Summary
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disease affecting the central nervous system, often characterized by the accumulation of irreversible clinical disability over time. In recent years, there has been a dramatic evolution in several key concepts of MS treatment. The demonstration of the effects of ocrelizumab, a selective monoclonal antibody against CD20+ B cells, has significantly modified our knowledge of the immune-pathophysiology of MS and has provided a new therapeutic target for relapsing and progressive MS patients. Emerging findings suggest that, besides its strong anti-inflammatory activity, ocrelizumab may limit disability progression and may exert beneficial effects on cognitive function, fatigue, and quality of life of MS patients. The significant reductions of the rate of global and regional brain atrophy and of serum neurofilament light chain levels, which were found to be partially independent of overt inflammatory activity, suggest that this treatment may also limit neuro-axonal damage. By discussing the most recent evidence regarding the effects of ocrelizumab on clinical measures as well as on magnetic resonance imaging and fluid biomarkers, this review summarizes current knowledge on the possible mechanisms underlying the effects of ocrelizumab in limiting MS progression and neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, and neurodegenerative disease affecting the central nervous system (CNS), often leading to the accumulation of irreversible clinical disability [1].
During recent years, there has been a dramatic evolution in several key concepts of MS treatment. The demonstration of the strong effects of ocrelizumab, a selective monoclonal antibody against CD20+ B cells, has provided a new therapeutic avenue for relapsing and progressive MS patients [2, 3]. The positive results from randomized controlled trials (RCTs) and observational studies with anti-CD20 therapies also improved understanding of MS pathophysiology, since they confirmed the evidence coming from experimental studies suggesting that B cells, typically involved in adaptive humoral immunity, are also critical for MS pathogenesis [4, 5].
Moreover, it is now clear that the reduction of overt inflammatory disease activity (i.e., clinical relapses, gadolinium-enhancing and new T2-hyperintense magnetic resonance imaging [MRI] lesions) should be combined with the prevention of chronic inflammation and neurodegenerative phenomena such as neuro-axonal damage that are likely to represent the main contributors to disability progression [6]. Furthermore, recent evidence challenges the dichotomy between relapsing and progressive disease courses, suggesting a continuum of the disease with an underlying progressive course and a highly variable superimposed accumulation of disability, resulting from relapses with incomplete recovery [7].
In this view, emerging findings suggest that besides the strong anti-inflammatory activity, ocrelizumab may contribute to limiting disability progression and may exert beneficial effects on cognitive function, fatigue, and quality of life of MS patients.
The use of MRI measures developed for the estimation of neurodegeneration (i.e., global and regional atrophy) and the quantification of serum neurofilament light chain (sNFL) levels have suggested that ocrelizumab promotes the reduction of both inflammation and the progression of neurodegeneration. By discussing the most recent evidence regarding the effects of ocrelizumab on clinical measures as well as on MRI and fluid biomarkers, this review summarizes current knowledge about the possible mechanisms underlying the effects of ocrelizumab in limiting MS worsening and neurodegeneration.
Mechanisms of Action
Ocrelizumab, a humanized monoclonal antibody, was approved by the Food and Drug Administration (FDA) in March 2017 and by the European Medicines Agency (EMA) in January 2018 at a dose of 600 mg i.v. twice per year, for the treatment of relapsing (R) and primary progressive (PP) MS patients.
Ocrelizumab selectively targets the extracellular loop of CD20-expressing B cells, causing antibody-dependent cell lysis. In particular, the mechanisms involved in apoptotic B-cell depletion are antibody-dependent cellular phagocytosis, antibody-dependent cellular cytotoxicity, and complement-dependent cytotoxicity [8].
The infusion of ocrelizumab promotes a depletion of CD20+ B cells within hours, mainly occurring in the liver [9]. This depletion reaches its nadir typically after 8 weeks and can be sustained for several weeks or months.
In RCTs of ocrelizumab in MS, the median time to B-cell repletion (i.e., returning to baseline or lower limit of normal [LLN]) is 72 weeks (range 27–175 weeks) after treatment [10]. In 90% of patients, B-cell repletion was within approximately 2.5 years (120 weeks) after the last infusion [10].
Disability Progression and Improvement
In MS, disability progression is usually measured using the Expanded Disability Status Scale (EDSS) [11], where confirmed disability progression (CDP) is defined as an increase in EDSS score confirmed at 12 or 24 weeks or later time points [12, 13]. Assessing composite confirmed disability accumulation (composite CDA), which evaluates worsening of EDSS, the 9-hole peg test (9-HPT) and timed 25-foot walk test (T25FWT) for the upper and lower extremity functions, respectively, better capture aspects of disease progression potentially missed with the EDSS, improving the sensitivity in detecting subtle progression [14,15,16,17,18,19]. In detail, composite CDA is defined as worsening of disability from baseline, evaluated with EDSS (increase ≥ 1.5 when baseline EDSS score was 0; increase ≥ 1.0 when baseline EDSS score was ≤ 5.5; or increase ≥ 0.5, when baseline EDSS score was ≥ 6.0), or ≥ 20% worsening in T25FW or in 9-HPT that was confirmed after 12 or 24 weeks [7].
In addition to its role in reducing overt inflammation (see [20] for a comprehensive review), compared to interferon beta 1a (IFN β-1a), recent evidence suggests that ocrelizumab may prevent disability accumulation in RMS [2, 7]. Of note, this was also observed in PPMS patients being treated with ocrelizumab compared to placebo [3, 21].
In two identical Phase III RCTs conducted in RMS (OPERA I, NCT01247324; OPERA II, NCT01412333), ocrelizumab showed a reduction in the prevalence of 12-week CDP (by 43% and 37%, respectively, p < 0.05) compared with IFN β-1a [2]. In the 7.5-year OPERA I/II follow-up open-label extension (OLE) interim analysis, the risk of 48-week CDP and of requiring a walking aid were 23% and 35% lower in patients who initiated ocrelizumab earlier vs those initially receiving IFN β-1a (14.5% vs 16.4% HR = 0.77 [95% confidence interval, CI 0.60–0.98]; p = 0.034; 5.2% vs 7.0%; HR = 0.65 [95% CI = 0.44–0.97]; p = 0.034, respectively) (Table 1, Fig. 1) [22].
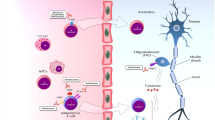
Schematic representation of the different outcomes supporting the beneficial effects of ocrelizumab beyond its anti-inflammatory activity. Ocrelizumab has been found to: A limit disability progression; B promote disability improvement; C prevent cognitive deterioration and improve fatigue and multiple sclerosis patients’ quality of life (QoL); D limit the accumulation of global and regional brain atrophy; E limit the occurrence of chronic active lesions and the accumulation of their microstructural abnormalities; F reduce the serum level of neurofilament light chain. See text for further details. Created with BioRender.com
In the phase III ORATORIO (NCT01412333) RCT in PPMS, compared with placebo ocrelizumab was associated with a significant reduction of 12-week CDP (32.9% vs 39.3%, 24% decrease, p = 0.03) (the primary endpoint of the study), confirmed with the 24-week CDP (29.6% vs 35.7%, 25% reduction, p = 0.04) [3]. The prevalence of worsening on T25FWT was also significantly decreased with ocrelizumab (38.9% vs 55.1%, 29% decrease, p = 0.04). An interim report from ORATORIO OLE has shown consistent and sustained treatment-associated benefit in multiple measures of CDP over a period of 8 years [23]. Both the risks of the first 48-week CDP EDSS and 48-week CDP-9HPT were reduced, respectively, by 29% and 34% in patients who started ocrelizumab earlier vs those initially receiving placebo (HR = 0.71 [95% CI = 0.57–0.87]; p = 0.001; HR = 0.66 [95% CI = 0.50–0.86]; p = 0.002) (Table 1, Fig. 1). Although these were promising findings, in a pre-specified subgroup analysis, the magnitude of the effect of ocrelizumab was larger in younger patients and in those with gadolinium (Gd)-enhancing lesions at baseline compared to older patients and those without disease activity [3]. The relatively young mean age and short disease duration, together with a substantial proportion of PPMS patients with disease activity present, may explain, at least partially, the significantly lower rate of disability progression in PPMS treated with ocrelizumab compared to placebo, which may be mainly secondary to the strong anti-inflammatory effect of this drug [3, 24].
There is growing evidence suggesting that disability worsening in MS may occur not only as a consequence of relapse-associated worsening (RAW), due to the occurrence of overt inflammatory activity, but also as progression independent of relapse activity (PIRA), this latter also being termed “silent progression” [7, 25, 26]. Using the same criteria proposed for CDA, PIRA was defined as worsening in EDSS, T25FWT, or 9-HPT, but independent of clinical relapses [7].
Disability worsening because of incomplete recovery following relapse was previously defined as the onset of confirmed worsening by 1.0 point or more in EDSS score within 180 days of a relapse [27]. Another definition of RAW is CDA events in which the initial increase of disability is preceded by any protocol-defined relapse in the last 90 days [7]. By evaluating pooled data from OPERA I/II (n = 1656) [7], ocrelizumab proved to be superior to IFN β-1a in preventing composite CDA as well as both RAW and PIRA. It is noteworthy that PIRA events were the main contributors to both 12-week and 24-week composite CDA after 96 weeks in patients treated with IFN β-1a (174/223 [78%] and 137/179 [81%]) and ocrelizumab (147/167 [88%] and 115/129 [89%]). Conversely, only a minority of MS patients had composite 12-week CDA explained by RAW events (93/239 [38.9%] in IFN β-1a and 43/145 [29.7%] in ocrelizumab). Very few MS patients experienced both RAW and PIRA events (17/390 [4.4%] for 12-week and 15/299 [5.0%] for 24-week composite CDA). Ocrelizumab vs IFN β-1a was associated with reduced risk of composite CDA (hazard ratio [HR] = 0.67, [95% CI = 0.55–0.82]; p < 0.001), confirmed PIRA (HR = 0.78 [95% CI = 0.63–0.98]; p = 0.03), and RAW (HR = 0.47 [95% CI = 0.29–0.78]; p = 0.003) events. Similar trends were observed across the individual components (EDSS, T25FWT, and 9-HPT) of the composite for PIRA and RAW. The association of ocrelizumab with reduced disability accumulation was also evident in the subgroup of patients defined by a higher risk of secondary progressive MS (EDSS ≥ 4 and pyramidal functional system score ≥ 2) [13], with a more pronounced reduction in the risk of 12-week and 24-week composite PIRA in this specific subpopulation [24].
Although slowing disability accumulation is a major objective in MS care, there is also growing interest in assessing confirmed disability improvement (CDI). This outcome has been introduced to support the beneficial effects of highly effective treatments for MS not only in reducing the occurrence of disease activity and disability progression but also in promoting recovery from disability possibly due to the enhancement of plasticity and repair mechanisms. CDI has been defined as an EDSS score decrease ≥ 1.0 in patients with a baseline score ≥ 2.0, confirmed after 12 or 24 weeks [28, 29].
Interestingly, in the pooled analysis of OPERA I/II RCTs, the proportion of patients with 12-week CDI, defined as an EDSS decrease confirmed at 12 weeks, was higher with ocrelizumab compared with IFN β-1a, although this was significant in the OPERA I (20.0% vs 12.4%, 61% difference, p = 0.01) but not in the OPERA II (21.4% vs 18.8%, 14% difference, p = 0.40) [2]. Moreover, the proportion of patients with 24-week CDI in patients with a baseline EDSS score of ≥ 2.0 was significantly higher in patients receiving continuous ocrelizumab compared with those switching from IFN β-1a to ocrelizumab (25.8% vs 20.6%; p = 0.046) [7].
Cognition, Fatigue, and Quality of Life
Although refinements to the clinical definition of progression have been proposed, a comprehensive assessment of MS patients, which also takes into consideration cognition and patient reported outcomes (e.g., evaluation of quality of life, fatigue), has recently been introduced [30].
Cognition
Cognitive impairment affects up to 70% of MS patients and can typically involve several cognitive domains, including information processing speed, executive function, and memory, whereas verbal fluency and visuospatial abilities are less frequently affected [31, 32].
Cognitive dysfunction occurs in all MS clinical phenotypes, from the earliest phases of the disease, becoming more prevalent and severe in patients with the progressive clinical phenotypes. Such impairment has an important impact on MS patients, families, and society, due to its detrimental effects on quality of life, employment, and engagement in social activities [31, 32].
Although little attention has been given to the influence of disease-modifying therapies (DMTs) on cognitive functions, there is emerging evidence from RCTs and observational studies for the beneficial effects of DMTs on cognitive functions in MS patients [33].
By evaluating pooled data from OPERA I and OPERA II, three recent studies showed the positive effects of ocrelizumab on cognitive functions [31, 34, 35]. Over 96 weeks, the ocrelizumab group (n = 766) showed a significantly reduced risk of 12-week (HR = 0.62 [95% CI = 0.48–0.79]; p < 0.001) and 24-week (HR = 0.61 [95% CI = 0.45–0.84]; p = 0.002) confirmed symbol digit modalities test (SDMT) worsening of ≥ 4 points compared to IFN β-1a group (n = 749) [35]. By evaluating only RMS patients at increased risk of progressive disease (i.e., with EDSS score ≥ 4 and pyramidal functional system score ≥ 2), ocrelizumab- vs IFN β-1a-treated patients showed a significantly greater improvement at SDMT (n = 180) (ocrelizumab group: n = 186; IFN β-1a group: n = 180; mean [SE] score improvement at SDMT = 6.2 [1.2] vs 2.6 [1.2]; p = 0.023) [34]. Interestingly, these findings were also confirmed in the sub-groups of RMS patients showing moderate SDMT impairment (i.e., scores ≥ 2 standard deviations below norms) at baseline (ocrelizumab group: n = 116; IFN β-1a group: n = 107; mean [SE] SDMT score improvement from baseline = 10.5 [1.4] vs 6.0 [1.4]; p = 0.011) [34] (Table 1, Fig. 1). These results support the hypothesis that the beneficial effects of ocrelizumab may also occur in MS patients with cognitive deficits who are also more likely to have a more severe disease course.
Another study on the same populations evaluated the effects of ocrelizumab in preventing cognitive decline due to RAW or PIRA [31]. RAW-SDMT events were defined as confirmed SDMT worsening occurring ≤ 90 days after onset of a relapse. SDMT scores were then “re-baselined” ≥ 30 days after each relapse, and PIRA-SDMT events were defined as confirmed SDMT worsening that occurred independent of relapse activity. Interestingly, 24-week confirmed SDMT worsening (≥ 4-point reduction) was almost exclusively related to PIRA, with SMDT worsening occurring in 7.0% vs 11.3% of ocrelizumab- and IFN β-1a-treated patients, respectively. Conversely, SDMT worsening secondary to RAW was experienced by only a minority (0.3%) of all RMS patients [31]. Furthermore, compared with IFN β-1a, ocrelizumab reduced the risk of 24-week confirmed SDMT worsening independent of disease activity (i.e., PIRA) (HR = 0.58 [95% CI 0.39 = 0.86]; p = 0.006), whereas no difference in 24-week confirmed SDMT due to relapses (i.e., RAW) was found between the two treatment groups [31]. These findings suggest that ocrelizumab may exert beneficial effects not only suppressing inflammatory activity, in terms of reducing cognitive relapses [36], but also possibly limiting the neurodegenerative processes independent of overt inflammation. Although no definitive data on cognition have been published in progressive MS patients, the 1-year interim analysis of the CONSONANCE RCT, which evaluated 629 patients (325 SPMS; 304 PPMS), suggested a possible beneficial effect of ocrelizumab in improving or at least stabilizing cognitive function in progressive MS [37]. However, a longer follow-up is needed to confirm these preliminary findings.
Fatigue and Quality of Life
Up to 80% of MS patients experience symptoms of fatigue, either persistently or sporadically [38].
In addition to the standard clinical outcome measures used to evaluate the efficacy of ocrelizumab, phase III RCTs included patient-reported outcome (PRO) measures to evaluate more comprehensively the effects of DMTs on fatigue, participants’ subjective experience of health status, and overall quality of life. In the OPERA I, OPERA II, and ORATORIO RCTs, the Medical Outcomes Study Short Form-36 (SF-36) [39], a generic health-related quality of life (HRQOL) questionnaire, was administered to the patients. The Physical Component Summary (PCS) of the SF-36 was selected as the secondary outcome measure.
In OPERA I, the mean change in SF-36 PCS in the ocrelizumab-treated participants did not differ from the mean change in the IFN β-1a-treated group at 96 weeks (mean change = 0.04 [−0.86 to 0.93] vs −0.66 [−1.59 to 0.28], p = 0.22). Conversely, in the OPERA II RCT, the adjusted mean change was higher in the ocrelizumab than IFN β-1a-treated group at the end of the 96-week trial (adjusted mean change = 0.33 [−0.55 to 1.20] vs −0.83 [−1.76 to 0.09], p = 0.04) [2]. There was no difference in the adjusted mean change in SF-36 PCS between ocrelizumab and placebo groups in the ORATORIO trial at 120 weeks (adjusted mean change = −0.7 with ocrelizumab and −1.1 with placebo, p = 0.60) (Table 1, Fig. 1) [3]. The use of a single summary score did not allow a detailed analysis of the effects of ocrelizumab on specific HRQOL domains that may be of particular interest to individuals with neurological diseases. To address this topic, a recent study investigated the impact of ocrelizumab on HRQOL in individuals with MS using both SF-36 and Neuro-QoL and detected improvements in fatigue and anxiety after 12 months of ocrelizumab treatment [40]. Interestingly, most of the observed changes were on the mental rather than the physical components of HRQOL, suggesting that improvements may not have been due to a reduction in physical disability alone.
The association of work productivity with ocrelizumab therapy should also be taken into consideration when choosing therapy for patients, particularly since the majority of patients with MS are young adults of working age [41]. In a recent study, ocrelizumab-treated patients were more likely to be employed and experience less impact on work productivity than patients treated with oral (teriflunomide, fingolimod, or dimethyl fumarate) or injectable (IFN β-1a, IFN β-1b, glatiramer acetate, or pegylated IFN β-1a) therapies [41].
Fluid and MRI Biomarkers
Neurofilaments
Neurofilaments (NfL) are abundant cytoskeletal components that are exclusively expressed in neurons. Pathological processes associated with neuro-axonal damage such as inflammation, demyelination, and neurodegeneration lead to an increased permeability or disruption of the neuro-axonal membrane with a subsequent release of neurofilaments into the interstitial fluid and eventually into the cerebrospinal fluid and blood.
NfL levels are associated with measures of disability progression and accumulation of irreversible brain damage at MRI in MS [42]. Accordingly, NfL level quantification in the cerebrospinal fluid (CSF) or in the blood has been suggested as a feasible and reliable biomarker for monitoring the effects of DMTs [42, 43].
In the Ocrelizumab Biomarker Outcome Evaluation study (OBOE; NCT02688985), 1-year analysis demonstrated a significant reduction of serum (s) NfL (−13.1%, −18.6%, and −30.8%) and CSF NfL levels (−24.5%, −40.0%, and −54.7%) in ocrelizumab-treated RMS patients at weeks 12, 24, and 52, suggesting a progressive and sustained reduction in axonal injury with this treatment [44].
When evaluating OPERA I/II (n = 1421) and ORATORIO (n = 596) RCTs, significant reductions in sNfL were observed 12 weeks after ocrelizumab initiation, compared with IFN β-1a (RMS, geometric mean ratio [GMR] = 0.80) or placebo (PPMS, GMR = 0.89) and sustained through to the end of controlled treatment (RMS, [96 weeks], GMR = 0.56, PPMS [120 weeks], GMR = 0.81, all p < 0.0001) [45] (Table 1, Fig. 1).
Interestingly, ocrelizumab significantly reduced sNfL levels independent of the presence of baseline clinical or MRI disease activity. By stratifying patients with or without disease activity at baseline (i.e., for RMS: presence of Gd-enhancing lesions on baseline MRI and/or relapse in prior 3 months for RMS; for PPMS: presence of Gd-enhancing lesions on baseline MRI), the age-adjusted sNfL levels after 96 weeks of ocrelizumab in treated RMS patients approached those of a cohort of healthy controls in the two sub-groups with or without baseline disease activity (geometric mean = 4.4 [4.2–4.6] pg/ml and 4.1 [4.0–4.3] pg/ml, respectively) [45]. Similarly, geometric mean sNfL levels after 120 weeks were significantly reduced from baseline (p < 0.005) both in ocrelizumab-treated PPMS patients with or without Gd-enhancing lesions at baseline MRI (geometric mean = 4.6 [4.1–5.1] pg/ml and 4.2 [4.2–4.4] pg/ml, respectively), although they remained elevated compared with healthy controls (p < 0.001) [45].
Of note, in the ORATORIO analysis, a tenfold increase in baseline sNfL in the control group was associated with increased risk of progression on 9-HPT (HR = 2.33, p = 0.036) and T25FWT (HR = 5.35, p = 0.003) [46].
The demonstration that ocrelizumab significantly decreased sNfL, which was independent of overt inflammatory activity, suggests that this treatment may also limit neuro-axonal damage.
Global and Regional Atrophy
Brain atrophy can be observed from the earliest phases of MS and is one of the main predictors of physical and cognitive disability [47, 48]. Reducing the rate of brain atrophy has been incorporated as an endpoint in several recent clinical trials in MS [49,50,51].
By evaluating the data from RCTs in RMS patients, significant differences in the percentage brain volume change (PBVC) (SIENA software) from week 24 to week 96 between the ocrelizumab- and the IFN β-1a group were observed in the OPERA I (PBVC = −0.57% vs −0.74%, 22.8% difference, p = 0.004), but not in the OPERA II (PBVC = −0.64% vs −0.75%, 14.9% difference, p = 0.09) [2].
In the 3-year follow-up OLE, RMS patients continuing ocrelizumab exhibited lower 5-year brain atrophy compared with those switching from IFN β-1a (PBVC = −1.87% vs −2.15%; p < 0.01), suggesting a role for ocrelizumab in limiting neurodegeneration if treatment is started early [52].
Similarly, in the ORATORIO trial, the rate of brain atrophy was significantly lower in PPMS patients treated with ocrelizumab vs placebo from week 24 to week 120 (PBVC = −0.90 vs −1.09, p = 0.02) [3]. However, in the OLE, rates of whole brain (adjusted PBVC = −3.1% vs −3.4%; p = 0.13) and cortical atrophy (adjusted Jacobian rate = −2.5% vs −2.6; p = 0.38) were not significantly different between the two groups from baseline to week 144 [21] (Table 1, Fig. 1).
The switch to ocrelizumab in the placebo cohort, the reduced frequency of MRI scans in the OLE phase, and the relatively small brain volume changes over the extended period may have contributed, at least partially, to the lack of significant differences between PPMS starting ocrelizumab early compared to those initially receiving placebo. Further studies performed in larger cohorts of patients are needed to confirm the efficacy of ocrelizumab in limiting brain atrophy.
Recent studies have evaluated the effects of ocrelizumab in specific CNS structures, such as the thalamus. The thalamus is a central hub highly connected with several brain regions, having a key role in locomotor and cognitive functions. Thalamic atrophy occurs early in the disease, has been suggested as a sensitive marker of overall brain damage, and is associated with disability progression [30, 53, 54].
By evaluating the pooled data from OPERA I/II (n = 1421), a significantly lower thalamic atrophy from baseline to week 96 was found in RMS patients treated with ocrelizumab compared to IFN β-1a independent of sNfL levels (low sNfL level [< 10.6 pg/ml]: ocrelizumab = −0.94%; IFN β-1a = −2.05%; high sNfL level [≥ 10.6 pg/ml]: ocrelizumab = −2.03%; IFN β-1a = −3.68%; p < 0.005 for all comparisons) [55]. There were similar findings for PPMS patients in the ORATORIO RCT (n = 596), where thalamic atrophy was significantly lower from baseline to week 120 in PPMS patients treated with ocrelizumab compared to placebo independent of sNfL levels (low sNfL level [< 10.3 pg/ml]: ocrelizumab = −1.12%; placebo = −1.46%; high sNfL level [≥ 10.3 pg/ml]: ocrelizumab = −1.95%; placebo = −3.10%%; p < 0.005 for all comparisons) [55].
In the OLE OPERA I and OPERA II pooled analysis, patients continuing ocrelizumab experienced less thalamic atrophy vs those initiating ocrelizumab later. Thalamic volume changes were significantly lower in ocrelizumab-ocrelizumab vs IFN β-1a-ocrelizumab patients at weeks 46, 94, 142, 190, 238 (−2.12%/ −2.88%, −2.36%/ −3.31%, −2.78%/ −3.61%, −3.03%/ −3.68%, and −3.41%/ −4.07%, respectively; p < 0.001 for all comparisons). Similar results were observed in the ORATORIO OLE, when comparing PPMS patients continuing ocrelizumab with those previously in the placebo group (−2.44%/ −3.46%, −2.61%/ −3.93%, −3.25%/ −4.30%, and −3.62%/ −4.86%; p < 0.001 for all comparisons) [56] (Table 1, Fig. 1).
Chronic Active Lesions
Pathological studies have shown that up to 57% of white matter lesions have a peripheral “rim” of iron-laden activated microglia/macrophages associated with ongoing demyelination and axonal loss, around an inactive core without blood–brain barrier damage [57,58,59,60]. These lesions, termed “chronic active,” reflect a compartmentalized chronic inflammatory pathological process that has been suggested to contribute to MS severity and progression [30, 61]. The potential effects of different DMTs on this more compartmentalized chronic inflammation have not been investigated so far, and they are not yet included in treatment monitoring [62].
Since chronic active lesions slowly increase in size over time (i.e., slowly evolving lesions [SEL]), a method for their assessment has been proposed based on the identification of white matter lesions showing a linear and progressive expansion over long-enough periods of time on conventional T1- and T2-weighted images [63,64,65].
In the pooled data from OPERA I and OPERA II (n = 1334) and ORATORIO (n = 555), SEL mean number (6.3 vs 4.6, p = 0.002), volume (1838 vs 1223 mm3, p < 0.001), and proportion of the volume among T2-hyperintense white matter lesions (11.3% vs 8.6%, p < 0.001) were significantly higher in PPMS compared with RMS [64]. Compared with non-SELs, SELs were also characterized by a lower T1 signal intensity and a significant longitudinal T1 signal intensity decline [64], suggesting more severe demyelination and axonal damage that accumulate at a faster rate compared to inactive white matter lesions.
In another study that evaluated 555 PPMS patients of the phase III ORATORIO RCT, SELs showed a larger decrease in mean normalized T1 signal intensity and a greater relative accumulation of T1-hypointense volume that predicted 12-week composite CDP (measured by EDSS worsening, ≥ 20% increase in T25FWT or in 9-HPT) from baseline to week 120 [63].
Of note, although the proportion of PPMS patients with ≥ 1 SEL was similar in ocrelizumab- and placebo-treated groups (73.2% vs 69.0%, respectively), the proportion of total pre-existing T2-hyperintense lesions identified as SELs from baseline to week 120 was significantly lower in ocrelizumab- vs placebo-treated PPMS patients (median = 2.5 vs 3.4, p = 0.044) [63]. Moreover, compared with placebo, ocrelizumab was associated with a lower T1-hypointense lesion volume increase from baseline to week 120 (p < 0.001), not only in new T2-hyperintense lesions (p < 0.001), but also in T2-hyperintense white matter lesions that were classified as SELs (+ 27% vs + 40%, p < 0.001) and non-SELs (+ 15% vs + 18%, p = 0.005) [63]. Finally, ocrelizumab was associated with a significantly reduced decrease in normalized T1 signal intensity in SELs from baseline to week 120 compared with placebo (− 0.24 vs − 0.28, p = 0.013) [63] (Table 1, Fig. 1).
The accumulation of chronic active lesions may represent one of the contributors to clinical disability independent of clinical relapses and overt inflammatory MRI activity (i.e., PIRA) [61]. Accordingly, the demonstration of a beneficial effect of ocrelizumab in limiting the number and volume of chronic active lesions and their microstructural abnormalities, which mainly reflect demyelination and axonal loss, may further support the use of this drug for limiting clinical worsening in MS patients.
Conclusions
The introduction of ocrelizumab in the MS armamentarium established an effective new therapeutic approach for the treatment of both RMS and PPMS patients. Growing evidence suggests that ocrelizumab may help to limit the progression of clinical disability and promote disability improvement.
Data from RCTs and their OLEs, and observational studies has shown that ocrelizumab has a strong anti-inflammatory action that is associated with an almost complete suppression of clinical relapses and MRI activity (i.e., new T2-hyperintense white matter lesions and Gd-enhancing lesions). In addition, it has been suggested that ocrelizumab also significantly reduces a substantial proportion of MS-related disability progression that is independent of overt inflammatory disease activity.
The use of composite scores to evaluate disability progression, not just the EDSS, but also measuring walking ability and manual dexterity, together with the evaluation of cognitive performance, severity of fatigue, and impact of MS patients’ quality of life, is allowing more accurate and comprehensive assessment of the beneficial effects of ocrelizumab.
The application of paraclinical tools, such as advanced MRI techniques and NfL quantification, which are more specific to the different pathological processes contributing to MS-related disability progression, is shedding light on the possible mechanisms underlying the positive effects of ocrelizumab. The significant reduction in the rate of global and regional brain atrophy and of NfL levels, which was found to be partially independent of overt inflammatory activity, suggests that this treatment may also limit neuro-axonal damage, which represents one of the most important pathological substrates leading to the accumulation of irreversible disability.
The significant reduction in the occurrence of microstructural tissue abnormalities in chronic active lesions suggests that ocrelizumab may be effective in reducing chronic and compartmentalized inflammation, which contributes to MS severity and progression.
Despite these promising results, further work is needed to confirm these findings, in longitudinal studies with a longer follow-up, and to validate the biomarkers so they can be applied in clinical settings.
References
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43.
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20.
Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–68.
Li R, Patterson KR, Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19(7):696–707.
Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019;18(12):905–22.
Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–40.
Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and Other CD20(+) B-cell-depleting therapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):835–41.
Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, et al. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest. 2013;123(12):5098–103.
Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–87.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52.
Kalincik T, Cutter G, Spelman T, Jokubaitis V, Havrdova E, Horakova D, et al. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138(Pt 11):3287–98.
Lorscheider J, Buzzard K, Jokubaitis V, Spelman T, Havrdova E, Horakova D, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–405.
Cadavid D, Cohen JA, Freedman MS, Goldman MD, Hartung HP, Havrdova E, et al. The EDSS-Plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult Scler. 2017;23(1):94–105.
Goldman MD, LaRocca NG, Rudick RA, Hudson LD, Chin PS, Francis GS, et al. Evaluation of multiple sclerosis disability outcome measures using pooled clinical trial data. Neurology. 2019;93(21):e1921–31.
Koch MW, Mostert JP, Wolinsky JS, Lublin FD, Uitdehaag B, Cutter GR. Comparison of the EDSS, timed 25-foot walk, and the 9-hole peg test as clinical trial outcomes in relapsing-remitting multiple sclerosis. Neurology. 2021;97(16):e1560–70.
Koch MW, Mostert J, Repovic P, Bowen JD, Uitdehaag B, Cutter G. Reliability of outcome measures in clinical trials in secondary progressive multiple sclerosis. Neurology. 2021;96(1):e111–20.
van Munster CE, Uitdehaag BM. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. 2017;31(3):217–36.
Uitdehaag BMJ. Disability outcome measures in phase III clinical trials in multiple sclerosis. CNS Drugs. 2018;32(6):543–58.
Margoni M, Preziosa P, Filippi M, Rocca MA. Anti-CD20 therapies for multiple sclerosis: current status and future perspectives. J Neurol. 2021.
Wolinsky JS, Arnold DL, Brochet B, Hartung HP, Montalban X, Naismith RT, et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):998–1009.
Giovannoni G, Kappos L, de Seze J, Hauser SL, Bonati U, Overell J, et al. Long-term reduction of relapse rate and confirmed disability progression after 7.5 years of ocrelizumab treatment in patients with relapsing multiple sclerosis in the OPERA OLE. Mult Scler J. 2021;27(2_suppl):606–7.
Wolinsky JS, Vermersch P, Hartung H-P, Naismith RT, Airas L, Townsend B, et al. Sustained reduction in 48-week confirmed disability progression in patients with PPMS treated with ocrelizumab in the ORATORIO OLE: 8-year follow-up. Mult Scler J. 2021;27(2_suppl):101–2.
Wolinsky JS, Montalban X, Hauser SL, Giovannoni G, Vermersch P, Bernasconi C, et al. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann Neurol. 2018;84(4):527–36.
University of California SFMSET, Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–66.
Lublin FD, Haring DA, Ganjgahi H, Ocampo A, Hatami F, Cuklina J, et al. How patients with multiple sclerosis acquire disability. Brain. 2022.
Chan A, Phillips JT, Fox R, Zhang A, Potts J, Kurukulasuriya N, editors. Differential recovery from relapse between treatment groups in the CONFIRM study of delayed-release dimethyl fumarate (P7.215). AAN; 2015: https://n.neurology.org/content/84/14_Supplement/P7.215 Poster P7.215. Accessed 8 Nov 2021.
Wiendl H, Spelman T, Butzkueven H, Kappos L, Trojano M, Su R, et al. Real-world disability improvement in patients with relapsing-remitting multiple sclerosis treated with natalizumab in the Tysabri Observational Program. Mult Scler. 2021;27(5):719–28.
Phillips JT, Giovannoni G, Lublin FD, O’Connor PW, Polman CH, Willoughby E, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler. 2011;17(8):970–9.
Filippi M, Preziosa P, Langdon D, Lassmann H, Paul F, Rovira A, et al. Identifying progression in multiple sclerosis: new perspectives. Ann Neurol. 2020;88(3):438–52.
Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19(10):860–71.
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302–17.
Preziosa P, Conti L, Rocca MA, Filippi M. Effects on cognition of DMTs in multiple sclerosis: moving beyond the prevention of inflammatory activity. J Neurol. 2021.
Benedict R, De Seze J, Hauser S, Kappos L, Wolinsky J, Zheng H, et al., editors. Impact of ocrelizumab on cognition in patients at increased risk of progressive disease (P1.420). AAN; 2018: https://n.neurology.org/content/90/15_Supplement/P1.420 Poster P1.420. Accessed 8 Nov 2021.
Cohan S, Benedict R, De Seze J, Hauser S, Kappos L, Wolinsky J, et al., editors. Time to cognitive worsening in patients with relapsing multiple sclerosis in ocrelizumab phase III trials (S44.005). AAN; 2018: https://n.neurology.org/content/90/15_Supplement/S44.005 Platform Presentation S44.005. Accessed 8 Nov 2021.
Benedict RH, Pol J, Yasin F, Hojnacki D, Kolb C, Eckert S, et al. Recovery of cognitive function after relapse in multiple sclerosis. Mult Scler. 2021;27(1):71–8.
Benedict R, Sormani MP, McGinley M, Arnold D, Bar-Or A, Bermel R, et al. A multicenter, open label, single-arm, phase 3b study (CONSONANCE) to assess efficacy of ocrelizumab in patients with primary and secondary progressive multiple sclerosis: year 1 interim analysis of cognition outcomes (P1–1.Virtual). Neurology. 2022;98(18 Supplement):647.
Lerdal A, Celius EG, Krupp L, Dahl AA. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol. 2007;14(12):1338–43.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Glanz BI, Zurawski J, Casady EC, Shamah R, Weiner M, Chitnis T, et al. The impact of ocrelizumab on health-related quality of life in individuals with multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7(2):20552173211007524.
Neuberger EE, Abbass IM, Jones E, Engmann NJ. Work productivity outcomes associated with ocrelizumab compared with other disease-modifying therapies for multiple sclerosis. Neurol Ther. 2021;10(1):183–96.
Preziosa P, Rocca MA, Filippi M. Current state-of-art of the application of serum neurofilaments in multiple sclerosis diagnosis and monitoring. Expert Rev Neurother. 2020;20(8):747–69.
Delcoigne B, Manouchehrinia A, Barro C, Benkert P, Michalak Z, Kappos L, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology. 2020;94(11):e1201–12.
Cross A, Bennett J, von Büdingen HC, Carruthers R, Edwards K, Fallis R, et al., editors. Ocrelizumab treatment reduced levels of neurofilament light chain and numbers of B cells in the cerebrospinal fluid of patients with relapsing multiple sclerosis in the OBOE study (S56.008). AAN; 2019. https://n.neurology.org/content/92/15_Supplement/S56.008 Platform Presentation S56.008. Accessed 8 Nov 2021.
Bar-Or A, Thanei G, Harp C, Bernasconi C, Bonati U, Cross AH, et al., editors. Ocrelizumab treatment induces a sustained blood NfL reduction in patients with PPMS and RMS. Mult Scler J 2020; 26 (S3): 178-179. ECTRIMS; 2020: MSVirtual 2020—Poster Abstracts P0125. Accessed 8 Nov 2021. https://journals.sagepub.com/doi/full/10.1177/1352458520974937
Bar-Or A, Thanei G, Harp C, Bernasconi C, Bonati U, Cross AH, et al., editors. Blood neurofilament light levels are lowered to a healthy donor range in patients with RMS and PPMS following ocrelizumab treatment. Mult Scler J 2019; 25 (S2): 52. ECTRIMS 2019—Oral Presentations 152. Accessed 8 Nov 2021. https://journals.sagepub.com/doi/10.1177/1352458519868070
Rocca MA, Battaglini M, Benedict RH, De Stefano N, Geurts JJ, Henry RG, et al. Brain MRI atrophy quantification in MS: from methods to clinical application. Neurology. 2017;88(4):403–13.
Sastre-Garriga J, Pareto D, Battaglini M, Rocca MA, Ciccarelli O, Enzinger C, et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. 2020;16(3):171–82.
Filippi M, Preziosa P, Rocca MA. Magnetic resonance outcome measures in multiple sclerosis trials: time to rethink? Curr Opin Neurol. 2014;27(3):290–9.
Rocca MA, Preziosa P, Filippi M. Application of advanced MRI techniques to monitor pharmacologic and rehabilitative treatment in multiple sclerosis: current status and future perspectives. Expert Rev Neurother. 2019;19(9):835–66.
Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75(1):43–9.
Hauser SL, Kappos L, Arnold DL, Bar-Or A, Brochet B, Naismith RT, et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology. 2020;95(13):e1854–67.
Eshaghi A, Prados F, Brownlee WJ, Altmann DR, Tur C, Cardoso MJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210–22.
Azevedo CJ, Cen SY, Khadka S, Liu S, Kornak J, Shi Y, et al. Thalamic atrophy in multiple sclerosis: a magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol. 2018;83(2):223–34.
Bar-Or A, Thanei G, Harp C, Bernasconi C, Bonati U, Cross AH, et al. Ocrelizumab reduces thalamic volume loss and clinical progression in ppms and rms independent of baseline nfl and other measures of disease severity. Mult Scler J 2020; 26 (S3): 177. ECTRIMS: MSVirtual 2020—Poster Abstracts P0123. Accessed 8 Nov 2021. https://journals.sagepub.com/doi/full/10.1177/1352458520974937
Arnold D, Sprenger T, Bar-Or A, Wolinsky J, Kappos L, Kolind S, et al. editors. Reduced thalamic atrophy in patients initiating earlier versus delayed ocrelizumab therapy: results from the OLE of OPERA I/II and ORATORIO. Mult Scler J 2020; 26 (S3): 8-9. ECTRIMS; 2020: MSVirtual 2020—Platform Presentations FC03.05. Accessed 8 Nov 2021. https://journals.sagepub.com/doi/full/10.1177/1352458520974936
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710–21.
Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 2018;135(4):511–28.
Dal-Bianco A, Grabner G, Kronnerwetter C, Weber M, Hoftberger R, Berger T, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133(1):25–42.
Dal-Bianco A, Grabner G, Kronnerwetter C, Weber M, Kornek B, Kasprian G, et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain. 2021;144(3):833–47.
Filippi M, Preziosa P, Barkhof F, Chard DT, De Stefano N, Fox RJ, et al. Diagnosis of progressive multiple sclerosis from the imaging perspective: a review. JAMA Neurol. 2021;78(3):351–64.
Preziosa P, Filippi M, Rocca MA. Chronic active lesions: a new MRI biomarker to monitor treatment effect in multiple sclerosis? Expert Rev Neurother. 2021;21(8):837–41.
Elliott C, Belachew S, Wolinsky JS, Hauser SL, Kappos L, Barkhof F, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain. 2019;142(9):2787–99.
Elliott C, Wolinsky JS, Hauser SL, Kappos L, Barkhof F, Bernasconi C, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler. 2019;25(14):1915–25.
Preziosa P, Pagani E, Moiola L, Rodegher M, Filippi M, Rocca MA. Occurrence and microstructural features of slowly expanding lesions on fingolimod or natalizumab treatment in multiple sclerosis. Mult Scler. 2020:1352458520969105.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
M. Margoni reports grants and personal fees from Almiral. She was awarded a MAGNIMS-ECTRIMS fellowship in 2020. P. Preziosa received speakers’ honoraria from Biogen Idec, Novartis, Bristol, Myers Squibb, Genzyme, Roche, and Excemed. P. Tortorella is an employee of Roche SpA. M. Filippi is Editor-in-Chief of the Journal of Neurology and Associate Editor of Radiology, Human Brain Mapping and Neurological Sciences; received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi, Almiral, Eli Lilly, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). M.A. Rocca received speakers’ honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Margoni, M., Preziosa, P., Tortorella, P. et al. Does Ocrelizumab Limit Multiple Sclerosis Progression? Current Evidence from Clinical, MRI, and Fluid Biomarkers. Neurotherapeutics 19, 1216–1228 (2022). https://doi.org/10.1007/s13311-022-01252-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01252-5