Abstract
Video-assisted thoracic surgery (VATS) has been widely used in lung cancer treatment. However, VATS left upper lobectomy (LUL) is complex due to the intricate branching pattern of the left pulmonary artery (PA). Nevertheless, VATS right upper lobectomy can be simplified through a bronchus-first and simultaneous vessel stapling technique. In this study, the learning curve was obtained while ensuring favorable oncological outcomes using bronchus-first method for VATS LUL. First, retrospective data of 148 consecutive patients who underwent VATS LUL (bronchus-first method) for non-small cell lung cancer (NSCLC) from March 2018 to October 2020 were analyzed. The learning curve was then assessed via cumulative sum (CUSUM) analysis. Moreover, data at different stages of the learning curve, including operation time, blood loss, postoperative hospital stay, lymph node harvested, thoracotomy conversion, postoperative complications, endoscopic stapler consumptions, and 3 year overall survival, were recorded. The learning curve was best modeled as the equation: y = − 7.78 + 2.05x−2.23 × 10−2x2 + 6.43 × 10−5x3, with a good-to-fit test R2 = 0.97. The surgeon entered the proficient stage (59th case–148th case) after consecutive operations of 58 cases (learning stage, 1st case–58th case). Notably, more lymph nodes were harvested in the proficient stage than in the learning stage (17.69 ± 1.47 vs. 15.53 ± 1.43, P < 0.01). Compared with the learning stage, the proficient stage was associated with shorter operation time (114.28 ± 8.56 min vs. 126.81 ± 7.30 min, P < 0.01), fewer blood loss (44.22 ± 7.75 mL vs. 57.41 ± 22.98 mL, P < 0.01), shorter postoperative hospital stay (6.02 ± 0.99 d vs. 7.22 ± 1.34 d, P < 0.01), and fewer endoscopic stapler consumptions (5.89 ± 0.64 vs. 6.53 ± 0.50, P < 0.01). However, thoracotomy conversion (4/90 vs. 5/58, P = 0.32), postoperative complications (10/90 vs. 11/58, P = 0.23) and 3 year overall survival (62.2% vs. 50.8%, log-rank test, P = 0.11) showed no significant difference between the two stages. The surgeon with former single-direction VATS lobectomy experience can master bronchus-first VATS LUL after attending to 58 cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. Moreover, video-assisted thoracic surgery (VATS) lobectomy is widely used to treat lung cancer [1,2,3]. Conventionally, VATS LUL is complicated and challenging, especially for junior surgeons with limited former VATS experience in treating highly variable branching pattern of the left pulmonary artery [4], which may cause massive hemorrhage risk due to vessel injuries. Previous studies showed that VATS right upper lobectomy [5, 6] and left upper lobectomy [7, 8] can be simplified via a bronchus-first and simultaneous vessel stapling technique. In this study, the operation procedure, the learning curve, and oncological outcomes were assessed by introducing this bronchus-first method to bi-port VATS LUL.
Methods
Retrospective data of 148 patients with postoperative pathological stage IB to stage IIIA lung cancer [9] who underwent bi-port VATS LUL using a bronchus-first technique between March 2018 and October 2020 in the Department of Thoracic Surgery, Henan Provincial Chest Hospital were analyzed. The patients received routine examinations, including thoracic computed tomography, cerebral magnetic resonance imaging, skeletal emission computed tomography, and abdominal color Doppler ultrasound (CDU). Besides, cardiopulmonary function was assessed via pulmonary function test, electrocardiogram, and cardiac CDU. The patients and their families signed informed consent forms, and the study was approved by the medical ethics committee of the hospital.
The inclusion criteria were: (1) patients with non-small cell lung cancer; (2) patients with cancer diameter less than 4 cm and without pleural dissemination and distant organ metastasis; (3) patients whose function of important organs, such as heart and lungs, could withstand lobectomy; (4) patients with no anti-tumor treatment history, such as radiotherapy, chemotherapy, and targeted drugs.
The exclusion criteria included: (1) patients with extensive dense adhesions or pleural calcification in the thoracic cavity that a decortication was a must via VATS or thoracotomy; (2) patients with tumors involving the chest wall or other organs that require simultaneous resection; (3) patients with interlobular tumor requiring combined lobectomy; (4) patients with central lung cancer of left upper lobe requiring bronchoplasty and/or angioplasty.
Operator experience: The selected surgeon had conducted about 100 cases of single-direction VATS of lobectomy (including 18 cases of single-direction VATS LUL) and participated in other 800 cases as assistants (including 100 cases of single-direction VATS LUL and 41 cases of bronchus-first VATS LUL). Moreover, the assistant had participated in about 600 single-direction VATS of lobectomy (including 100 cases of single-direction VATS of LUL and 41 cases of bronchus-first VATS LUL). The scrub nurses and the anesthetists were relatively fixed, and postoperative management was completed by the assistant and the specialized nursing team.
Surgery procedure
First, the patients were fixed to lateral decubitus position after double-lumen tracheal intubation. A 3 cm ~ 4 cm incision was made at the 4th intercostal space of the anterior axillary line as the utility port, and another incision (about 1.5 cm) was made at the 7th intercostal space of the mid-axillary line as the camera port.
The primary procedures included lobectomy and systemic lymphadenectomy. The main anatomical structure was processed in the following sequence: interlobar lingual artery, posterior artery (Fig. 1A), left upper bronchus (Fig. 1B, C), and all residual vessels of left upper lobe (Fig. 1D, E).
The interlobar lingual artery and the posterior artery dorsal to the bronchus were separated and ligated along the oblique fissure in case of a well-developed oblique fissure (Fig. 1A). A dissection was then made along the anterior hilum. A gentle blunt dissection was used to separate superior pulmonary veins (SPV) from the anterior surface of the left upper bronchus. The surgeon should allow the clamp to pass superficial to branches of PA when passing a clamp from the “entrance” between SPV and the left upper bronchus to avoid injury while ensuring that there is adequate space posterior as “exit” for the stapler positioning. The left upper bronchus was stapled after confirmation via lung dilation (Fig. 1B, C), followed by the simultaneous stapling of SPV and all residual artery branches of left upper lobe (Fig. 1D, E), and specimen removal.
Notably, the “tunnel method” was used if the oblique fissure was not fully developed. Briefly, a tunnel was established from the lower margin of the lingual vein to the superficial margin of the interlobar lingual artery, then the anterior oblique fissure was opened using an endoscopic stapler. Another tunnel was established along the upper margin of the dorsal artery to the posterior hilum, and posterior oblique fissure was opened using an endoscopic stapler. The following procedures were the same as in the case of a well-developed fissure.
A complete radical lymphadenectomy was performed at station 10#, 7#, 5#, 6#, 11#, and 12# lymph nodes. The station 10# lymph nodes of the anterior hilum and 11# lymph nodes could be dissected along the oblique fissure, while the 11# and 12# lymph nodes surrounding the bronchus could be removed from the specimen.
After pushing the left lower lobe to ventral side by forceps, the posterior hilum and subcarinal area could be fully exposed, and station 10# lymph nodes could be removed. Then the pleura is incised along the inner side of descending aortae, and the gap between the descending aortae and pericardium is then enlarged with an ultrasonic scalpel, and station 7# lymph nodes could be removed. Then move to the posterior hilum, and gradually open the pleura below aortic arch with ultrasonic scalpel. Meanwhile identify the recurrent laryngeal nerve by blunt separation and protect it when dissecting station 5# and 6# lymph nodes.
CUSUM analysis
CUSUM analysis was conducted based on four indicators: operation time (δ1), intraoperative blood loss (δ2), number of dissected lymph nodes (δ3), and levels of dissected lymph nodes (δ4). The δ value for each surgery was determined using this formula: δ = xi− × 0. The mean values of the indicators above were used as the target value. The failure rates (× 0) of these indicators were determined as follows: × 0 = failure cases/total cases. The target value was reached at xi = 0; otherwise, xi = 1. The failure rates of the above indicators were 0.45, 0.49, 0.43, and 0.36, respectively. The cumulative score for each surgery was determined as follows: ∑ = δ1 + δ2 + δ3 + δ4. The learning curve was assessed by polynomial curve fitting. The cutoff point was noted when the slope of the curve changed from positive to negative [10,11,12,13].
Statistical analysis
SPSS 20.0 for Windows (SPSS, Inc., Chicago, IL, USA) and Microsoft Office Excel 2016 (Microsoft, Inc., Redmond, TX, USA) were used for all statistical analyses. Continuous variables were expressed as mean ± standard deviation (‾x ± s). The independent sample t test was used after normality tests for inter-group comparisons, while Chi-square (χ2) test was used for categorical variables. The overall survival was analyzed using Kaplan–Meier analysis. The difference between the two stages was compared via the log-rank test. P < 0.05 was considered statistically significant level.
Results
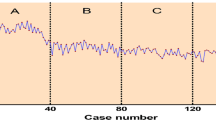
Although all surgeries were successfully completed, 9 cases of thoracotomy conversion (5 hemorrhage cases and 4 cases of nail lymph nodes) and 21 cases of overall morbidity (12 cases of pulmonary infections occurred. However, they recovered and were discharged after treatment with no perioperative death. The best fitting equation of CUSUM learning curve was y = − 7.78 + 2.05x−2.23 × 10−2x2 + 6.43 × 10−5x3, with a good-to-fit R2 = 0.97 (Fig. 2A). The slope of this equation changed from positive to negative after 58 cases of surgeries. The surgeon entered the proficient stage (59th case–148th case) after consecutive operations of 58 cases (learning stage, 1st case–58th case). More lymph nodes were harvested in the proficient stage than in the learning stage (17.69 ± 1.47 vs. 15.53 ± 1.43, P < 0.01). Compared with the learning stage, the proficient stage was associated with shorter operation time (114.28 ± 8.56 min vs. 126.81 ± 7.30 min, P < 0.01), fewer blood loss (44.22 ± 7.75 mL vs. 57.41 ± 22.98 mL, P < 0.01), shorter postoperative hospital stay (6.02 ± 0.99 d vs. 7.22 ± 1.34 d, P < 0.01), and fewer endoscopic stapler consumptions (5.89 ± 0.64 vs. 6.53 ± 0.50, P < 0.01). However, thoracotomy conversion (4/90 vs. 5/58, P = 0.32), postoperative complications (10/90 vs. 11/58, P = 0.23), and 3 year overall survival (62.2% vs. 50.8%, log-rank test, P = 0.11) were not significantly different between the two stages. Also, the baseline characteristics, such as age, gender, pathological type, and postoperative TNM staging, were not significantly different between the two stages (P > 0.05, Table 1).
A total of 21(21/148, 14.2%) patients developed complications more than Grade 2 based on Clavien–Dindo classification, and they all recovered after treatments, including 12 cases with postoperative pneumonia requiring an antibiotic therapy, 4 cases with prolonged air leakages lasting more than 3 days, and 3 cases with empyema requiring prolonged chest tube drainage. The complication rates (PS vs. LS, 11.1% vs. 19.0%, P = 0.23) were not differed among the two phases of VATS lobectomy. No mortality occurred in perioperative period in any of the two phases. There were no 30-day operative mortalities, and no cases of bronco-pleural fistula were observed in our study.
In our study, the survival data were derived from a cohort of patients with different stages, including 38 cases (22.6%) in stage I, 66 cases (39.2%) in stage II, and 44 cases (26.1%) in stage III. The median duration of follow-up was 51.6 months. The median overall survival (OS) was 36.0 months in LS stage and 37.0 months in LS stage. The 3 year OS in the PS and LS (62.2% vs. 50.8%, log-rank test, P = 0.11) showed no significant difference between the two stages.
Discussion
The learning curve refers to the gradual process of mastering a certain skill through continuous learning. The learning curve of surgical procedures has been widely analyzed using CUSUM analysis since the 1970s [10,11,12,13,14]. In our study, a surgeon could enter the proficient stage after 58 cases of consecutive operations, meanwhile data from other studies showed competency being achieved after conducting 25 to 140 cases [15,16,17,18,19,20]. Data from different surgeons with different backgrounds often vary enormously. Liu X, et. al. [16] found that after approximately 60 cases, a single center could reach proficiency for VATS uniportal lobectomy. However, the individual characteristics of the surgeons in the learning process was not given. Other studies noted data from the learning curve of a single or two surgeons. Li, et.al. [17] revealed that proficiency of VATS lobectomy could be both achieved by 2 senior surgeons after more than 100 cases of surgeries. Liang, et.al. [18] found that the junior surgeon had a shorter learning curve than the senior surgeon in single-port thoracoscopic lobectomy, with competency achieved at 25 cases vs. 37 cases. Yao, et.al. [15] described the learning curve for VATS lobectomy by a single surgeon, and found that 26 cases were required to achieve proficiency. In another study, Song, et.al. [19] presented that a single surgeon could achieve efficacy after 35 cases and proficiency was achieved after 53 cases. Vieira, et.al. [20] noted that 140 cases of lobectomies were required to master the approach by a single surgeon.
Conventional studies mainly focused on the evaluation of single perioperative parameter (operation time, e.g.) [15,16,17, 19, 20], and multi-dimensional parameter analysis was rare [18]. However, data from a single parameter would make statistic job easier, but proficiency of parameters (e.g., operation time, blood loss, postoperative hospital stay, lymph node dissection) would not always be achieved synchronously, and bias may produce even when describing the learning procedure of a same surgeon. In our study, four indicators were used as the evaluation criteria, and we could obtain a more precise assessment of this learning procedure. Moreover, the overall survival may also be another indicator of the learning curve [12] since the above differences may not be distinguished during the perioperative period. Besides, postoperative overall survival can reflect these differences several years later. In this study, this method simplified the operation and also showed oncologic efficacy, with 3 year overall survival over 50% in the two stages of the learning curve, necessitating further clinical follow-up.
The learning curve for VATS lobectomy has been widely researched, notably case requirements may be higher in the absence of mentoring at high-volume centers. However, high-fidelity simulation may flatten the learning curve of VATS of lobectomy due to technology advances [21, 22]. Moreover, surgical videos can facilitate the acquisition of new surgical skills and accelerate the learning of new techniques [23]. High operation frequency helps surgeons to attain proficiency. In this study, the operation frequency of 40–50 cases of VATS LUL a year could not rapidly enhance proficiency. Therefore, the team participated in relative scholar meetings, exchanging opinions on technical details with experts, repeated reviewing of the surgical videos of the experts and ours to formulate key surgical points. Moreover, a fixed surgical team, including the assistant, anesthesiologists, and scrub nurses, continuously optimized the surgical process. Finally, a strict intraoperative guidance by the corresponding author ensured the uniformity and the safety of the operation.
The procedures in single-direction VATS LUL included stapling of SPV, anterior artery, left upper bronchus, apicoposterior artery, interlobar lingual artery and oblique fissure [24, 25]. Our method divided the VATS LUL into two main procedures: the stapling of bronchus and the stapling of the vessels. Compared with single-direction VATS LUL, this procedure sequence was similar to a “reverse direction” VATS LUL [8]. After bronchus stapling, the left upper lobe could be flexibly rotated to a safer and easier position for simultaneous stapling of SPV, anterior artery, and apicoposterior artery. In this study, the vascular sheath were opened, and the lymph nodes firmly attached to them were reflected to distal of the specimen. Therefore, these lymph nodes could be easily retrieved after incision closure, thus reducing the vessel injury risk and operation time. However, vessel injury risk may still occur when separating and stapling the left upper bronchus, especially for novice surgeons in the learning stage. Moreover, this operation procedure was not always fixed, and could even be unsuitable for patients with mediastinal lingular artery in about 30% of VATS LUL [24, 25]. Therefore, a close scrutiny of the chest computed tomography or three-dimensional computed tomography bronchography and angiography (3D-CTBA) is necessary before surgery to explicit dangerous anatomy variants that may be encountered during the operation [26,27,28]. Furthermore, arteriovenous fistula (AVF) risk may occur when multiple vessels are simultaneously stapled. However, no AVF data were found during the follow-up, necessitating further clinical observation.
This study has some limitations. First, this is a retrospective study performed in a single center, and postoperative survival benefits of the two stages require long-term follow-up. Finally, the success of the surgical procedure depends on the whole surgical team members, including the surgeon, assistant, anesthetists, and scrub nurses. Surgeons can enter the proficient stage from the learning stage after rigorous training, standardized operation practice, and a certain number of cases [29, 30]. The surgeons can gradually perform VATS LUL (bronchus-first method) under supervision once the basic skills are learned.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Berfield KS, Farjah F, Mulligan MS (2019) Video-assisted thoracoscopic lobectomy for lung cancer. Ann Thorac Surg 107(2):603–609. https://doi.org/10.1016/j.athoracsur.2018.07.088
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (2021) Lung cancer. Lancet 398:535–554. https://doi.org/10.1016/S0140-6736(21)00312-3
Murota M, Yamamoto Y, Satoh K, Ishimura M, Yokota N, Norikane T, Mitamura K, Takami Y, Fujimoto K, Nishiyama Y (2020) An analysis of anatomical variations of the left pulmonary artery of the interlobar portion for lung resection by three-dimensional CT pulmonary angiography and thin-section images. Jpn J Radiol 38:1158–1168. https://doi.org/10.1007/s11604-020-01024-1
Xu H, Zhang L (2019) The bronchus first and vessels simultaneously stapled technique: a safe and simple method for video-assisted right upper lobe lobectomy. Thorac Cardiovasc Surg 67(2):131–136. https://doi.org/10.1055/s-0037-1620276
Zhang L, Xu H (2018) Video-assisted thoracic surgical right upper lobectomy with bronchus-first and simultaneous vessel stapling technique. Thorac Cardiovasc Surg 66(2):177–179. https://doi.org/10.1055/s-0037-1602261
Grismer JT, Read RC (1995) Evolution of pulmonary resection techniques and review of the bronchus-first method. Ann Thorac Surg 60(4):1133–1137. https://doi.org/10.1016/0003-4975(95)00602-h
Zhang M, Sihoe AD, Du M (2016) A “reverse direction” technique of single-port left upper pulmonary resection. J Thorac Dis 8(8):2252–2255. https://doi.org/10.21037/jtd.2016.06.79
Goldstraw P, Chansky K, Crowley J, Nicholson AG, Groome P, Mitchell A, Bolejack V, International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions (2016) The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009
Chaput de Saintonge DM, Vere DW (1974) Why don’t doctors use cusums? Lancet 1(7848):120–121. https://doi.org/10.1016/s0140-6736(74)92345-9
Hopper AN, Jamison MH, Lewis WG (2007) Learning curves in surgical practice. Postgrad Med J 83:777–779. https://doi.org/10.1136/pgmj.2007.057190
Wohl H (1977) The cusum plot: its utility in the analysis of clinical data. N Engl J Med 296(18):1044–1045. https://doi.org/10.1056/NEJM197705052961806
Valsamis EM, Chouari T, O’Dowd-Booth C, Rogers B, Ricketts D (2018) Learning curves in surgery: variables, analysis and applications. Postgrad Med J 94(1115):525–530. https://doi.org/10.1136/postgradmedj-2018-135880
Puri V, Gaissert HA, Wormuth DW, Grogan EL, Burfeind WR, Chang AC, Seder CW, Fernandez FG, Brown L, Magee MJ, Kosinski AS, Raymond DP, Broderick SR, Welsh RJ, DeCamp MM, Farjah F, Edwards MA, Kozower BD (2019) defining proficiency for the society of thoracic surgeons participants performing thoracoscopic lobectomy. Ann Thorac Surg 107(1):202–208. https://doi.org/10.1016/j.athoracsur.2018.07.074
Yao F, Wang J, Yao J, Hang F, Cao S, Cao Y (2017) Video-assisted thoracic surgical lobectomy for lung cancer: description of a learning curve. J Laparoendosc Adv Surg Tech A 27(7):696–703. https://doi.org/10.1089/lap.2016.0636
Liu X, Chen X, Shen Y, Wang H, Feng M, Tan L, D’Amico TA (2018) Learning curve for uniportal video-assisted thoracoscopic surgery lobectomy-results from 120 consecutive patients. J Thorac Dis 10(8):5100–5107. https://doi.org/10.21037/jtd.2018.08.87
Li X, Wang J, Ferguson MK (2014) Competence versus mastery: The time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 147:1150–1154
Liang M, Wu P, Xu C, Zheng B, Chen C (2023) Junior surgeons are quicker to master the single-port thoracoscopic lobectomy: comprehensive analysis of the learning curve and oncological outcomes. World J Surg Oncol 21(1):134. https://doi.org/10.1186/s12957-023-03017-6
Song Z, Yuan Y, Cheng C, Chen S, Zhang X (2023) The learning curve on uniportal video-assisted thoracoscopic lobectomy with the help of postoperative review of videos. Front Oncol 13:1085634. https://doi.org/10.3389/fonc.2023.1085634
Vieira A, Bourdages-Pageau E, Kennedy K, Ugalde PA (2020) The learning curve on uniportal video-assisted thoracic surgery: an analysis of proficiency. J Thorac Cardiovasc Surg 159(6):2487–95.e2482. https://doi.org/10.1016/j.jtcvs.2019.11.006
Jensen K, Bjerrum F, Hansen HJ, Petersen RH, Pedersen JH, Konge L (2017) Using virtual reality simulation to assess competence in video-assisted thoracoscopic surgery (VATS) lobectomy. Surg Endosc 31(6):2520–2528. https://doi.org/10.1007/s00464-016-5254-6
Nashaat A, Sidhu HS, Yatham S, Al-Azzawi M, Preece R (2019) Simulation training for lobectomy: a review of current literature and future directions. Eur J Cardiothorac Surg 55(3):386–394. https://doi.org/10.1093/ejcts/ezy276
Ibrahim AM, Varban OA, Dimick JB (2016) Novel uses of video to accelerate the surgical learning curve. J Laparoendosc Adv Surg Tech A 26(4):240–242. https://doi.org/10.1089/lap.2016.0100
Liu L, Che G, Pu Q, Ma L, Wu Y, Kan Q, Zhuge X, Shi L (2010) A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 19(2):e71–e77. https://doi.org/10.1016/j.suronc.2009.04.005
Liao H, Mei J, Lin F, Liu C, Pu Q, Liu L (2018) Single-direction thoracoscopic lobectomy: left side. J Thorac Dis 10(10):5932–5934. https://doi.org/10.21037/jtd.2018.09.34
Li Z, Wu W, Kong Y et al (2023) Analysis of variations in the bronchovascular pattern of the lingular segment to explore the correlations between the lingular segment artery and left superior division veins. Front Surg 10:1173602. https://doi.org/10.3389/fsurg.2023.1173602
He H, Chen P, Chen X, Wang PY, Liu SY, Wang F (2021) Analysis of anatomical variations of the lingular artery of the left upper lobe using 3D computed tomography angiography and bronchography. J Thorac Dis 13(8):5035–5041. https://doi.org/10.21037/jtd-21-1141
Hagiwara M, Shimada Y, Kato Y, Nawa K, Makino Y, Furumoto H, Akata S, Kakihana M, Kajiwara N, Ohira T, Saji H, Ikeda N (2014) High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery. Eur J Cardiothorac Surg 46(6):e120–e126. https://doi.org/10.1093/ejcts/ezu375
Grossi S, Cattoni M, Rotolo N, Imperatori A (2023) Video-assisted thoracoscopic surgery simulation and training: a comprehensive literature review. BMC Med Educ 23(1):535. https://doi.org/10.1186/s12909-023-04482-z
Liang H, Liang W, Lei Z, Liu Z, Wang W, He J, Zeng Y, Huang W, Wang M, Chen Y, He J (2018) Three-dimensional versus two-dimensional video-assisted endoscopic surgery: a meta-analysis of clinical data. World J Surg 42(11):3658–3668. https://doi.org/10.1007/s00268-018-4681-z
Acknowledgements
Not applicable.
Funding
The study was funded by Henan Medical Science and Technology Research Project (No: 2018020545).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Compliance with ethical standards
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved in accordance with the requirements of the Helsinki Declaration and relevant laws and regulations. The study has been approved by the ethics committee of Henan Provincial Chest Hospital (No.201803–16). Informed consent was obtained from all individual participants included in the study. The participant has consented to the submission of the case report to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, G., Zhang, H. & Qian, R. The learning curve of a bronchus-first method in bi-port video-assisted thoracoscopic surgery for left upper lobe lung cancer. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01826-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13304-024-01826-2