival following tumor resection in patients with recurrent high-grade glioma: a randomized clinical trial. JAMA Oncol. 2020;6:1939–46. https://doi.org/10.1001/jamaoncol.2020.3161.
Aghi M, Vogelbaum M, Kalkanis S, Bota D, Piccioni D, Elder B, Engh J, Kaptain G, Landolfi J, Cloughesy T. OS09.6 Long-term follow-up data from 126 patients with recurrent high grade glioma from three phase 1 trials of Toca 511 and Toca FC: update and justification for a phase 2/3 trial. Neuro Oncol. 2017;19:iii19. https://doi.org/10.1093/neuonc/nox036.065.
Cloughesy TF, Landolfi J, Vogelbaum MA, Ostertag D, Elder JB, Bloomfield S, Carter B, Chen CC, Kalkanis SN, Kesari S, Lai A, Lee IY, Liau LM, Mikkelsen T, Nghiemphu P, Piccioni D, Accomando W, Diago OR, Hogan DJ, Gammon D, Kasahara N, Kheoh T, Jolly DJ, Gruber HE, Das A, Walbert T. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018;20:1383–92. https://doi.org/10.1093/neuonc/noy075.
Allen C, Opyrchal M, Aderca I, Schroeder MA, Sarkaria JN, Domingo E, Federspiel MJ, Galanis E. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2013;20:444–9. https://doi.org/10.1038/gt.2012.62.
ClinicalTrials.gov identifier: NCT00390299. Viral therapy in treating patients with recurrent glioblastoma multiforme. 2020.
Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, Rozenman-Yair S, Panet A, Libson E, Irving CS, Galun E, Siegal T. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221–8. https://doi.org/10.1016/j.ymthe.2005.08.016.
Csatary LK, Gosztonyi G, Szeberenyi J, Fabian Z, Liszka V, Bodey B, Csatary CM. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67:83–93. https://doi.org/10.1023/b:neon.0000021735.85511.05.
Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, Neumann JO, Schoning T, Husing J, Beelte B, Kiprianova I, Roscher M, Bhat R, von Deimling A, Bruck W, Just A, Frehtman V, Lobhard S, Terletskaia-Ladwig E, Fry J, Jochims K, Daniel V, Krebs O, Dahm M, Huber B, Unterberg A, Rommelaere J. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther. 2017;25:2620–34. https://doi.org/10.1016/j.ymthe.2017.08.016.
Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–9. https://doi.org/10.1158/0008-5472.CAN-04-3227.
Patel DM, Foreman PM, Nabors LB, Riley KO, Gillespie GY, Markert JM. Design of a phase I clinical trial to evaluate M032, a genetically engineered HSV-1 expressing IL-12, in patients with recurrent/progressive glioblastoma multiforme, anaplastic astrocytoma, or gliosarcoma. Hum Gene Ther Clin Dev. 2016;27:69–78. https://doi.org/10.1089/humc.2016.031.
Shah AC, Parker JN, Gillespie GY, Lakeman FD, Meleth S, Markert JM, Cassady KA. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Ther. 2007;14:1045–54. https://doi.org/10.1038/sj.gt.3302942.
Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, Fueyo J. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77:3894–907. https://doi.org/10.1158/0008-5472.CAN-17-0468.
Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, Curiel DT, Lesniak MS. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. https://doi.org/10.1089/hum.2007.002.
Kemp V, van den Wollenberg DJM, Camps MGM, van Hall T, Kinderman P, Pronk-van Montfoort N, Hoeben RC. Arming oncolytic reovirus with GM-CSF gene to enhance immunity. Cancer Gene Ther. 2019;26:268–81. https://doi.org/10.1038/s41417-018-0063-9.
Desjardins A, Gromeier M, Friedman H, Landi D, Friedman A, Ashley DM, Curry W, Butowski N, Sloan A, Wen P, Cavaliere R, Gerstner E, Berger M, Buerki R, Chiocca EA, Sauvageau E, Kelly A, Nichols WG, Mixson L, Dickinson A, Orr K, Learn C, Bigner D. IMMU-26. Safety and efficacy of pvsripo in recurrent glioblastoma: long-term follow-up and initial multicenter results. Neuro Oncol. 2021;23:vi97. https://doi.org/10.1093/neuonc/noab196.385.
Foloppe J, Kempf J, Futin N, Kintz J, Cordier P, Pichon C, Findeli A, Vorburger F, Quemeneur E, Erbs P. The enhanced tumor specificity of TG6002, an armed oncolytic vaccinia virus deleted in two genes involved in nucleotide metabolism. Mol Ther Oncolytics. 2019;14:1–14. https://doi.org/10.1016/j.omto.2019.03.005.
Yang X, Saito R, Nakamura T, Zhang R, Sonoda Y, Kumabe T, Forsayeth J, Bankiewicz K, Tominaga T. Peri-tumoral leakage during intra-tumoral convection-enhanced delivery has implications for efficacy of peri-tumoral infusion before removal of tumor. Drug Deliv. 2016;23:781–6. https://doi.org/10.3109/10717544.2014.914987.
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bahr O, Phuphanich S, Sepulveda JM, De Souza P, Sahebjam S, Carleton M, Tatsuoka K, Taitt C, Zwirtes R, Sampson J, Weller M. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–10. https://doi.org/10.1001/jamaoncol.2020.1024.
Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16:e534–42. https://doi.org/10.1016/S1470-2045(15)00088-1.
Gromeier M, Brown MC, Zhang G, Lin X, Chen Y, Wei Z, Beaubier N, Yan H, He Y, Desjardins A, Herndon JE II, Varn FS, Verhaak RG, Zhao J, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Lipp ES, Nair SK, Khasraw M, Peters KB, Randazzo D, Sampson JH, McLendon RE, Bigner DD, Ashley DM. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun. 2021;12:352. https://doi.org/10.1038/s41467-020-20469-6.
Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M, Selby P, Melcher A, Harrington K, Vile RG. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res. 2009;15:561–9. https://doi.org/10.1158/1078-0432.CCR-08-1688.
Pol JG, Atherton MJ, Stephenson KB, Bridle BW, Workenhe ST, Kazdhan N, McGray AR, Wan Y, Kroemer G, Lichty BD. Enhanced immunotherapeutic profile of oncolytic virus-based cancer vaccination using cyclophosphamide preconditioning. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-000981.
Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–504. https://doi.org/10.1158/0008-5472.CAN-07-5312.
Adusumilli PS, Stiles BM, Chan MK, Chou TC, Wong RJ, Rusch VW, Fong Y. Radiation therapy potentiates effective oncolytic viral therapy in the treatment of lung cancer. Ann Thorac Surg. 2005;80:409–17. https://doi.org/10.1016/j.athoracsur.2005.01.048.
Advani SJ, Markert JM, Sood RF, Samuel S, Gillespie GY, Shao MY, Roizman B, Weichselbaum RR. Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther. 2011;18:1098–102. https://doi.org/10.1038/gt.2011.61.
Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, Whitley RJ, Markert JM, Roizman B, Weichselbaum RR. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–22.
Penheiter AR, Russell SJ, Carlson SK. The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies. Curr Gene Ther. 2012;12:33–47. https://doi.org/10.2174/156652312799789235.
Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18:498–513. https://doi.org/10.1038/s41577-018-0014-6.
Park AK, Fong Y, Kim SI, Yang J, Murad JP, Lu J, Jeang B, Chang WC, Chen NG, Thomas SH, Forman SJ, Priceman SJ. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med. 2020. https://doi.org/10.1126/scitranslmed.aaz1863.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shoaf, M.L., Desjardins, A. Oncolytic Viral Therapy for Malignant Glioma and Their Application in Clinical Practice. Neurotherapeutics 19, 1818–1831 (2022). https://doi.org/10.1007/s13311-022-01256-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01256-1