Abstract
The use of 3D printing is gaining considerable success in many medical fields including surgery. Here, the technology was introduced for increasing the level of anatomical understanding thanks to the inherent characteristics of 3D printed models: these are highly accurate and customized reproductions, being obtained from own radiological imaging of patients, and are solid graspable objects allowing for free manipulation on part of the user. The resulting tactile feedbacks significantly help the comprehension of anatomical details, especially the spatial relations between structures. In this regard, they proved to be more effective than conventional 2D imaging and 3D virtual models. To date, an increasing number of applications have been successfully tested in many surgical disciplines, extending the range of possible uses to pre-operative planning, counselling with patients, education of students and residents, surgical training, intraoperative navigation and others; in recent years, 3D printing was also employed for creating surgical tools and reproducing anatomical parts to be used, respectively, as templates or guides for specific tasks of the surgery and individualized implantable materials in reconstructive procedures. Future expectations concern on one side the reduction of manufacturing costs and time to further increase the accessibility of 3D printing, while on the other the development of novel techniques and materials suitable for 3D printing of biological structures by which recreating the architecture and functionality of real human organs and tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decades, the increasing development of novel computer-based technologies has led to the introduction of striking innovations in many fields of medicine. Radiology has marked a significant step forward when digital revolution has made available highly detailed information thanks to sophisticated tools for the acquisition and elaboration of medical images [1]. Despite the high quality and resolution of those obtained from current cross-sectional scanners, a comprehensive knowledge of the real anatomy is often difficult to infer [2]. In surgery, a clear understanding of the target anatomy should be obtained in the preoperative setting with the aim of planning the operation accordingly. The crucial steps of surgery often arise from prior interpretation of the available imaging. This ability is routinely based on the analysis of 2D pictures derived from conventional radiology. Surgeons are, therefore, supposed to mentally reconstruct what they watch on flat monitors into a 3D architecture where all details are likely to be represented as they would appear intraoperatively [3]. This is then reinforced or modified by the continuous visual feedbacks that the surgeon receives while operating. It is plausible that no other field of medicine is subjected to such complex process that gets gradually acquired as the experience of the operator increases, thus making junior surgeons less prone to it. In addition, the more the anatomy is irregular due to inherent variations or disease-related distortions, the harder its comprehension results. Which may turn out to be even harder for novices.
3D virtual (3DV) reconstructions have been created to facilitate anatomical understanding [1, 3], but have limited availability due to the need for dedicated software and personnel [3]. Moreover, they are still flat images projected on screens that the user must scroll all around to get different points of view from which evaluating the interspatial relationship between different areas of the target anatomy.
3D printing (3DP) technology has been recently applied to surgery for obviating the above shortcomings [4,5,6]. It has rapidly expanded in the industrial field for manifold uses prior to be introduced in medicine and it is still in constant evolution [7]. As to the production of 3D printed anatomical models, the process starts from a common volumetric dataset acquired by multi-detector computed tomography (MDCT) or magnetic resonance (MRI), and moves through the generation of a virtual 3D model of the anatomy of interest, that can then be translated into a physical object by means of the most suitable 3DP technology [2, 3]. The benefit of such manufactures comes from the augmented sensory perception that sense of touch conveys to the user [8]; the tactile feedbacks obtained by simply manipulating the model combine with visual inputs increasing the amount and accuracy of perceived details [3, 9]. The process of retrieving crucial information from the 3D printed model is not only more effective but, but it also takes less time to be generated with respect to 2D/3D imaging assessment [10].
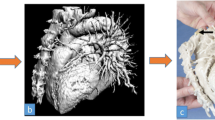
New devices and materials are being continuously developed to test novel areas of applicability and to speed up the printing process, furthermore, providing less expensive though highly realistic anatomical models (Fig. 1). To date, the spectrum of possible 3DP applications has been extended to various surgical specialties for multiple purposes: pre-operative planning, teaching to medical students and residents, counselling of patients, training of naive surgeons, simulation of surgical tasks and supplying of specifically designed models suitable for being used as implants or prosthesis [11, 12].
What follows aims at outlining the current state of art about the clinical use of 3DP in surgery by revisiting the most important applications experienced so far and trying on this base to figure out which perspectives may arise in the future.
Methods
Creating a 3D printed anatomical model is done through a series of technical steps and implies that a few essential requirements are fulfilled. First, high quality images must be obtained from MDCT or MRI scans to build a valuable 3DV reconstruction. Slice thickness of acquired images must not exceed 2 mm, with an optimal value below 1 mm [2, 3]. Thick slices would result in poor accuracy of the 3D model and in the loss of the finest details, e.g. small calibre vessels. The enhancement of the anatomical structures depends on the use of a medium contrast dye and on the specific contrastographic phase. Accordingly, each structure is processed in the phase in which it has the highest visibility, thus involving the use of registration techniques to restore the right spatial positioning of the structures [3].
Image elaboration for 3DP starts with a segmentation process, whose aim is to diminish the complexity of the original image by selectively marking the anatomy that is meant to be printed which is, therefore, extrapolated from the rest [3]. This is made by labelling each target structure in every single slice, by means of automatic or semi-automatic algorithms evolving within each slice and through the slices: algorithms’ evolution is guided by the natural contrast of different tissue densities and by morphological parameters, e.g. the smoothness of the contours or thresholds on the grey levels. The evolution stops once the structure of interest is completely identified. Then, segmentation labels are interpolated and a 3D rendering of the whole surface of the target anatomy is finally obtained. Such 3DV model reconstruction is navigable, allowing each structure to be rotated, hidden or coloured to enhance the interaction between different parts [3]. At this stage smoothing of the surface may correct irregularities or sharp edges to ensure homogeneity and consequently high quality of the 3D rendering.
In the next step, the 3DV model is exported as a surface triangulation language (STL) file which describes the spatial geometry of the object through a series of oriented triangular facets called mesh [2, 3]; this format is the current standard file format which can be processed by all 3DP software. The smaller is the size of these triangles, the more detailed is the surface of the 3DV model [3]. At this stage, smoothing of the surface may be required to correct irregularities or sharp edges; moreover, further elaboration of the STL file should be carried out according to the final aim of the printed object, like the creation of interlocking parts to enable the assembling/disassembling of the model which is then ready for being 3D printed. The key concept of 3DP manufacturing process is the creation of objects through a layer by layer process: the 3DV model is sliced into a series of 2D layers that are deployed one after the other by the 3D printer. This “additive” approach is the expedient by which 3D printers can manage highly complex geometries, likewise anatomical models [2, 3, 5].
3D printers can be distinguished according to the type of deposition and curing approach (e.g. Material Jetting, Material Extrusion, etc.), each implying a wide range of usable materials with different characteristics as to the degree of transparency, stiffness or deformability, mechanical strength, chromatic yield and so on [2, 11, 12] (Figs. 2, 3). In some cases, a support structure or devoted support material might be employed to support the building and can be removed or dissolved once the printing process is completed [2, 12]. In rare cases, due to the challenges in the cleaning and post-processing of complex anatomies, each structure can be printed separately and then stuck together to recreate the final object: however, this approach should be avoided due to the possible misalignments during the assembly of the 3D printed components [3].
3D printed model, made of photopolymer resin using a Material Jetting printer (Objet 260 Connex 3—Stratasys), showing a left adrenal gland tumor and its relationship with the corresponding kidney. The renal parenchyma is printed in a transparent resin to enable the visibility of inner structure whereas vessels are deformable and hollow
Results
The available evidence proves the increasing spread of 3DP in many surgical specialties over the last few years. Data obtained from the existing literature show that almost no area of surgery has remained immune to it [9, 12, 13]. The range of possible applications has broadened as the potentiality of the system has been fully understood by clinicians who have been encouraged to test 3D printed objects for novel usages that better fitted their needs.
Within the published literature there is a sort of consolidated tradition in grouping the known applications of 3DP for surgery by a few main categories which synthetize the specific end-uses of the technology [2, 11, 12]. Namely, these include: pre-operative planning, intraoperative navigation, education and training, patient’s counselling, surgical simulation, creation of anatomical phantoms, surgical instruments or implantable materials.
One of the most investigated is undoubtedly pre-operative planning [3, 10,11,12, 14]. 3D printed anatomical models are extremely accurate reproductions of the target anatomy built into a solid volume that can be observed and manipulated by the user until visual and tactile feedbacks have co-operated the transfer of the essential details [3, 10]. The steps of surgery can be traced or revisited directly on the model, anticipating technical challenges due to unfavourable anatomy or to disease-related alterations if any [3, 10, 14]. Several reports from different specialties have proven the effectiveness of this application in pre-surgical decision-making such as brain, cardiovascular, maxillofacial, transplant and general surgery, and orthopaedics. [11, 12, 14]. Here, 3DP helped localizing critical at-risk structures, defining resection lines or dissection planes, assessing the extent of disease to be ablated and, briefly, to deeply familiarize with patient’s anatomy prior to surgery [3, 11, 12, 14]. The retrieved level of accuracy was confirmed in some studies by the extremely low margin of error in terms of mm when users were asked to estimate measures (i.e. distances, lengths or volumes) both on 3D printed models and other image platforms such as MDCT or 3DV [10, 15]; in another study, the volume of resected liver did perfectly correspond to that predicted with 3DP before surgery in a series of 22 elective hepatectomies [7].
A very fine line is between the use of 3D printed anatomical models for pre-operative planning or intraoperative navigation. One of their advantages is that they are portable objects which the surgeon can bring in the operating room for intraoperative reassessment in case of difficult orientation [3, 10, 14]. In robotic surgery, where the operator sits unscrubbed at the console, this use is easily accessible with no restrictions; for other settings, there are a lot of printing materials suitable for being sterilized thus extending the use to the operative field [11, 12].
The potentiality of 3DP for educational purposes has been largely tested at many levels [2, 11, 12, 14, 16,17,18,19,20,21]. The learning of human anatomy by medical students is traditionally based on plain pictures or drawings taken from books or atlases with poor comprehension of details and spatial relation, being cadaver dissection infrequent. Surgical residents and naïve surgeons are exposed to daily practice in the operating room, but deep anatomical knowledge takes time to be achieved considering the inter-individual variations and the pathology-based distortions that are often encountered. Being 3D printed models built on patient-specific imaging data, they allow for detailed recognition of anatomical landmarks regardless of any deviation from the norm. Their effectiveness has been proven by a number of recent experimental studies, including a few randomized controlled trials, where participants of various expertise (alternately medical students, residents, junior or senior surgeons, radiologists) were asked to evaluate a given anatomy on 3D printed models and other platforms (CT scans, 3DV reconstructions or cadavers according to the specific study) whose results were compared [10, 16,17,18]. 3D printed models obtained better scores than 2D or 3DV formats and were not inferior to cadaveric materials in correctly identifying key anatomical elements. In addition, participants greatly appreciated the added value of 3D printed models in the learning process, as shown by the average high rates that were given in specific surveys [3, 18, 19].
The 3DP reproduction of a certain anatomical region or structure can be shared by physicians of different specialties to improve the understanding of a complex procedure or the management of a challenging case. A 3D solid, graspable object endowed with specific features such as transparency or detachable parts which make inner structures visible may effectively level off gaps between professionals in assessing conventional 2D imaging.
In addition, 3D printed models have turned out to be useful tools for preoperative counselling with patients and families, facilitating physicians in explaining the issues related either to the disease or the surgery [3, 10]. Our group recently collected highly positive feedbacks from patients and their relatives when the counselling was done with the corresponding anatomical model which they could see and handle as desired; indeed, the model inspired further questions on their part, thus encouraging the discussion about technical details and alternative options if any [3]. We found that a much higher comprehension can be perceived by patients about how the intervention is carried out, what kind of outcomes should be expected, which challenges might be faced intraoperatively or which complications may occur afterwards, if these issues are discussed on their own 3D printed model. When such a full understanding is achieved, the acceptance of the proposed treatment is likely to be increased, whereas the rate of possible medico-legal controversies might reduce accordingly.
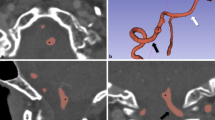
The education and training concept that 3DP embodies in surgery is much wider than described above. Recent technological advances enable the reproduction of hollow structures such as the heart or vessels of various size, from the aorta to small collateral branches of arteries and veins, which can be made of soft, deformable photopolymer resins mimicking the mechanical properties of real human tissue [2, 22]. Such kind of models not only augment the sensory perception on part of the user, but most importantly mark the beginning of a new era for surgical simulation. Surgeons of any expertise, from trainees to highly skilled operators, can train themselves in as many tasks as these models can reproduce whatever the purpose of simulation might be: improving a certain skill through multiple repetitions of the same exercise, testing novel surgical devices outside the operating room or getting ready for a real intervention before it takes place on the patient. We have recently attempted preoperative simulation of complex interventions (i.e. robotic live-donor nephrectomy and robotic aneurysmectomy of splenic artery aneurysm) on patient-specific 3D printed models where the vascular anatomy was made of deformable material which allowed vessels’ clamping, stapling and anastomosis [23] (Fig. 4). The resulting experience was positively valuated by the surgeon: the tested models appeared adequately realistic as to the mechanical performance of the material and proved to be effective for the completion of the tasks; moreover, training on the same anatomy that would have been found intraoperatively increased the confidence in succeeding the surgery.
Surgical simulation with daVinci Surgical Robot (Intuitive Inc., Sunnyvale, CA): a 3D printed model of a splenic artery aneurysm case is used to train robotic aneurysmectomy prior to real surgery. On the right, detail of simulated robotic vascular reconstruction (end-to-end arterial anastomosis performed with interrupted sutures)
The overall concept is that 3DP currently allows for a customized training that users of any expertise can tailor according to their own needs of proficiency and to the unique anatomical configuration of a given patient thanks to the accuracy of the printed manufactures. Other authors have already attested the feasibility and usefulness of this novel application in many operative settings: endovascular procedures were simulated with the aim of testing the equipment, selecting puncture site for catheter insertion and practicing the overall task; neurosurgeons built sophisticated 3D platforms of simulation that were either made of multiple materials for tissues of different texture (i.e. skin, bone, tumor, etc.) or implemented with electric conducting materials to reproduce vital structures such as cranial nerves which may alert the trainee with acoustic signals in case of damage during the simulated exercise [24,25,26].
The layout for such simulation might be further improved making it more realistic: a 3D phantom reproduction of the anatomical area of interest (i.e. the abdominal cavity, the cranium, the thorax or a smaller part of them) can be printed from the same patient’s dataset of radiological imaging to accommodate the corresponding 3D printed model [4, 5]. This kind of platform, which partly recalls the concept of the classic pelvic trainer, enables to practice additional tasks other than those related to the target of the operation: these concern the access to the target itself. In some situations, the way to reach a deep anatomical site can be an issue, especially if confined into a narrow space or surrounded by delicate structures. In others, the constrains imposed by the technique or by patient’s complexion may pose challenges in determining the easiest, less traumatic and most effective surgical access. For instance, in minimally invasive surgery the use of these 3D printed architectures might be of help for determining optimal trocars’ configuration or contriving technical solutions to improve the exposure of the area of interest though still in a simulation environment.
The types of tools for surgical purposes that can be printed in 3D has virtually no limits, which paves the way for a boundless number of applications. This is especially the case of reconstructive skeletal surgery not only because the solidity of bone tissue can be easily reproduced with 3DP, but also because many of the tools and devices that are routinely used (such as implants, prostheses or surgical instruments) are themselves suitable for being printed in 3D, furthermore with a patient-specific shape, and employed in place of the corresponding standard industrial products [11, 14]. To date, several kinds of templates, guides, jigs and other contour-shaped devices have been printed in 3D to aid specific steps of the operation: drilling of bone at predetermined depth and sites, measuring the length of screws or other fixating systems, outlining the related trajectories and angles, guiding their placement in the correct position and so on [2, 11, 13]. Other devices are purposely printed for being used as implantable materials that are left in place indefinitely [11, 12]. Unlike conventional prosthesis, 3DP technology allows for the patient-specific design of manufactures which perfectly fit with the anatomy that is meant to be replaced. Exact curvatures, shapes or symmetry can guarantee strict adherence to the needs of the given case, not least cosmetic issues. In fact, such highly customized implants make possible accurate, lifelike reconstructions of visible parts as head, face or limbs whose social impact is invaluable for patients [11, 12]. On the other hand, the more the implant fits the anatomy, the greater is the functional result as to possible side effects such as chronic postoperative discomfort or motor disability that one may experience as after joint replacement or reconstructive surgery.
In addition, when not directly implanted to replace damaged structures, these tailor-made tools can be used as precise templates or scaffolds on whose shape other types of prosthetic material can be moulded before being grafted to the patient [11, 12]. The use of 3D printed scaffolds have been also investigated to produce biological tissue implants generated through stromal cell migration and proliferation within the 3D architecture of the scaffold itself [7, 11, 12]. This novel frontier of printing 3D tissues, as well as the 3D printing of cells (bio-printing) with the final aim of directly building living tissues, is still at its early stage of investigation, although promising results can be foreseen according to the preliminary data available in the literature [2, 7, 11, 12]. The same applies for whole organ’s printing that many authors have already advocated, but whose actualization seems even farer due to the complexity of multiple cell types deployment during the bio-printing process and the intricate vasculature that is required to maintain viability of tissue.
Discussion
The increasing number of possible applications of 3DP in surgery makes its present and future role in the clinical practice beyond doubt. Thanks to the recent technological advancement as to image elaboration, printing techniques and available materials, it is now possible to create 3D anatomical models and tools for surgical use to cover needs and address issues of the current clinical practice.
The range of applicability of 3DP manufactures encompasses both the pre-surgical phase and the intraoperative setting for multiple uses. Whatever is the aim they have been printed for, these products are available for the user before surgery takes place. In most cases this allows to save time intraoperatively, because much of their contribution has been already exploited outside the operating room where the pressure for succeeding is lower; as a matter of fact, by relieving tension and reducing the time spent for reaching the target, taking decisions, evaluating solutions, calculating angles, defining dissection planes or performing critical manoeuvres, the surgeon has time enough to concentrate on other key elements resulting in a smoother, quicker and safer operation [13, 14]. Different degrees of transparency, chromatic resolution, extent of modularity, array of compactness and deformability as well as the different architectures by which these objects can be realized greatly contribute to their effectiveness and flexibility of use.
Time and costs of production still limit the widespread diffusion of 3DP in the clinical setting. The available literature reports that the overall time to complete the printing process, from image elaboration to the final output, can range between about half a day to several weeks [11, 12, 14]. In a previous experience of our group, it took a bit more than 24 h of work to create 3D printed models of patient-specific spleens scheduled for laparoscopic splenectomy [3]. The average time has been reduced to about 13 h for such cases relying on a different 3DP technology (namely binder jetting), but most complex reproductions are still much longer than this. At this stage, 3DP remains, therefore, suitable only for elective surgery [11, 12]. In addition, according to the final use that is aimed (i.e. planning, training or simulation), 3D printed objects shall necessarily be prepared on time enough to ensure they get fully exploited.
Costs vary a lot according to the type of printing technique and materials used, but also to the need for specialized staff and software [11, 14]. Being in-house production more cost-effective, the higher investment is usually attributed to the printer and software purchase, though open-access software is currently available [11]. For costs containing, it is advocated that the same platform of production is shared by multiple groups of users thus distributing the expense [11]. However, the rationale for justifying the extra cost of 3DP shall be balanced with the alternative available options; for instance, the cost of a conventional surgical simulator for minimally invasive technique may largely exceed that of a 3D printed anatomical phantom which adds the benefit of perceiving tactile feedback and training on a patient-tailored anatomy.
Minor drawbacks include the inability of reproducing extremely small or thin structures which is due both to the limitations imposed by most of printing materials, resulting in excessively weak printed products unsuitable for many applications, and to the resolution of current cross-sectional scanners for CT or MRI which is the major responsible for the final accuracy of the 3DV model [12, 14].
With further development of technology in the future it is likely that the above issues, especially time and costs of manufacturing, will be overcome and that the multiple known uses of 3DP will be steadily implemented in the routine clinical practice accordingly. The same applies to radiological investigations and techniques for image analysis whose additional refinement might drive the growth of novel applications of 3DP in medicine. Currently, the greatest expectations concern the possibility of creating printed biological human tissues and organs suitable for being effectively transplanted likewise the available grafts from donors. This will entail that not only technical issues will be resolved, but also that ethical implications will be addressed and regulatory measures defined on part of the pertaining authorities to guarantee patients’ safety. Despite preliminary data would indicate it as feasible [11], more evidence is required to assess the effectiveness and sustainability of 3DP technology for this aim.
References
Doi K (2006) Diagnostic imaging over the last 50 years: research and development in medical imaging science and technology. Phys Med Biol 51:R5–R27
Rengier F, Mehndiratta A, von Tengg-Kobligk H, Giesel FL (2010) 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010(5):335–341
Pietrabissa A, Marconi S, Peri A, Pugliese L, Auricchio F (2015) From CT scanning to 3-D printing technology for the pre-operative planning in laparoscopic splenectomy. Surg Endosc 30(1):366–371
Tack P, Victor J, Annemans L (2016) 3D-printing techniques in a medical setting: a systematic literature review. BioMed Eng Online 15:115
Kim GB et al (2016) Three-dimensional printing: basic principles and applications in medicine and radiology. Korean J Radiol 17(2):182–197
Ventola CL (2014) Medical applications for 3D printing: current and projected uses. Pharm Ther 39:704–711
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM (2014) Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem 86:3240–3253
Kappers AM (2011) Human perception of shape from touch. Philos Trans R Soc Lond B Biol Sci 366:31063114
Meijer F, Van der Lubbe RH (2011) Active exploration improves perceptual sensitivity for visual 3D objects in visual recognition tasks. Vision Res 51:2431–2439
Marconi S, Pugliese L, Botti M et al (2017) Value of 3D-printing for the comprehension of surgical anatomy. Surg Endosc 31(10):4102–4110
Malik HH, Darwood ARJ, Shaunak S, Kulatilake P, El-Hilly AA, Mulki O, Baskaradas A (2015) Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res 199:512–522
Li Chi, Cheung Fung, Fan Vei Chen, Ka Kit Leung G (2017) Application of three-dimensional printing in surgery. Surg Innov 24(1):82–88
Diment E, Thompson M, Begmann J (2017) Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ Open 7:e016891
Martelli N, Serrano C, van den Brink H et al (2016) Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery 159(6):1485–1500
Olivieri LJ, Krieger A, Loke YH, Nath DS, Kim PCW, Sable CA (2015) Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy. J Am Soc Echocardiogr 28:392–397
Kong X, Nie L, Zhang H et al (2016) Do three-dimensional visualization and three-dimensional printing improve hepatic segment anatomy teaching? A randomized controlled study. J Surg Educ. 73:264–269
Lim KH, Loo ZY, Goldie SJ, Adams JW, McMenamin PG (2016) Use of 3D printed models in medical education: a randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat Sci Educ. 9:213–221
Fasel JH, Aguiar D, Kiss-Bodolay D et al (2016) Adapting anatomy teaching to surgical trends: a combination of classical dissection, medical imaging, and 3D-printing technologies. Surg Radiol Anat 38:361–367
Jones DB, Sung R, Weinberg C, Korelitz T, Andrews R (2016) Three-dimensional modeling may improve surgical education and clinical practice. Surg Innov. 23:189–195
Garcia J, Yang Z, Mongrain R (2018) Lachapelle K (2018) 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Stel 4:27–40
Langridge B, Mamin S, Coumbe B et al (2018) Systematic review of the use of 3 dimensional printing in surgical teaching assessment. J Surg Educ 75:209–221
Rasheed K, Mix D, Chandra A (2015) Numerous applications of 3D printing in vascular surgery. Ann Vasc Surg 29(4):643–644
Pugliese L, Marconi S, Peri A, Botti M, Auricchio F, Pietrabissa A. Value of 3D-printing in the management of visceral aneurysms. In: Marone EM et al., editors 3D-printing for complex vascular disease, in press (Minerva Ed.)
Itagaki MW (2015) Using 3DP models for planning and guidance during endovascular intervention: a technical advance. Diagn Intervent Radiol 21(4):338–341
Waran V, Narayanan V, Karuppiah R, Owen SL, Aziz T (2014) Utility of multimaterial 3D printers in creating models with pathological entities to enhance the training experience of neurosurgeons. J Neurosurg 120:489
Grunert R, Strauss G, Moeckel H et al (2006) Elephant-an anatomical electronic phantom as simulation-system for otologic surgery. Conf Proc IEEE Eng Med Biol Soc 1:4408
Acknowledgements
The presented activity falls under the framework of 3D@UniPV project (http://www.unipv.it/3d), one of the strategic research area at the University of Pavia. The work is supported by the project PE-2013-02358887 on pancreatic ductal adenocarcinoma, funded by Italian Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Pugliese, L., Marconi, S., Negrello, E. et al. The clinical use of 3D printing in surgery. Updates Surg 70, 381–388 (2018). https://doi.org/10.1007/s13304-018-0586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-018-0586-5