Abstract
Introduction
Convection-enhanced delivery (CED) is a method of targeted, local drug delivery to the central nervous system (CNS) that bypasses the blood-brain barrier (BBB) and permits the delivery of high-dose therapeutics to large volumes of interest while limiting associated systemic toxicities. Since its inception, CED has undergone considerable preclinical and clinical study as a safe method for treating glioblastoma (GBM). However, the heterogeneity of both, the surgical procedure and the mechanisms of action of the agents studied—combined with the additional costs of performing a trial evaluating CED—has limited the field’s ability to adequately assess the durability of any potential anti-tumor responses. As a result, the long-term efficacy of the agents studied to date remains difficult to assess.
Materials and methods
We searched PubMed using the phrase “convection-enhanced delivery and glioblastoma”. The references of significant systematic reviews were also reviewed for additional sources. Articles focusing on physiological and physical mechanisms of CED were included as well as technological CED advances.
Results
We review the history and principles of CED, procedural advancements and characteristics, and outcomes from key clinical trials, as well as discuss the potential future of this promising technique for the treatment of GBM.
Conclusion
While the long-term efficacy of the agents studied to date remains difficult to assess, CED remains a promising technique for the treatment of GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Convection-enhanced delivery (CED) is a promising method of targeted, local drug delivery to the central nervous system (CNS). CED uses the stereotactic placement of one or more catheters within an area of interest for the direct infusion of therapeutics. These catheters are connected to pumps which provide a continuous, positive-pressure micro-infusion of the desired agents through the target tissues via principles of ‘bulk flow’ [1, 2]. Importantly, direct infusion via CED bypasses the challenge posed by the blood–brain barrier (BBB) to drug distribution within the CNS when administered via intravenous (IV) or oral delivery methods (Fig. 1). Similarly, by capitalizing on the restrictive function of the BBB, CED permits treatment with higher doses of therapeutic agents to the CNS while eliminating dose-related systemic toxicities [3].
Overview of CED. Direct infusion via CED bypasses the challenge posed by the BBB to drug distribution within the CNS when administered via intravenous (IV) or oral delivery methods. The BBB is comprised of continuous tight and adherens junctions along cerebral capillary endothelial cells buttressed by astrocyte end-foot processes and functions to protect the CNS from infections and toxic substances. The BBB also limits the distribution of many anticancer agents. CED uses stereotactically placed catheters, connected to mechanical pumps to provide a continuous, positive-pressure micro-infusion of desired agents through target tissues
Since its conceptualization, there has been considerable preclinical and clinical interest in incorporating CED for the treatment of diffusely infiltrating gliomas. Clinical studies to date have focused extensively on the treatment of high-grade gliomas (HGGs), and specifically glioblastoma (GBM) as current treatment standards remain inadequate and tumor recurrence is invariable. Even after safe, maximal resection and use of adjuvant chemotherapy and radiation, the complex molecular heterogeneity and diffusely infiltrative nature of these tumors continues to limit survival [4,5,6,7,8,9,10]. Furthermore, the challenges of the BBB limit potentially effective therapeutics from reaching the CNS.
CED offers the unique ability to treat large brain volumes, including both radiographically defined regions of tumor and surrounding infiltrated brain tissues, and to expand the armamentarium of potentially effective therapeutic agents. This review provides an up-to-date summary of the results of clinical trials using CED for the treatment of GBM and discusses variations in the published techniques.
Background
In 1994, a group of clinicians and engineers led by neurosurgeon Edward Oldfield, published the first paper describing CED for the local delivery of drugs that were either limited by the BBB or too large to diffuse effectively [1]. Research at the time had become very interested in bypassing the BBB to expand the arsenal of potentially effective treatments for CNS diseases. CED offered an alternative to the diffusion-mediated delivery systems being evaluated. Diffusely infiltrating gliomas, and particularly GBM, allowed a unique opportunity to capitalize on the strengths of CED. The infiltrative nature of these tumors requires larger treatment volumes than can be resected during surgery or treated effectively with diffusion-based approaches that rely on a compound’s concentration gradient and diffusivity within a targeted tissue and often achieve a limited volume of distribution of only a few millimeters.
In comparison, CED distributes agents via a pressure gradient generated by a pump-catheter system that enables the delivery of compounds to large volumes of both, targeted and adjacent tissues independent of the size and diffusivity of the compound [11, 12]. Moreover, CED permits the delivery of a wide range of compounds [13,14,15,16].
Interest in CED has expanded significantly as a number of advantages over diffusion-mediated approaches have been identified. These advantages include: (1) CED expands the intratumoral distribution of drugs as pressure gradients drive agents evenly over larger volumes than possible with diffusion-based approaches; (2) CED permits delivery of a homogenous concentration of drug throughout its volume of distribution (Vd) as it does not rely on a steep concentration gradient to drive flow; and (3) CED occurs independent of an agent’s molecular weight or diffusivity.
Physical principles
The biophysical properties of CED are unique among methods of local delivery and important to consider for the treatment of GBM. Conceptually, CED relies on principles of ‘bulk flow’. Bulk flow refers to the extracellular flow of fluid distributed via a pressure gradient. Bulk flow is best modeled using Darcy’s law, \(v=- K\nabla p\) under the assumption that the treated tissue is a hydrated, porous medium consisting of both fluid and solid phases. Under this model, the velocity of a molecule is directly related to the pressure gradient (\(\nabla p\)) and the hydraulic conductivity (K), or flow conductance of the medium infused. Compared with diffusive methods, distribution via bulk flow is independent of molecular weight, and very high concentrations are not required to ensure therapeutic levels of an agent over large volumes of targeted tissues. As a result, CED enables homogeneous delivery of high concentration therapeutics with tissue penetration up to a few centimeters in contrast to a few millimeters as with diffusion-based methods [12]. The large Vd achievable with CED is critical when considering the treatment of malignant gliomas where local invasion and subsequent recurrence from these invasive tumor cells commonly occurs within centimeters of the original tumor [17, 18]. It is important to note that the concentration fall-off at the border of the convective volume is steep and within these areas, delivery becomes more diffusion based than within the region of the pressure gradient adding an additional few millimeters of treatment [19].
The blood brain barrier in GBM
The BBB isolates the systemic circulation from the CNS via both physical and biochemical barriers [20,21,22]. The BBB is comprised of continuous tight and adherens junctions along cerebral capillary endothelial cells buttressed by astrocyte end-foot processes, and does not exist elsewhere in systemic circulation (Fig. 1). The BBB forms a physical barrier limiting CNS entry of bacteria, monoclonal antibodies, antibody–drug conjugates, large molecules weighing > 40 kD, and hydrophilic molecules. Nutrients, lipophilic molecules, and the removal of waste metabolites traverse the BBB via various active transporters. While the functions of the BBB are to protect the CNS from infections and toxic substances, they also limit the distribution of many anticancer agents [20, 23]. Drugs able to penetrate the CNS often cannot achieve effective concentrations as the required systemic concentrations carry unacceptable associated toxicities [19]. As a result, bypassing the BBB remains a significant hurdle to ensuring the delivery of the majority of approved and experimental oncologic drugs to the CNS. Mechanisms of local delivery capitalize on the bidirectional nature of the BBB. In fact, the most effective agents for local delivery are those that are not well transported across the BBB as these will not travel back into systemic circulation. This permits high concentrations of select agents to be delivered within the CNS with little risk of the drug leaking back into the systemic circulation and causing systemic toxicity.
BBB integrity is naturally disrupted by most brain tumors. This is best represented by conventional contrast-enhanced magnetic resonance imaging (MRI) following administration of gadolinium-based contrast agents where contrast leaks out of regions of BBB breakdown and identifies the bulk of the tumor. However, it is critical to understand that diffusely infiltrative lesions such as GBM contain tumor burden within the surrounding non-enhancing regions of brain where the BBB remains intact as evidenced from studies of MRI and positron emission tomography (PET) imaging [20]. These adjacent areas of protected infiltration are frequently the sources of tumor recurrence and progression and have a profound influence on the efficacy of any therapy. As such, they may provide an excellent target for CED.
Procedural nuances and characteristics of CED
CED Procedure
There is currently no standardized protocol for implementing CED as pre-clinical and clinical investigations have varied in their techniques [3, 6, 7, 9, 10, 24,25,26,27,28,29,30,31,32,33]. The fundamental procedure however involves the stereotactic placement of one-or-more small-diameter catheters directly into either brain parenchyma or tumor using image-guided neuronavigation (Fig. 1). Most trials have used flexible, single-lumen catheters and decisions regarding the number of catheters in published studies have been at the surgeon’s discretion, keeping in mind the volume of the intended target. In general, most clinical studies have limited infusions to tumors less than 4 cm in their largest dimension to ensure adequate distribution of infusate, and to limit potential side effects of infusing more volume into a region already burdened by mass effect [3, 6, 7, 9, 10, 24,25,26,27,28,29]. Catheter position is key to ensuring an adequate Vd and consensus suggests that optimal catheter positioning should be safely away from the ventricles and subarachnoid space to prevent the preferential distribution of infusate into cerebrospinal fluid (CSF) spaces.
Catheters are subsequently attached to an infusion pump which generates a pressure gradient that drives the flow of the infusate directly into the extracellular matrix of the targeted tissue (Fig. 1). Published studies have universally relied on external pumps that require the catheter to be tunneled out of the scalp and the patient to limit their movement or carry the pump with them for the duration of the study. Unfortunately, this has resulted in infusions being limited to a few days to mitigate infectious risks. A trial of a completely internalized, refillable pump-catheter system is ongoing which would permit chronic delivery and allow infusions to be continued in an outpatient setting (NCT03154996).
As the field of CED is relatively young and the results of reported case studies and clinical trials difficult to interpret, there is insufficient evidence to suggest whether catheters should be placed within pathologic tissue or adjacent to it (Table 1). This decision depends on the mechanism of the chosen therapeutic agent to be infused, the tissue characteristics (e.g., the presence of hemorrhage, nearby resection cavities or pial surfaces, proximity to ventricle, adjacent edema, etc.), and the treatment plan. Similarly, infusion rates and schedules have been variable in the literature (Table 1). While this variability is difficult to interpret, published flow rates have demonstrated that the brain parenchyma can tolerate small and large infusion volumes with relatively few associated symptoms due to mass effect or edema (Table 1). Fortunately, these symptoms are often reduced with administration of steroids and none have been fatal.
Flow direction, rates, Vd and Vi
The volume of distribution (Vd) to volume of infusion (Vi) ratio (Vd:Vi) is a critical parameter to consider for CED (Fig. 2). An understanding of this variable for specific anatomical regions or tumor types allows prediction of the required Vi [30]. While increases in Vd are approximately linearly related to Vi initially, preclinical evidence suggests that with prolonged infusion, this relationship changes reaching an equilibrium between infusion and clearance [31,32,33]. Understanding this relationship is complicated as it may be altered by intra-tumor and peri-humoral edema, intratumoral heterogeneity, and location-specific variables commonly encountered in the treatment of GBM and elaborated on below.
T1-weighted non-enhanced image demonstrating intratumoral infusion of chemotherapy with gadolinium tracer. The tumor volume has been segmented out and highlighted in pink. The gadolinium tracer correlating to the volume of distribution of infusate is segmented and highlighted in green. Analysis of the Vd demonstrates an irregular shaped Vd conforming to structural restrictions to flow such as pial boundaries. Infusing 15cm3 of drug resulted in a Vd of 30cm3 with an associated Vd/Vi ratio of 2. In this case, the Vd/tumor volume ratio is 20. Interestingly, a large Vd was achievable despite evidence of back flow into the subarachnoid space (blue arrow)
Backflow, sometimes referred to as reflux, along the catheter tract remains a large determinant of the achievable Vd (Fig. 2). Backflow can result in the loss of infusate into the CSF space, thus eliminating the pressure gradient and rendering the Vd independent of the Vi [34]. Backflow resistant catheters have been developed to combat this problem and are discussed further below.
Molecular features specific to the infusate also influence the Vd:Vi ratio. An agent’s lipophilicity, its susceptibility to enzymatic degradation, and the extent to which it binds cell surface receptors may all influence the Vd. Importantly, in preclinical studies investigating prolonged infusion using an implantable system and a surrogate imaging marker, an equilibrium between infusion and clearance appears to arise within 24—48 h resulting in a shrinking of the final Vd relative to the maximum Vd established despite continued infusion [31, 33]. An understanding of how this equilibrium influence the Vd:Vi of the agent delivered is important for treatment planning for longer term infusions.
Resistance to flow within a targeted tissue can alter the direction of flow from the catheter tip and can thus influence Vd. Distribution from a single point typically results in an elliptical-to-spherical distribution but is limited by physical barriers such as pial surfaces [19]. As a result, studies that have incorporated a tracer to study the achievable Vd have demonstrated irregular shaped volumes of distribution conforming to structural restrictions to flow (Fig. 2).
The properties of white matter versus gray matter also influence flow, and thus Vd:Vi [35]. White matter shows less resistance to bulk flow and flow direction is affected by the direction of white matter tracts. Mathematical models and tracer studies within clinical trials have also demonstrated preferential movement of infusate along paths of pre-existent white matter edema seen in the setting of malignancy which also contributes to the unpredictability of the Vd:Vi ratio [36,37,38]. Catheter induced edema can also influence the flow patterns surrounding the catheter [36]. Further study is required to better clarify the relationship of Vd to Vi and ultimately, these relationships may vary independently with each different treatment protocol on a case-by-case basis.
Clinical trials of CED for GBM
A number of early Phase clinical trials using CED to treat GBM have been reported. The infusion characteristics and results of these trials are summarized in Table 1.
CED trials of conventional chemotherapy
A number of studies of CED of conventional chemotherapies unable to cross the BBB have been performed. Lidar et al. performed CED of Paclitaxel, an anti-microtubule drug, to treat 15 patients with recurrent high-grade gliomas (13 GBMs, 2 anaplastic astrocytomas), and showed an imaging response in 11 of 15 treated patients [26]. Median overall survival (OS) for the group was reportedly 7.5 months.
Topotecan (TPT) is a topoisomerase-I inhibitor that has demonstrated significant antitumor effects in preclinical trials despite minimal antitumor effects and significant dose-limiting toxicities when delivered intravenously in humans [39,40,41,42]. TPT CED was initially evaluated in a Phase Ib trial to treat 16 patients with recurrent high-grade gliomas (10 GBMs, 6 anaplastic gliomas), and showed significant antitumor effects as determined by radiographic images, while extending median OS and progression-free survival (PFS) to 60 and 23 weeks, respectively [3]. No systemic toxicity was noted and neurologic deficits related to CED were limited to patients receiving only the highest doses. A follow-up trial of chronic TPT CED using a refillable, implantable pump-catheter system capable of delivering metronomic pulses of CED over 1 month is currently underway (NCT03154996).
Additional work delivering a mixture of TPT and gadolinium through a novel multipart catheter found that the achievable Vd depended on catheter location, with intratumoral placement resulting in a smaller Vd than when the catheter was placed in tumor-infiltrated brain despite the same flow rates for each location [32]. Infusions were well tolerated. An arm of this study looking at rate escalation for intratumoral delivery is still recruiting (NCT03927274).
CED trials of conjugated toxins
The first clinical trial of CED for recurrent GBM which demonstrated its safety and efficacy in a clinical setting was performed by Edward Oldfield’s group at the National Institutes of Health (NIH) [6]. The study evaluated the targeted toxin TF-CRM107, a human transferrin (TF) conjugated to diphtheria toxin (CRM107) with a point mutation that abolishes nonspecific binding to mammalian cells. The study reported a reduction in tumor size of at least 50% in 9 of 15 patients with limited associated toxicity in patients treated at higher concentrations. There were no systemic toxicities. Unfortunately, a Phase II arm of the study published in 2003 produced less encouraging results with only 39% of patients who completed the treatment demonstrating a complete or partial radiographic response [29]. Similarly, a Phase III study involving TF-CRM107 was aborted as an interim analysis again demonstrated a 39% response rate [43]. Median OS was 37 weeks following treatment in the Phase II arm of the study likely reflecting the poor tumor response rate.
IL-4 Pseudomonas exotoxin (NBI-3001) is a recombinant fusion protein composed of interleukin-4 (IL-4) and Pseudomonas exotoxin that capitalizes on the overexpression of IL-4 in malignant gliomas. In 2000, the first clinical trial using CED of IL-4 Pseudomonas exotoxin to treat recurrent GBM reported evidence of tumor necrosis in 6 of 9 patients [7]. A similar trial treated 25 patients with recurrent GBM and 6 with WHO Grade III anaplastic astrocytomas (AA) with NBI-3001 and showed a median OS of 5.8 months for GBM patients despite the majority of patients demonstrating extensive tumor necrosis on MRI suggestive of treatment response [44]. Minimal adverse effects attributed to treatable edema and no systemic toxicities were observed in either study.
Epidermal growth factor receptor (EGFR) overexpression has also been targeted through clinical trials of CED using TP-38, a chimeric protein containing a TGF-\(\alpha\) binding domain that binds to EGFR, and Pseudomonas exotoxin which induces apoptosis. The first study of TP-38 was plagued by issues of ventricular and subarachnoid leakage of infusate with failed parenchymal distribution [45]. As a result, median OS was 28 weeks for 20 patients with recurrent or progressive malignant brain tumors and 17 GBMs. While the study demonstrated the safety of infusion, it also became the first study to mark the importance of monitoring the efficacy of CED infusion in future trials.
The PRECISE trial was a Phase III CED clinical trial studying IL13-PE38QQR (Cintredekin besudotox), another chimeric protein made of IL-13 receptor alpha 2 chain (IL-13R \(\alpha\) 2) and a truncated form of Pseudomonas exotoxin A (PE38QQR) [46]. PRECISE enrolled 296 patients who received either IL13-PE38QQR via CED 96 h following resection, or CCNU-containing Gliadel wafers implanted at the time of resection. No benefit in median OS was identified relative to Gliadel-treated patients. However, the study was only powered to detect a greater than 50% benefit in survival and any smaller benefits would have required a larger cohort. Additional criticism of the trial pointed out that 11% of patients did not fulfill inclusion criteria and only 27% had complete resections prior to treatment [47]. Follow-up studies looked into other concerns of problems with catheter position and IL-13 receptor levels to explain the underperformance for improving OS. Despite the identified difficulties, these studies did find an improvement in PFS from 11 to 18 weeks with IL-13PE38QQR [47].
CED trials involving liposomes
Liposomes are small, spherically-shaped artificial vesicles composed of a lamellar-phase lipid bilayer that can be synthesized to deliver therapeutics gradually. Additionally, liposomes can undergo endocytosis or phagocytosis, permitting intracellular drug delivery of agents normally incapable of crossing cell membranes [48, 49]
Several trials have assessed CED of genes to tumor cells using liposomal carriers. Initial attempts involved direct intratumoral injection to implant vector-producing cells that generate retroviruses carrying the herpes simplex virus-thymidine kinase (HSV-TK) gene [50, 51]. Difficulties producing sufficient cellular transduction in these studies and others led to trials using CED to deliver the HSV-TK gene [52, 53]. The first trial involved 8 patients and found that HSV-TK CED resulted in a reduction in tumor size of greater than 50% in 2 patients as determined on radiographic studies, and a median OS of 28 weeks for all treated patients with no associated morbidity [28]. The reported Vd achievable in this trial was small and may have limited its efficacy. Another study using liposomes carrying nonreplicating Semliki Forest virus with IL-12 for activating natural killer cells produced inconclusive results [54]. In addition, investigations of the topoisomerase inhibitor irinotecan (CPT-11) embedded in nanoliposomes are ongoing (clinicaltrials.gov; NCT02022644).
CED trials involving viruses
Recently, the therapeutic potential of PVSRIPO, a live attenuated poliovirus type 1 (Sabin) vaccine with its cognate internal ribosome entry site replaced with that of human rhinovirus type 2 was evaluated for the treatment of GBM [10]. PVSRIPO is known to cause a sustained proinflammatory cytokine response and activation of the function of antigen-presenting cells. These responses enable T-cell stimulation in preclinical in vitro assays and may counter tumor-induced immunosuppression while instigating antitumor immunity as a result of its tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus [55]. The trial, utilizing CED to overcome the limitations of the BBB treated 61 patients with recurrent GBM and reported median OS of 12.5 months following treatment, with only 19% of patients experiencing a PVSRIP-related adverse event of grade 3 or higher.
Physical limitations and challenges of CED
Despite the clinical promise of CED for GBM, a number of physical and technical limitations and challenges still remain. The most prevalent issues are summarized below:
Backflow
Backflow occurs when infusate flows along the catheter insertion tract rather than entering the surrounding tissues and remains the biggest challenge to effective CED (Fig. 2). In the setting of backflow, infusate exits the targeted tissue and can spread into unintended areas of the brain or disperse through communicating CSF spaces. This results in a decrease in the intended dose, a limited volume of parenchymal distribution, and unintended distribution of the agent through the CNS. The exact causes of backflow are not fully understood, but have been associated with pressure spikes during infusion, catheter insertion techniques, catheter design, and the presence of air bubbles in the infusion line or in the site of implantation [4].
Evidence suggests that soft catheters with thin diameters are less likely to cause mechanical disruption and thus less backflow [56,57,58]. Novel catheter designs including “step-design” catheters, porous membrane catheters, and valve-tip catheters are currently being investigated to reduce the problems of end-port occlusions and its potential effects on causing pressure spikes that may lead to backflow [57, 59]. The majority of these designs remain in pre-clinical trials however and most clinical trials to date have used small diameter, single exit port soft catheters.
In addition to these pending advancements, most centers have employed guidelines to place catheters at least 2 cm from pial surfaces and resection cavities. Additional attempts to reduce backflow include minimizing insertion trauma, slowly ramping up the infusion rate from a low rate to a higher rate to keep a constant positive pressure during the infusion period as well as give the tissue time to adapt, as well as delaying infusion to allow adjacent tissue to acclimate to the presence of the catheter [6, 28]. There remains a need for the development of catheter materials to make them less prone to scar formation around the catheter entry site to help push the limits of the infusion duration.
Air within the infusion line or pushed into the tissue parenchyma can also disrupt the flow of infusate causing unpredictable flow patterns with alterations to the desired Vd [57, 60]. As a result, air may also contribute to backflow. Priming the cannula prior to insertion prevents air bubbles from occurring at the catheter tip.
Pathologic conditions
The treatment of GBM requires additional considerations related to the increased interstitial pressure within the tumor which can compromise intra-tumoral drug distribution [61]. Similarly, the interstitial pressures associated with peritumoral edema as compared with the surrounding tissues and their mitigation with steroid use should be considered [62]. Increased interstitial pressure within the tumor can diminish the pressure gradient that drives convective flow making it difficult for infusate to penetrate the interstitium during CED. In addition, some tumors may be fibrous or thick from scarring from previous interventions and this may also affect flow distribution. Furthermore, these tumors contain a heterogeneous distribution of blood vessels which can alter the flow trajectory and disrupt the predicted Vd as infusate preferentially flows through perivascular spaces [63, 64].
Infusions into or around tumors with extensive necrosis or cysts results in pooling of the delivered agents within these areas and results in an unequal distribution of infusate to nonviable tissues. Tumor proximity to the ventricles should also be noted and care taken to avoid catheter placement too close to the ependymal surface as penetration of infusate into the ventricle results in a loss of the pressure gradient and distribution through communicating CSF spaces. The effects of steroids on infusion remain an unanswered question in clinical trials. Preclinical evidence suggests that infusions are enhanced by treatment with steroids prior to CED [62, 65]. However, further research is needed on the effects of steroids on the Vd attainable.
Choice of agent for CED
While significant and necessary attention has been given to the technological development of CED, delivery of the appropriate agent remains critical to the ultimate success of CED. Importantly, CED increases the flexibility of future drug design as the method permits a diverse group of agents to be delivered. However, although local delivery improves treatment delivery while avoiding systemic toxicities associated with high doses of toxic chemotherapies, peritumoral brain with tumor infiltration contains regions of tumor within functional parenchyma that must be preserved. This is the reason for the interest in molecularly targeted agents whose toxicity is limited to tumor cells, as well as chemotherapies like Topotecan which act specifically on infiltrating tumor cells within regions of healthy brain given the higher cellular proliferation within regions harboring active tumor.
It is interesting to note that while the procedural optimization of CED remains critical to fully realizing the potential of any selected agent, the function of any specific agent may alter the desired procedural implementation of CED. As a result, it is likely that advancement of CED will continue with concurrent improvements in procedural, technological, and biochemical systems in the future.
Future improvements
Next generation catheter design
The majority of clinical trials investigating CED have used either rigid or soft, small-diameter, single lumen catheters within the targeted tissue. However, catheter design is an area of active study and a number of novel catheters have been developed to improve infusion rates, reduce backflow, and to increase the achievable Vd.
Given findings that larger-diameter catheters lead to more problems with backflow, stepped catheters in which the distal tip is smaller in diameter than the rest of the cannula have been investigated which allow improved flow rates with less backflow [66,67,68]. The risk of backflow is not completely eliminated however, and is still a risk with higher infusion rates. Catheters have been designed with multiple-holes which are theorized to provide better pressure outputs [19]. However, flow from these catheters is unpredictable and the infusate often flows only through the most proximal port making the remaining ports useless. Hollow-fiber catheters with multiple ports have been developed which contain millions of tiny openings along its wall (on the order of 0.45 m\(\mu\)) [66, 68]. These catheters have shown promising results, increasing the amount of infusate transferred by up to threefold, improving uniformity of distribution, and reducing backflow. Renishaw (Wotton-under-Edge, UK) has developed ultrafine, tissue-compatible, anti-backflow catheters which produce minimal tissue damage and overcome the issues of floppiness often associated with soft, thin catheters, as well as backflow [4, 66]. Balloon-tipped catheters can be inflated to fill the resection cavity and force infusate into the desired region and away from the catheter tip thereby limiting backflow [4, 66]. Recently, the Cleveland Multiport Catheter which deploys 4 independent delivery microcatheters was developed and evaluated in 3 patients over 96 h. This catheter demonstrated minimal backflow although the infusion rates were also relatively low [32]. Current trials examining higher flow rates are ongoing.
Long-term infusions via CED will require flexible catheters which are rigid during insertion, navigable, and safe for prolonged implantation. In addition to optimizing catheter design to reduce backflow, the further refinement of catheter materials to make them less prone to scar formation around the catheter may also improve the efficacy of delivery via CED.
Prolonged delivery via subcutaneous pump implantation
To date, clinical trials of CED for GBM have used externalized catheters attached to pumps to drive infusion. The use of externalized catheters is believed to increase the risk of infection as the length of therapy increases. As a result, most trials investigating CED have used short infusion lengths. Successful intracranial drug delivery will likely need to be repeated in a cyclical manner to target tumor cells that are not dividing at the time of infusion, or that did not uptake the drug at the time of the initial treatment. A Semi-permanent, refillable pump-catheter implants is in development that will allow augmentation of infusions schedules to allow periods of washout of infusate [31, 33]. This ensures the safety of surrounding functioning brain and prevents excessive drug accumulation. These implantable mechanical pumps generate an infusion force and are refillable. Refillable pumps also allow multiple chemotherapies to be infused over the chronic treatment schedule. The entire pump-catheter system can be moved to different sites, or removed entirely when the treatment schedule is complete. Chronic CED via subcutaneous pump-catheter implantation has been investigated in preclinical animal studies with great success and is currently being evaluated in a Phase I trial treating recurrent GBM with Topotecan [31, 33]. The approach shows great potential as an implantable semi-permanent mechanism of CED may allow for constant or intermittent regional infusions of a wide variety of agents to occur in an outpatient setting and can be removed in its entirety when long-term treatment is completed.
Imaging and modeling
Optimizing outcomes for a particular agent delivered via CED depends on the adequate delivery of the agent through the desired tissues. As reported above, many clinical trials have suffered from poor convection and attempts to assess the efficacy of delivery have been limited [26,27,28, 45, 69]. However, there remains no standardized method of monitoring the efficacy of CED. Infusion monitoring is currently via direct or indirect methods.
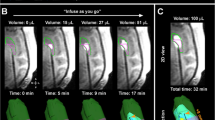
Direct monitoring relies on labeling the infusate itself with a detectable radioisotope or using a surrogate tracer such as gadolinium-DPTA, or gadoteridol-loaded liposomes mixed with the infusate (Fig. 3). This allows real-time imaging of the distribution pattern of therapy [23, 63]. Tracer methods presume that the distribution of the contrast agent, as shown on CT or MRI, equals that of the drug’s distribution. Directly labeling agents alters the size and potentially the charge and tissue affinity of the agent and therefore may affect the potential Vd.
T1-weighted, non-enhanced image demonstrating CED infusion of topotecan and Gd-DTPA for 48 h following resection of a right frontal HGG. Direct infusion monitoring is possible by co-infusing topotecan with a surrogate tracer such as gadolinium-DPTA. This allows real-time imaging of the distribution pattern of therapy. Tracer methods presume that the distribution of the contrast agent, as shown on CT or MRI, equals that of the drug’s distribution. The resection cavity is marked with an *
Indirect methods rely on radiographic alterations as a result of fluid administration or drug-induced tissue effects [70]. However, GBMs often start with T2 hyperintense regions that would complicate indirect approaches. Moving forward, more accurate methods are required to validate accurate cannula placement, track distribution of infusate, and permit treatment adjustments, including the addition of extra targets.
Similarly, the spatial distribution of infusate remains unpredictable. Understanding the direction of flow is critical to targeting CED. Currently, software developed by BrainLab exists that uses MRI obtained data to calculate the desired drug distribution volume and a 3-dimensional visualization of the plan of treatment including the number and position of catheters accounting for anatomic and physiologic variability [71]. Using this software on the data from the PRECISE trial demonstrated its utility for 85% of catheters simulated after placement. As methods of imaging improve, software inputs will be more accurate and produce more accurate and useful simulations.
Procedure cost
Studying CED includes time in the operating room, multiple MRIs, catheters and the stereotactic platforms necessary to place them, infusates, pumps, time of the staff performing the procedure and associated inpatient monitoring. These factors and their associated costs may limit its applicability to clinical trials [43]. As with any intervention, procedural costs are reduced with the availability of the innovation. Attaching CED to other procedures allows for insurance coverage of the operating room time and hospital stay. However, performing CED at the same time as the initial covered procedure is challenging as biopsies at the site of CED can create blood products or air that can adversely affect infusions. Similarly, craniotomies and resections immediately prior to CED creates a cavity which can similarly interfere with infusions. Pre-treatment of the region surrounding a tumor followed by resection of the lesion remains to be examined in large series.
Conclusions
Despite both the physical and biological challenges described above, CED remains a safe and viable treatment method to bypass the BBB and to deliver a multitude of agents to large, targeted regions within the CNS, despite the physical characteristics of the agents. It remains particularly attractive for the treatment of GBM where maximal surgical resection in conjunction with standard chemotherapy and radiation has been shown to improve survival, but fails to treat the infiltrative tumor cells within the surrounding functional brain parenchyma responsible for recurrences. In the setting of GBM, CED has demonstrated the targeted treatment of large areas of functional brain with high-dose agents while avoiding systemic toxicities. To date, several therapies have been shown to be safe and somewhat effective in preclinical and clinical studies. However, discouraging results in two randomized Phase III studies reveal technical shortcomings that remain to be addressed. At this point, the heterogeneity of the studied treatments has limited the ability to adequately assess the durability of any potential anti-tumor responses. As a result, the long-term efficacy of these treatments remains unknown. Before CED is able to fully realize its therapeutic potential, further optimization of the technical aspects of CED and standardization leading to reproducibility is necessary. After technically optimizing CED, the field can focus on which therapeutic or therapeutics will derive the greatest benefits for the treatment of GBM. Essential aspects for future consideration are catheter design, number of catheters, placement of catheters, infusion rate, start-up infusion protocol, duration of infusion, type of drug infused, potential drug encapsulation, and methods of evaluation for drug distribution. For now, CED remains a promising and powerful method for treating GBM.
References
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91:2076–2080
D'Amico RS, Kennedy BC, Bruce JN (2014) Neurosurgical oncology: advances in operative technologies and adjuncts. J Neurooncol 119:451–463. https://doi.org/10.1007/s11060-014-1493-3
Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, Sands SA, Surapaneni K, Lai R, Yanes CL, Bagiella E, DeLaPaz RL (2011) Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 69:1272–1279; discussion 1279-1280 https://doi.org/10.1227/NEU.0b013e3182233e24
Mehta AM, Sonabend AM, Bruce JN (2017) Convection-enhanced delivery. Neurotherapeutics 14:358–371. https://doi.org/10.1007/s13311-017-0520-4
Kunwar S (2003) Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl 88:105–111
Laske DW, Youle RJ, Oldfield EH (1997) Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 3:1362–1368
Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK (2000) Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res 6:2157–2165
Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, Friedman HS, Herndon JE, 2nd, Kunwar S, Marcus S, McLendon RE, Paolino A, Penne K, Provenzale J, Quinn J, Reardon DA, Rich J, Stenzel T, Tourt-Uhlig S, Wikstrand C, Wong T, Williams R, Yuan F, Zalutsky MR, Pastan I (2003) Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol 65:27–35
Weber FW, Floeth F, Asher A, Bucholz R, Berger M, Prados M, Chang S, Bruce J, Hall W, Rainov NG, Westphal M, Warnick RE, Rand RW, Rommell F, Pan H, Hingorani VN, Puri RK (2003) Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl 88:93–103
Desjardins A, Gromeier M, Herndon JE, 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD (2018) Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 379:150–161. https://doi.org/10.1056/NEJMoa1716435
Jain RK (1989) Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst 81:570–576. https://doi.org/10.1093/jnci/81.8.570
Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH (2015) Convection-enhanced delivery to the central nervous system. J Neurosurg 122:697–706. https://doi.org/10.3171/2014.10.JNS14229
Ksendzovsky A, Walbridge S, Saunders RC, Asthagiri AR, Heiss JD, Lonser RR (2012) Convection-enhanced delivery of M13 bacteriophage to the brain. J Neurosurg 117:197–203. https://doi.org/10.3171/2012.4.JNS111528
Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Roberts B, Larson RF, Yamashita Y, Krauze M, Noble CO, Drummond D, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS (2008) Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg 108:989–998. https://doi.org/10.3171/JNS/2008/108/5/0989
Huynh NT, Passirani C, Allard-Vannier E, Lemaire L, Roux J, Garcion E, Vessieres A, Benoit JP (2012) Administration-dependent efficacy of ferrociphenol lipid nanocapsules for the treatment of intracranial 9L rat gliosarcoma. Int J Pharm 423:55–62. https://doi.org/10.1016/j.ijpharm.2011.04.037
Szerlip NJ, Walbridge S, Yang L, Morrison PF, Degen JW, Jarrell ST, Kouri J, Kerr PB, Kotin R, Oldfield EH, Lonser RR (2007) Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg 107:560–567. https://doi.org/10.3171/JNS-07/09/0560
Barker FG, 2nd, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, Wilson CB (1998) Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42:709–720; discussion 720-703
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874. https://doi.org/10.3171/jns.1987.66.6.0865
Zhou Z, Singh R, Souweidane MM (2017) Convection-enhanced delivery for diffuse intrinsic pontine glioma treatment. Curr Neuropharmacol 15:116–128
Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, Giannini C, Burns TC, Kizilbash SH, Laramy JK, Swanson KR, Kaufmann TJ, Brown PD, Agar NYR, Galanis E, Buckner JC, Elmquist WF (2018) Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol 20:184–191. https://doi.org/10.1093/neuonc/nox175
Fokas E, Steinbach JP, Rodel C (2013) Biology of brain metastases and novel targeted therapies: time to translate the research. Biochim Biophys Acta 1835:61–75. https://doi.org/10.1016/j.bbcan.2012.10.005
Theodorakis PE, Muller EA, Craster RV, Matar OK (2017) Physical insights into the blood-brain barrier translocation mechanisms. Phys Biol 14:041001. https://doi.org/10.1088/1478-3975/aa708a
Sampson JH, Raghavan R, Brady M, Friedman AH, Bigner D (2011) Convection-enhanced delivery. J Neurosurg 115:463–464; discussion 465-466 https://doi.org/10.3171/2010.11.JNS101801
Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I (2011) Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13:132–142. https://doi.org/10.1093/neuonc/noq142
Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrie M, Meng Y, Richard M, Parizot C, Laigle-Donadey F, Gorochov G, Psimaras D, Sanson M, Tibi A, Chinot O, Carpentier AF (2010) Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol 12:401–408. https://doi.org/10.1093/neuonc/nop047
Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, Hadani M, Ram Z (2004) Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100:472–479. https://doi.org/10.3171/jns.2004.100.3.0472
Patel SJ, Shapiro WR, Laske DW, Jensen RL, Asher AL, Wessels BW, Carpenter SP, Shan JS (2005) Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery 56:1243–1252; discussion 1252-1243
Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, Kracht L, Coenen HH, Sturm V, Wienhard K, Heiss WD, Jacobs AH (2003) Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 54:479–487. https://doi.org/10.1002/ana.10688
Weaver M, Laske DW (2003) Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol 65:3–13
Souweidane MM (2014) Editorial: convection-enhanced delivery for diffuse intrinsic pontine glioma. J Neurosurg Pediatr 13:273–274. https://doi.org/10.3171/2013.10.PEDS13421
Sonabend AM, Stuart RM, Yun J, Yanagihara T, Mohajed H, Dashnaw S, Bruce SS, Brown T, Romanov A, Sebastian M, Arias-Mendoza F, Bagiella E, Canoll P, Bruce JN (2011) Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol 13:886–893. https://doi.org/10.1093/neuonc/nor051
Vogelbaum MA, Brewer C, Barnett GH, Mohammadi AM, Peereboom DM, Ahluwalia MS, Gao S (2018) First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: results of pilot trial 1. J Neurosurg 130:1–10: https://doi.org/10.3171/2017.10.JNS171845
D'Amico RS, Neira JA, Yun J, Alexiades NG, Banu M, Englander ZK, Kennedy BC, Ung TH, Rothrock RJ, Romanov A, Guo X, Zhao B, Sonabend AM, Canoll P, Bruce JN (2019) Validation of an effective implantable pump-infusion system for chronic convection-enhanced delivery of intracerebral topotecan in a large animal model. J Neurosurg 1:1–10: https://doi.org/10.3171/2019.3.JNS1963
Chen PY, Ozawa T, Drummond DC, Kalra A, Fitzgerald JB, Kirpotin DB, Wei KC, Butowski N, Prados MD, Berger MS, Forsayeth JR, Bankiewicz K, James CD (2013) Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol 15:189–197. https://doi.org/10.1093/neuonc/nos305
Raghavan R, Brady ML, Rodriguez-Ponce MI, Hartlep A, Pedain C, Sampson JH (2006) Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 20:E12. https://doi.org/10.3171/foc.2006.20.4.7
Healy AT, Vogelbaum MA (2015) Convection-enhanced drug delivery for gliomas. Surg Neurol Int 6:S59–67. https://doi.org/10.4103/2152-7806.151337
Linninger AA, Somayaji MR, Mekarski M, Zhang L (2008) Prediction of convection-enhanced drug delivery to the human brain. J Theor Biol 250:125–138. https://doi.org/10.1016/j.jtbi.2007.09.009
Lonser RR, Warren KE, Butman JA, Quezado Z, Robison RA, Walbridge S, Schiffman R, Merrill M, Walker ML, Park DM, Croteau D, Brady RO, Oldfield EH (2007) Real-time image-guided direct convective perfusion of intrinsic brainstem lesions: technical note. J Neurosurg 107:190–197. https://doi.org/10.3171/JNS-07/07/0190
Bruce JN, Falavigna A, Johnson JP, Hall JS, Birch BD, Yoon JT, Wu EX, Fine RL, Parsa AT (2000) Intracerebral clysis in a rat glioma model. Neurosurgery 46:683–691
Burris HA, 3rd (1998) Topotecan: Incorporating It Into the Treatment of Solid Tumors. Oncologist 3:1–3
Kaiser MG, Parsa AT, Fine RL, Hall JS, Chakrabarti I, Bruce JN (2000) Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery 47:1391–1398; discussion 1398-1399
Matsumoto Y, Fujiwara T, Honjo Y, Sasaoka N, Tsuchida T, Nagao S (1993) Quantitative analysis of DNA topoisomerase I activity in human and rat glioma: characterization and mechanism of resistance to antitopoisomerase chemical, camptothecin-11. J Surg Oncol 53:97–103
Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK (2017) Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg 126:191–200. https://doi.org/10.3171/2016.1.JNS151591
Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S, Bruce J, Hall W, Rainov NG, Westphal M, Warnick RE, Rand RW, Floeth F, Rommel F, Pan H, Hingorani VN, Puri RK (2003) Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol 64:125–137
Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, Friedman HS, Greer K, Herndon JE, 2nd, Kunwar S, McLendon RE, Paolino A, Petry NA, Provenzale JM, Reardon DA, Wong TZ, Zalutsky MR, Pastan I, Bigner DD (2008) Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol 10:320–329. https://doi.org/10.1215/15228517-2008-012
Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK, Group PS (2010) Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol 12:871–881. https://doi.org/10.1093/neuonc/nop054
Mueller S, Polley MY, Lee B, Kunwar S, Pedain C, Wembacher-Schroder E, Mittermeyer S, Westphal M, Sampson JH, Vogelbaum MA, Croteau D, Chang SM (2011) Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol 101:267–277. https://doi.org/10.1007/s11060-010-0255-0
Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL (1994) Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem 269:2550–2561
Zhu J, Zhang L, Hanisch UK, Felgner PL, Reszka R (1996) A continuous intracerebral gene delivery system for in vivo liposome-mediated gene therapy. Gene Ther 3:472–476
Klatzmann D, Valery CA, Bensimon G, Marro B, Boyer O, Mokhtari K, Diquet B, Salzmann JL, Philippon J (1998) A phase I/II study of herpes simplex virus type 1 thymidine kinase "suicide" gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther 9:2595–2604. https://doi.org/10.1089/hum.1998.9.17-2595
Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH (1997) Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 3:1354–1361
Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, Southgate TD, Klatzmann D, Lassmann H, Castro MG, Lowenstein PR (1999) Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med 5:1256–1263. https://doi.org/10.1038/15207
Eck SL, Alavi JB, Alavi A, Davis A, Hackney D, Judy K, Mollman J, Phillips PC, Wheeldon EB, Wilson JM (1996) Treatment of advanced CNS malignancies with the recombinant adenovirus H5.010RSVTK: a phase I trial. Hum Gene Ther 7:1465–1482. https://doi.org/10.1089/hum.1996.7.12-1465
Ren H, Boulikas T, Lundstrom K, Soling A, Warnke PC, Rainov NG (2003) Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene—a phase I/II clinical protocol. J Neurooncol 64:147–154
Brown MC, Holl EK, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, Bigner DD, Gromeier M, Nair SK (2017) Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med 9:eaan4220. https://doi.org/10.1126/scitranslmed.aan4220
Casanova F, Carney PR, Sarntinoranont M (2014) Effect of needle insertion speed on tissue injury, stress, and backflow distribution for convection-enhanced delivery in the rat brain. PLoS ONE 9:e94919. https://doi.org/10.1371/journal.pone.0094919
Sillay KA, McClatchy SG, Shepherd BA, Venable GT, Fuehrer TS (2014) Image-guided convection-enhanced delivery into agarose gel models of the brain. J Vis Exp. https://doi.org/10.3791/51466
Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, Morrison PF, Brechbiel MW, Oldfield EH (2002) Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg 97:905–913. https://doi.org/10.3171/jns.2002.97.4.0905
Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, Berger MS, Bankiewicz K (2005) Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg 103:923–929. https://doi.org/10.3171/jns.2005.103.5.0923
Allard E, Passirani C, Benoit JP (2009) Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials 30:2302–2318. https://doi.org/10.1016/j.biomaterials.2009.01.003
Boucher Y, Baxter LT, Jain RK (1990) Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res 50:4478–4484
Yang X, Saito R, Nakamura T, Zhang R, Sonoda Y, Kumabe T, Forsayeth J, Bankiewicz K, Tominaga T (2016) Peri-tumoral leakage during intra-tumoral convection-enhanced delivery has implications for efficacy of peri-tumoral infusion before removal of tumor. Drug Deliv 23:781–786. https://doi.org/10.3109/10717544.2014.914987
Raghavan R, Brady ML, Sampson JH (2016) Delivering therapy to target: improving the odds for successful drug development. Ther Deliv 7:457–481. https://doi.org/10.4155/tde-2016-0016
Brady ML, Raghavan R, Alexander A, Kubota K, Sillay K, Emborg ME (2013) Pathways of infusate loss during convection-enhanced delivery into the putamen nucleus. Stereotact Funct Neurosurg 91:69–78. https://doi.org/10.1159/000342492
Yang W, Barth RF, Huo T, Nakkula RJ, Weldon M, Gupta N, Agius L, Grecula JC (2014) Radiation therapy combined with intracerebral administration of carboplatin for the treatment of brain tumors. Radiat Oncol 9:25. https://doi.org/10.1186/1748-717X-9-25
Debinski W, Tatter SB (2009) Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother 9:1519–1527. https://doi.org/10.1586/ern.09.99
Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH (1999) Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 90:315–320. https://doi.org/10.3171/jns.1999.90.2.0315
Bidros DS, Liu JK, Vogelbaum MA (2010) Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol 6:117–125. https://doi.org/10.2217/fon.09.135
Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, Aldape KD (2007) Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 25:837–844. https://doi.org/10.1200/JCO.2006.08.1117
Mehta AI, Choi BD, Raghavan R, Brady M, Friedman AH, Bigner DD, Pastan I, Sampson JH (2011) Imaging of convection enhanced delivery of toxins in humans. Toxins (Basel) 3:201–206. https://doi.org/10.3390/toxins3030201
Sampson JH, Raghavan R, Brady ML, Provenzale JM, Herndon JE, 2nd, Croteau D, Friedman AH, Reardon DA, Coleman RE, Wong T, Bigner DD, Pastan I, Rodriguez-Ponce MI, Tanner P, Puri R, Pedain C (2007) Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro Oncol 9:343–353. https://doi.org/10.1215/15228517-2007-007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author, Randy S. D’Amico, certify that this manuscript is a unique submission and has not been previously published elsewhere, nor is it under consideration for publication, in part or in full, with any other source in any medium. All authors of this manuscript have contributed to, read, and approved of the manuscript and its submission for publication. The authors will be happy to provide the required forms should the manuscript be accepted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
D’Amico, R.S., Aghi, M.K., Vogelbaum, M.A. et al. Convection-enhanced drug delivery for glioblastoma: a review. J Neurooncol 151, 415–427 (2021). https://doi.org/10.1007/s11060-020-03408-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03408-9