Abstract
Predictors of response to neoadjuvant chemotherapy are not available for gastric and oesophago-gastric junction carcinoma. HER-2 over-expression in breast cancer correlates with poor prognosis and high incidence of recurrence. First aim of this study was to evaluate if the HER-2 expression/amplification is predictive of response to neoadjuvant chemotherapy in terms of pathologic regression. Secondary aim was to evaluate if HER-2 expression varies after neoadjuvant treatment. Thirty-five patients with locally advanced gastric or oesophago-gastric junction carcinoma underwent preoperative chemotherapy and surgical resection at San Raffaele Scientific Institute and Spedali Civili of Brescia. HER-2 expression/amplification was evaluated on every biopsy at diagnosis time and on every surgical sample after neoadjuvant chemotherapy. Pathologic response to chemotherapy was evaluated according to TNM classification (ypT status and ypN status) and Mandard’s tumour regression grade classification. In our series 10 patients (28.6%) showed a reduction in HER-2 overexpression and in 6 of them (17.1%) HER-2 expression completely disappeared. Only three of the six patients with HER-2 disappearance had a complete pathological response to neoadjuvant chemotherapy. There was a strong correlation between HER-2 negativity on biopsy and absence of lymph node metastasis in surgical samples after neoadjuvant chemotherapy, irrespective of nodal status before chemotherapy. A direct correlation between HER-2 reduction after neoadjuvant chemotherapy and pathologic regression (primary tumour and lymph nodes) in surgical samples was found. HER-2 negativity may represent a predictor of pathologic response to neoadjuvant chemotherapy for gastric and oesophago-gastric junction adenocarcinoma. Neoadjuvant treatment can reduce HER-2 overexpression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The EGF receptors are a family of tyrosine kinase proteins that are critical in proliferation and differentiation of normal cells. HER-2 is the most potent oncoprotein in the EGF-R family and many studies have clearly indicated that HER-2 over-expression plays a pivotal role in oncogenic transformation. The association of HER-2 over-expression with cancer has been demonstrated initially in breast cancer: HER-2 over-expression in breast cancer correlates with poor prognosis and high incidence of recurrence. HER-2 confers a strong proliferative advantage to tumour cells. HER-2 gene amplification and protein over-expression have been found in many other malignancies including gastric cancer [1, 2].
Since the publication of the MAGIC trial [3], patients affected by gastric and oesophago-gastric junction cancers often undergo neoadjuvant chemotherapy in Europe. At the time of surgery, patients responsive to preoperative chemotherapy have significantly smaller tumour size and lower stage. However, the patients who suffer severe toxicity during chemotherapy will not benefit from this approach. And the risk of delaying surgery in patients who do not respond to chemotherapy may negatively influence clinical outcome. Patients, furthermore, may develop progressive disease whilst on chemotherapy, which would compromise their tumour resection [4].
Recent studies have tried to investigate HER-2 over-expression pre and post neo-adjuvant chemotherapy, as well as hormone receptors, in breast cancer [5, 6]. After neoadjuvant chemotherapy hormone receptors levels, HER2 status and the intrinsic subtype had changed in a considerable number of patients, but the changes in IHC markers did not significantly correlate with therapeutic response. These studies show that chemotherapy could change HER-2 expression status in a variable percentage of patients.
In this context, we developed this study in order to evaluate if the pre-neoadjuvant chemotherapy levels of HER-2 expression/amplification in locally advanced adenocarcinoma of the stomach and oesophago-gastric junction have a role in predicting survival and pathologic response to neoadjuvant chemotherapy. Our secondary aim was to compare HER-2 expression/amplification before and after neoadjuvant chemotherapy in gastric cancer.
Methods
Patient population
Thirty-five patients with locally advanced adenocarcinoma of stomach and oesophago-gastric junction who had received preoperative chemotherapy and surgical resection between January 2007 and April 2013 at San Raffaele Scientific Institute and at Spedali Civili of Brescia where included in this retrospective study. Eligibility criteria included no distant metastases, other than minimal peritoneal seeding, and no previous chemotherapy or radiotherapy. A diagnosis of carcinoma was established through endoscopic biopsy of the primary lesion. Pre-treatment work-up included endoscopy with endosonography, and computed tomography (CT) scan of the thorax and abdomen. In some cases 18F-FDG PET, magnetic resonance (MRI) and staging laparoscopy were performed. Patients received at least two cycles of neoadjuvant treatment. Treatment schedules administered were regimens containing cisplatin and/or fluoropirimidine (5-FU or Capecitabine). After that, patients underwent appropriate surgery: subtotal gastrectomy, total gastrectomy and oesophagectomy with D2 lymphadenectomy. Thirty out of 35 patients did not have distant metastases. We also included five patients with evidence of minimal peritoneal seeding, because they had a successful clinical response of primary lesion to chemotherapy and they underwent surgical resection. These cases were submitted to Hypertermic Intraperitoneal Chemotherapy (HIPEC) concomitant or subsequent to surgical resection. All procedures were carried out with the prior informed consent of the patients. The Ethic Committee expressed positive regarding the present study.
Histopathological investigations
Both samples of primary tumours obtained by surgical resection and samples from biopsies obtained at the time of diagnosis were fixed in buffered formalin (formaldehyde, 4% wt/vol, and acetate buffer, 0.05 mol/L) and routinely processed to paraffin wax. The histopathological investigation included evaluation of TNM staging [7] and classification of histological types [8]. Tumour regression of the primary lesion was assessed in post-surgical samples using the tumour regression grading (TRG) system of Mandard et al. [9] as follows: TRG1, the absence of residual cancer and extensive fibrosis; TRG2, rare residual cancer cells scattered through the fibrosis; TRG3, increased residual cancer cells but fibrosis still predominating; TRG4, residual cancer outgrowing fibrosis; TRG5, the absence of regressive changes.
Immunohistochemistry
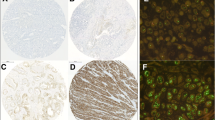
The HercepTest system for the detection of HER-2 expression came from DAKO (Hamburg, Germany). Immunohistochemistry (IHC) was performed using a standard biotin–streptavidin method with the appropriate antigen retrieval method. The HercepTest was applied strictly according to the manufacturer’s instructions. HER-2 over-expression was indicated by an intense brown staining (using diaminobenzidine as chromogen) of the cell membrane. To determine HER-2 protein overexpression only the membrane staining pattern and intensity of invasive tumour cells were scored. The proportion of positively stained invasive cells was estimated. Immunostaining was scored according to the criteria specified by DAKO for the interpretation of the HercepTest, which have also been used in trials of Herceptin [10–13]. The score ranged from 0 (negative) to 3+ (strong membrane signal—positive).
Fluorescence in situ hybridization
In case the result of IHC for HER-2 was 2+, fluorescence in situ hybridization (FISH) analysis was performed. Moreover, FISH was performed in case of disappearance of HER-2 overexpression after neoadjuvant chemotherapy. Therefore, 25 specimens obtained from biopsy at diagnosis and 18 specimens from resective surgery underwent to FISH analysis. The procedure was performed according to the instructions of the kit supplier with slight modifications. The ratio of the HER-2/neu-specific signal to the centromere-17-specific signal was estimated in 60 nuclei per sample. The threshold for amplification was set at 2.0. Nuclei without any HER-2 or centromere-specific signal were excluded from analysis. Up to 15 signals per nucleus were counted. When signal swarms were found indicating a high level of gene amplification, the signal number was arbitrarily set to a value of 16 [14].
Statistical analysis
Correlations between categorical variables were done with Pearson’s χ 2 test or Fisher exact test when appropriate. p values <0.05 were defined as statistically significant. The statistical analyses were performed with SPSS software 20.0 (SPSS, Chicago, IL, USA).
Results
Clinical characteristics
Twenty-seven out of the 35 patients were men and 8 were women. The mean age was 61 years (range 33–77 years). According to Eastern Cooperative Oncology Group criteria, the performance status was good in all patients (ECOG 0–1). Data regarding tumour location, clinical stage, type of surgery and chemotherapeutic regimen are depicted in Table 1. All patients except one (97%) had lymph node involvement. Distant metastases were observed in five patients (three of them had peritoneal involvement and two had non loco-regional nodes involvement).
Histopathology
The results of HER-2 over-expression evaluation performed on biopsy at diagnosis time were the following (Table 2): one patient had a score of 0 (2.9%), 7 patients had a score of 1+ (20%), 25 patients had a score of 2+ (71.4%) and 2 patients had a score of 3+ (5.7%). The cases with a score of 2+ underwent to FISH analysis: the gene was amplified in 28% of these patients. Nine patients (25.7%) resulted HER-2 positive and 26 patients (74.3%) HER-2 negative at diagnosis time.
At the pathological examination, the mean tumour size was 3.3 ± 2 cm (range 0–9 cm). Four tumours (11.4%) infiltrated adjacent structures (ypT4b), 5 tumours (14.3%) invaded serosa (ypT4a), 11 tumours (31.4%) penetrated subserosal connective tissue without invasion of serosa (ypT3), 4 tumours (11.4%) invaded no further than the muscularis propria (ypT2), 5 tumours (14.3%) invaded no further than the submucosa (ypT1b) and 2 tumours (5.7%) invaded no further than lamina propria. In four cases (11.4%) there was no evidence of residual disease (ypT0). The median number of lymph nodes resected was 31 ± 15 (range 12–86). Lymph node metastases were found in 20 (57.1%) out of the 35 cases. Data regarding pathologic stage are shown in Table 3.
More than half (n = 19, 54.3%) of the tumours were poorly differentiated (grade 3). None was undifferentiated (G4) and 12 cases were G2 (34.3%). In the remaining 4 cases it was impossible to determine the grade of differentiation because of the lack of residual tumour in the surgical specimen. With respect to the Lauren classification, 24 cases (68.6%) were intestinal, 8 (22.9%) were diffuse and 3 cases (8.6%) were unclassifiable. The grade of tumour regression after neoadjuvant chemotherapy was evaluated according to Mandard classification. Four patients (11.4%) had a complete histological regression (TRG 1) at the primary tumour after therapy (one of these cases was ypN1). Four patients (11.4%) had TRG 2 and 10 patients (28.6%) had TRG 3. Ten patients (28.6%) had TRG 4. Finally, 4 cases (11.4%) did not show any regressive change (TRG 5). In three cases was not possible to evaluate TRG. However, Mandard et al. considered responders to chemotherapy the grades 1, 2 and 3 and non-responders the grades 4 and 5. In our experience, responders were 18 (51.4%) and non-responders were 14 (40%).
As shown in Table 2, the results of HER-2 over-expression evaluation on surgical specimens show that six patients (17.1%) scored 0, 8 patients (22.9%) scored 1+, 19 patients (54.3%) scored 2+ and 2 patients (5.7%) scored 3+. In almost all cases of score 2+, FISH analysis was performed. In 11 cases (31.4%) the gene was not amplified, while in 7 patients (20%) the gene was amplified. According to ToGA-trial criteria [15], patients with a score of 3+ or 2+ and gene amplification had been considered as HER-2 positive while patients with a score of 0, 1+ or 2+ and gene not amplified had been considered as HER-2 negative. Compared with the results of preoperative biopsy, 10 patients (28.6%) showed a reduction in HER-2 overexpression score while 25 patients (71.4%) did not. Moreover, in 6 cases (17.1%), HER-2 expression completely disappeared (score 0). Three of them had a complete pathological response to neoadjuvant chemotherapy (TRG 1), whereas in the other three cases the disappearance of HER-2 expression was associated with a persistence of tumour cells.
Clinical and pathological characteristics related to HER-2 over-expression
Table 4 shows the relationship between HER-2 status, grouped in HER-2 positive (score 3+ and 2+ with gene amplification) and HER-2 negative (score 0, 1+ and 2+ without gene amplification), and clinical–pathological characteristics evaluated in the study. Age, sex, ypT-stage, grading, histologic type according Lauren classification, TRG and complete pathological response were not correlated with HER status (p value >0.05). Regarding HER-2 status at diagnosis time we observed a correlation with nodal status in surgical samples (χ 2 = 3.93, p value = 0.026).
Furthermore we analysed the relationship between HER-2 reduction after neoadjuvant chemotherapy and pathological characteristics (Table 5): HER-2 reduction had a significant correlation with ypT-downstaging (χ 2 = 5.90, p value: 0.005), nodal sparing (χ 2 = 10.15, p value = 0.000), good response according Mandard’s TRG (χ 2 = 4.88, p value: 0.009) and complete pathological response (χ 2 = 2.55, p value 0.029). HER-2 reduction did not correlate with age, sex, grading and histologic type according Lauren classification (p value >0.05).
Discussion
The role of neoadjuvant chemotherapy remains controversial in gastric cancer. Current evidence suggests that preoperative treatment may improve surgical outcome in case of locally advanced cancer. However, only patients who respond to preoperative therapy with tolerable toxicity will potentially benefit from this approach. Some patients, furthermore, may develop progressive disease on chemotherapy, which would compromise their tumour resection. Hence, there is the need to find new markers of response to neoadjuvant treatment. The evaluation of pathologic response to chemotherapy provides a useful intermediate endpoint to identify potential factors of response to anticancer drugs. The present clinical study was undertaken to determine whether the HER-2 expression could affect the pathologic response of gastric and oesophago-gastric junction carcinoma to neoadjuvant chemotherapy. Secondarily we aimed to compare HER-2 expression/amplification before and after neoadjuvant chemotherapy in gastric cancer.
A lot of evidence about HER-2 role in cancer biology is derived from breast cancer studies [16]. Ever since 1987 HER-2 over-expression in breast cancer has been shown to correlate with poor prognosis, high incidence of recurrence, lymph node spread and high proliferative rate. Recent studies tried to investigate HER-2 over-expression pre- and post-neoadjuvant chemotherapy, as well as hormone receptors. After neo-adjuvant treatment HER-2 status changed in a considerable number of patients, but the changes in IHC markers did not significantly correlate with therapeutic response. Moreover, HER-2 expression on biopsy at diagnosis is not predictive of pathologic complete response [17]. These studies show that chemotherapy could change HER-2 expression status in a variable percentage of patients. Such observation suggests that chemotherapy can alter the tumour gene expression and that this event is an important point in drug sensitivity [18].
In contrast to breast carcinoma, in gastric cancer HER-2 over-expression and erbB-2 amplification is heterogeneous. It is common to find, within the same tumour, areas with moderate to strong reactivity and areas without reactivity. With this study, we described a change in HER-2 expression due to neoadjuvant treatment in gastric cancer and eventually the role of HER-2 overexpression as a predictive marker of response to this treatment.
In our series, 10 patients (28.6%) showed a reduction in HER-2 overexpression and in 6 of them (17.1%) HER-2 expression completely disappeared (score 0). Three of the six patients with HER-2 disappearance had a complete pathological response to neoadjuvant chemotherapy (TRG 1).
According to our data, there is a strong correlation between HER-2 negativity on biopsy at diagnosis time and nodal sparing in surgical samples after neoadjuvant chemotherapy, irrespective of nodal status before chemotherapy. Therefore, HER-2 negativity at diagnosis time may be a predictor of nodal sparing after neoadjuvant chemotherapy. This evidence, in accordance with literature results, supports that HER-2 over-expression is correlated with lymph node metastases and poor prognosis [19].
In our series, there is a strong correlation between the reduction of HER-2 expression after neoadjuvant chemotherapy and pathologic response in term of ypT-downstaging, Mandard’s TRG, nodal sparing and complete pathologic response. According to literature data, this evidence suggests that HER-2 changes due to neoadjuvant treatment could be a surrogate marker of pathologic regression [20, 21]. We hypothesise that neoadjuvant chemotherapy acts mainly on tumour–cell population with a high grade of HER-2 over-expression/amplification. Cells with a high HER-2 over-expression are probably characterised by a high proliferative rate and biological aggressiveness. This particular cell population probably significantly contributes to tumour development, tumour progression and eventually nodal invasion.
A particularly important topic is the correlation between HER-2 over-expression and TRG. In our study we found a strong correlation between reduction in HER-2 expression before and after chemotherapy and tumour regression (TRG 1, 2 and 3). We also found a correlation between HER-2 disappearance and tumour regression (TRG 1, 2 and 3). This is easily understood for the three patients who, after neoadjuvant chemotherapy, had a complete pathological response. However, three patients showed HER-2 disappearance despite a non-complete pathological response (TRG 2 or 3). The above-mentioned effect can be explained by the preferential activity of neoadjuvant chemotherapy on HER-2 expressing cells.
The limits of this study are its retrospective design and the low number of the study sample. Further prospective trials would be useful to pursue this issue.
In conclusion, HER-2 over-expression/amplification in gastric and oesophago-gastric junction cancer patients is correlated with pathologic response to neoadjuvant chemotherapy, in particular for nodal involvement. To the best of our knowledge we are the first to describe a reduction and disappearance of HER-2 expression induced by neoadjuvant chemotherapy in gastric cancer. Further follow-up studies are necessary to define weather HER-2 expression is actually a marker of long-term survival. This is particularly important to define HER-2 reduction as an objective of neoadjuvant chemotherapy and to develop new therapeutic strategies.
References
Hynes NE, MacDonald G (2009) ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 21(2):177–184
De Bono JS, Rowinsky EK (2002) The ErbB receptor family: a therapeutic target for cancer. Trends Mol Med 8(4 Suppl):S19–S26
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355(1):11–20
De Manzoni G, Baiocchi GL, Framarini M, De Giuli M, D’Ugo D, Marchet A et al (2014) The SIC-GIRCG 2013 consensus conference on gastric cancer. Updates Surg 66(1):1–6
Kumaki N, Umemura S, Tang X, Saito Y, Suzuki Y, Tokuda Y et al (2011) Alteration of immunohistochemical biomarkers between pre- and post-chemotherapy: hormone receptors, HER2 and Ki-67. Breast Cancer 18(2):98–102
Kinsella MD, Nassar A, Siddiqui MT, Cohen C (2012) Estrogen receptor (ER), progesterone receptor (PR), and HER2 expression pre- and post- neoadjuvant chemotherapy in primary breast carcinoma: a single institutional experience. Int J Clin Exp Pathol 5(6):530–536
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Organization, W.H. (2000) Tumours of the stomach. In: Hamilton SR, Altonen LA (eds) World Health Organization classification of tumours. Tumours of the digestive system. IARC Press, Lyon, pp 37–68
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73(11):2680–2686
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J (1998) Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58(13):2825–2831
Baselga J (2001) Phase I and II clinical trials of trastuzumab. Ann Oncol 12(Suppl 1):S49–S55
Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D et al (1998) Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 16(8):2659–2671
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Fusco N, Rocco EG, Del Conte C, Pellegrini C, Bulfamante G, Di Nuovo F et al (2013) HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol 26(6):816–824
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN et al (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14(4):320–368
Li X, Liu M, Zhang Y, Wang J, Zheng Y, Li J et al (2011) Evaluation of ER, PgR, HER-2, Ki-67, cyclin D1, and nm23-H1 as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer. Med Oncol 28:S31–S38
Tiezzi DG, De Andrade JM, Cândido dos Reis FJ, Marana HR, Ribeiro-Silva A, Tiezzi MG et al (2006) Apoptosis induced by neoadjuvant chemotherapy in breast cancer. Pathology 38(1):21–27
Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S et al (1991) Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res 51(3):1034–1038
Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J et al (2011) Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 253(5):934–939
Becker K, Reim D, Novotny A, Zum Büschenfelde CM, Engel J, Friess H et al (2012) Proposal for a multifactorial prognostic score that accurately classifies 3 groups of gastric carcinoma patients with different outcomes after neoadjuvant chemotherapy and surgery. Ann Surg 256(6):1002–1007
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical approval
This research involving human participants was in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human participants and/or animals
This research involved human participants who supplied their own consent. This study was performed in accordance with the ethical standards of the Declaration of Helsinki. All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chiari, D., Orsenigo, E., Guarneri, G. et al. Effect of neoadjuvant chemotherapy on HER-2 expression in surgically treated gastric and oesophagogastric junction carcinoma: a multicentre Italian study. Updates Surg 69, 35–43 (2017). https://doi.org/10.1007/s13304-017-0423-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-017-0423-2