Abstract
In recent years, laparoscopic pancreatoduodenectomy (LPD) has been gaining a favorable position in the field of pancreatic surgery. However, its role still remains unclear. This review investigates the current status of LPD in high-volume centers. A literature search was conducted in PubMed, and only papers written in English containing more than 30 cases of LPD were selected. Papers with “hybrid” or robotic technique were not included in the analysis. Out of a total of 728 LPD publications, 7 publications matched the review criteria. The total number of patients analyzed was 516, and the largest series included 130 patients. Four of these studies come from the United States, 1 from France, 1 from South Korea, and 1 from India. In 6 reports, LPDs were performed only for malignant disease. The overall pancreatic fistula rate grades B–C were 12.7%. The overall conversion rate was 6.9%. LPD seems to be a valid alternative to the standard open approach with similar technical and oncological results. However, the lack of many large series, multi-institutional data, and randomized trials does not allow the clarification of the exact role of LPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since widespread adoption of minimally invasive surgery (MIS) in the late 1980s, surgical technique has undergone a major transformation and there are very few abdominal disease processes and operations that have not been impacted. MIS techniques have been demonstrated to be safe and effective in the treatment of benign and malignant disease [1–3].
Today, the role of laparoscopic approach in pancreatic surgery is still debated. In 1994, Cuschieri [4] reported the first laparoscopic distal resection and, in the same year, Gagner and Pomp [5] reported the first laparoscopic pancreatoduodenectomy (LPD). Two decades later, laparoscopic distal resection has been widely adopted and becomes the technique of choice to treat pancreatic pathology of the body and tail by most experienced pancreatic surgeons. By contrast, the diffusion of LPD remains confined to a very few high-volume centers with no clear advantages over open pancreaticoduodenectomy (OPD). The slow progression of this technique is likely related to two major aspects. First, the retroperitoneal position of the pancreas and the close relation to major central mesenteric vasculature make dissection difficult and potentially hazardous. Second, biliary and pancreatic reconstruction is particularly challenging, requiring advanced surgical skills and additional time.
In the recent years, several series of LPD have been published. However, many reports include only a small amount of patients, are retrospective in nature, and often include a variety of LPD procedures, such as hybrid or combined approaches. In this review, we summarize what is currently known about LPD from the published literature with respect to the patients selected to undergo the procedure, the operative and postoperative outcomes, and the pathologies treated.
Materials and methods
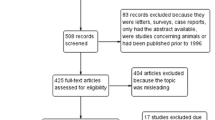
A literature review was performed using PubMed Central with the search terms “laparoscopic pancreatoduodenectomy”, “laparoscopic Whipple”, “minimally invasive pancreatoduodenectomy”, and “minimally invasive Whipple”. In addition, cross-referencing was performed, and the relevant articles were reviewed. The final search was made on May 5, 2016. Only articles written in English were included. The minimum number of LPD reported was 30. In the case of multiple articles from the same institution, only the most recent article with the most complete data was included to avoid any overlap in the data analysis. Articles with “hybrid” technique or robotic-assisted pancreatoduodenectomy (PD) were excluded from our review.
The following variables were collected in all the studies: patient demographics, including number of LPD, inclusion period, institution, country, age, sex, body mass index (BMI), and malignant indication; operative variables, including operative time (OT), intraoperative estimated blood loss (EBL), vascular resection, conversion rate, and conversion to hybrid or robotic technique; pathological reports, including tumor size, pathology, benign indications, margin status (R0), and number of lymph nodes resected; and postoperative outcomes, including overall complication, major complication, postoperative pancreatic fistula (POPF), delay gastric empting (DGE), postoperative pancreatic hemorrhage (PPH), intraabdominal abscess, hospital length of stay (LOS), re-intervention, and mortality.
Our previous report [6] assessed postoperative morbidity using the expanded Accordion Severity Grading System described by Strasberg et al. [7]. In five studies [8–12], the postoperative complications were addressed using the Clavien–Dindo classification system [13]; in one article [8], only grades ≥3 are reported, in two [9, 12], grades 3–4 are reported, in one [11], grades >3, and in one [10], all the grades are reported. To determine major complications, we used grades ≥3 in the Clavien–Dindo classification and >3 in the Strasberg classification. The paper from Paniccia et al. [14] did not use the standard classification for postoperative complications. In addition, all studies used the International Study Group of Pancreatic Surgery (ISGPS) consensus definitions for POPF [15]. Regarding DGE [16], the definition from the ISGPS was used in four papers [6, 8, 10, 11], but not specified in the other papers [9, 12, 14]. PPH [17] under the ISGPS definition was used in three papers [6, 8, 11], and the other papers used different definitions of postoperative hemorrhage.
Results
Patients’ selection
In a total of 728 papers, 7 articles [6, 8–12, 14] fulfilled our inclusion criteria for this review and included 516 patients who underwent LPD. All studies were single center reports, and no multi-institutional or prospective reports were identified. The mean observational period of the studies was 6 years (range 1–15), with reports coming from four countries on three continents. The demographic information for review articles is listed in Table 1.
At the turn of the century, laparoscopy for the treatment of pancreatic disease was only for staging and palliation of pancreatic malignancies [18, 19]. Since then, the feasibility and safety of LPD have been clearly demonstrated.
Palanivelu et al. presented the first large series of LPD in 2007 [20], and this group was the first to propose that not only was it possible and safe to perform LPD, but there may also be advantages in comparison with open resection. As expected, careful patient selection was performed during the exploration and early adoption phase of this technique.
In 2009, the same group reported a larger series of 75 patients to demonstrate the oncological adequacy and safety of LPD [21]. Indeed, the vast majority of LPD in the included studies have been performed for patients with underlying malignant disease, and no large series containing treatment of benign disease by LPD in large numbers have been published.
Regarding the age, sex, and BMI of those undergoing LPD, there were no differences in the recruitment of the patients. In fact, there were no common univocal selection or exclusion criteria for LPD amongst the studies. In our previous report [6], regarding our personal experience with LPD, the decision to perform LPD was not based only on clinical factors or preoperative diagnosis. In addition, the patients’ preferences were taken into consideration when deciding the surgical approach. However, as common sense dictates, large lesions with major vascular involvement or clearly hostile abdomen were excluded from LPD. Similar criteria were adopted by Kendrik and Cusati in their report [22]. Song and colleagues [10] included in their series only patients with periampullary tumors without preoperative T4 or M1 staging, suspicion of vascular involvement, high cardiopulmonary morbidity, severe obesity (>30 kg/m2 for men; >35 kg/m2 for women), or expected severe adhesion or inflammation. Delitto et al. [12] adopted similar criteria regarding BMI, with a cutoff of BMI ≥40. In addition to the previous exclusion criteria, Dokmak and colleagues [9] proposed that further contraindications to LPD included the need for multiple frozen sections, such as for intraductal papillary mucinous neoplasms or the division of a median arcuate ligament. Only in the reports published by Croome et al. [8], Mesleh et al. [23], and Senthilnathan et al. [11] was vascular involvement not exclusion criteria for LPD.
Operative details
Operative variables are summarized in Table 2. The reported OT was between 4 and 10 h. In the studies reported by Croome et al. [8] and Delitto et al. [12], there were no differences in OT comparing LPD vs OPD. On the contrary, Asbun and Stauffer [6], Dokmak et al. [9], and Song et al. [10] report that OT in LPD is greater than in OPD. This difference is significant in all three series with longer median OT of 139, 78, and 129 min, respectively. However, all the series described a reduction in the OT with increased experience. Song et al. described that, after the initial 50 cases of LPD, OT were decreased, becoming similar to that of OPD (15). Kendrick and Cusati reported a mean OT of 7.7 h for the first 10 patients, which improved to 5.3 h for the last 10 patients in their initial series of 62 patients [22]. It should also be noted that there was a learning curve not only for the surgeon, but also for all those involved in the procedure, including operating room staff, anesthesia team, and surgical assistants. Increased OT is the necessity of a meticulous dissection and precise reconstruction in LPD and may also be related to training new surgeons in the performance of the procedure.
The majority of the studies report a significant decrease in EBL when compared to OPD. This appears to be inherent to the laparoscopic technique, where any significant bleeding would obscure that the operative field and decreased blood loss may be one of the greatest advantages to LPD. EBL is directly related to a need for intraoperative blood transfusions, and therefore, many series also show a decreased use of blood transfusions for LPD. For patients with malignancy, minimizing blood transfusion has been reported to have a positive effect on the long-term survival [24–30].
Despite many who would consider vascular involvement requiring reconstruction as exclusion criteria for LPD, 2 series reports experience with vascular resections during LPD for 22 patients [8] and 1 patient [11]. In the author’s series comparing cost between 75 LPD and 48 OPD, there were no differences in the rate of vein resection between the laparoscopic (17%) and open (15%) procedures [23]. Croome et al. [31] and Palanisamy et al. [32] more recently reported their experience demonstrating the feasibility of major vascular resection during LPD. The description from Palamisamy et al. [32] is a report of a single case of portal resection with type IV vascular reconstruction. The aim of this paper was to show the technical feasibility of this procedure. Croome et al. [31] reported a large experience in vascular resections (31 patients) with different types of vascular involvement and reconstruction with a comparison between laparoscopic and open vascular resections. Laparoscopic resection had less EBL than open approach, but with longer clamping time (LPD 46.8 ± 30.8 min vs OPD 25.1 ± 16.2 min, p < 0.001). No differences were found between the two groups in terms of postoperative outcomes.
The conversion rate is lower than 10% in all the series. A common cause of conversion is reported to be the unexpected major vascular infiltration. Several groups report conversion to a hybrid technique [6, 8, 9, 22]. Croome et al. [8] report the experience of five patients undergoing LPD using robot-assisted methods as an adjunct for reconstructions after total LPD. Some authors suggest adopting the hybrid technique during the learning curve to improve the MIS technique at the beginning of the center’s experience. In addition, conversion to hybrid or OPD should not be considered as a failure as long as is it not at the expense of increased postoperative morbidity [6]. It is important to make the conversion before massive bleeding occurs, or when persistent failure to progress will result in an unreasonable and excessive increase in OT. This concept is stressed by the authors of all the series.
Postoperative outcomes
Postoperative outcomes are summarized in Table 3. The overall postoperative complication rate was 26.8–74%. However, major complication rates reported for Clavien–Dindo grades ≥3 [9–12] were 8.2–28%. Croome et al. [8] only reported those patients with Clavien–Dindo grades ≥3b, accounting for a major complication rate 5.6%. In our previous report [6], using the expanded Accordion Severity Grading System described by Strasberg et al. [7], major complications (Clavien–Dindo grades ≥3) occured in 13 patients (24.5%). In general, complication rates reported in these series are comparable to most other high-volume centers reporting open pancreatoduodenectomy outcomes. While it is difficult to compare the data for specific complications, such as POPF, DGE, and PPH, due to incomplete data information, the reported rates are similar to those reported in the previous literature, such as for the overall morbidity. Reported rates of POPF grades B–C were 6.3–44% in the different series. Dokmak et al. [9] reported the incidence of the overall POPF as 48% (grade A: 4%; grade B: 20%; grade C: 24%). In the same paper, they report the overall POPF rate after OPD as 41% (grade A: 9%; grade B: 26%; grade C: 6%). While there were no significant differences between LPD and OPD in the overall POPF rate, the rate of POPF grade C was higher in the LPD group. The authors suggested that this resulted from the difficult pancreatic anastomosis required for LPD in some periampullary tumors with a soft pancreas and a non-dilated small main pancreatic duct. However, besides this single experience, the other series report a lower POPF grades B and C rates for LPD ranging from 8.4 to 24% (Table 3).
Most series report a decreased LOS for LPD except for Dokmak et al. [9]. For PD, however, LOS is generally predicated on the presence or absence of major complications. As major complications are generally driven by POPF, it is worth noting that decreased LOS will be accomplished by minimizing the POPF and major complication rates. The difference in LOS seen in the included studies may also be due to a more aggressive postoperative management of the patients undergoing LPD in comparison with OPD rather than the laparoscopic approach itself.
Several series show that patients treated by LPD had a faster recovery, leading to an earlier start of adjuvant therapy. Croome et al. [8] reported a median time between surgery and adjuvant treatment of 48 (17–116) days for LPD vs 59 (25–302) days for OPD (p = 0.001). Song et al. [10] reported that 81.8% of the patients resected with LPD received adjuvant treatment compared to 69.7% of patients resected with OPD. Conversely, Nussbaum et al. [33] reported that LPD was not associated with an earlier initiation of adjuvant chemotherapy and that the interval time was more due to the major postoperative complications, not the surgical approach. Similar to this study, we have recently reported our long-term outcomes for pancreatic ductal adenocarcinoma after LPD and note that there was no advantage to the initiation of adjuvant therapy for LPD (Stauffer et al. 2016 Surg Endoscopy).
In a direct cost analysis of LPD vs OPD, LPD was found to be associated with increased costs within the operating room due to operating time and supply costs [23]. However, the overall cost between the two techniques was noted to be similar due to decreased postoperative admission costs for LPD.
Pathology
Extended pathological reports are listed in Table 4. The tumor dimensions were similar for all series, ranging from 1.2 to 4 cm. In general, LPD was associated with small-to-moderate dimension tumors and with smaller tumor size than OPD in most comparative series. As noted previously, a large proportion of patients in all series underwent LPD for malignant indications. Periampullary adenocarcinoma is the most frequent indication for LPD, with pancreatic ductal adenocarcinoma being the most common.
Negative margins were achieved in 60–100%, with four series reaching ≥90% R0 margin rate. Delitto et al. [12] reported higher negative margins for LPD compared to OPD (90.4 vs 74%, p = 0.03), and there may be a potential advantage with a precise dissection performed under direct magnified vision. The mean lymph node retrieval after LPD was 15–23 lymph nodes. Our series for patients with pancreatic adenocarcinoma showed higher lymph node retrieval for patients undergoing LPD compared to OPD, although this was related to the time period in which these operations were performed [34].
The lack of prospective or randomized trials and the relatively short follow-up prevents definitive conclusions regarding the overall long-term survival of patients with malignant disease treated with LPD. The results presented by Senthilnathan et al. [11] represent the largest series with the longest follow-up of patients undergoing LPD for periampullary malignancy. They describe 130 LPD from 1999 to 2013 with a median survival rate of 33 months. This survival rate was lower than that reported in their previous experience (24). The authors justify this decreased survival rate with the widened criteria of patient selection for LPD with growing expertise, as compared with highly selective cases in earlier series. The 5-year survival rate was 29.42% [11]. Our group recently reported our long-term survival for patients undergoing LPD for pancreatic ductal adenocarcinoma. Five-year survival was noted to be 32%, comparing favorably, but statistically similar, to OPD (15%) [34].
Discussion
Despite the increasing number of publications and interest regarding LPD, few large series are available and experience is still limited to a small number of highly specialized surgeons and institutions. In the majority of these centers and when performed by experienced surgeons, LPD has been proven to be feasible and safe with no inferiority to the open technique and in certain aspects, showing some benefits, such as EBL. This, however, may not be the case when analyzing results at lower volume centers. Sharpe and colleagues [35] analyzed the US National Cancer Database and found that LPD was performed for pancreatic ductal adenocarcinoma for 9% of patients (384 LPD vs 4037 OPD) in 2010 and 2011, 75.7% of hospitals performed OPD only, 23.3% performed <10 LPD, and 1% performed ≥10 LPD. Interestingly, the majority of LPD procedures (96.2%) were performed in low-volume centers (<10 LPD/year). Subgroup analysis revealed an alarming finding of a high mortality (7.5%) for low-volume centers performing LPD, underscoring the potential risks of this operation possibly resulting from being performed without the appropriate training or experience [35].
The data compiled in this review were highly selected to avoid the inclusion of a heterogeneous group of patients. There are many publications with smaller numbers of patients, as well as series that include hybrid or a composite of MIS techniques. A systematic review on robotic PD written by Cirocchi and colleagues [36] demonstrated how in different studies, there were a variety of techniques reported in a single group of patients. The authors concluded that the paper reported no difference in outcomes and safety in patients undergoing standard open, laparoscopic, or robotic PD. A recent paper published by Boggi et al. [37] described the safety and feasibility of pancreatic robotic resection in 200 consecutive robotic resections performed in 7 years. Within this patient group, the results of 83 robotic PD were reported with similar outcomes between open and robotic approach [37]. Zureikat et al. have recently reported a large multi-institutional series of robotic PD vs OPD [38] and found that many of the advantages and disadvantages of LPD are similar to the robotic approach. They found no difference for mortality, POPF, and length of stay, but found less major complications for the robotic platform.
In selected papers, case-matched controlled analysis was used to better compare LPD to OPD [9, 10]. Other authors used national or administrative database registries to evaluate the current status of minimally invasive PD [35, 39]. These studies have a larger number of patients with statistical power, but they miss the granular details of the patient’s actual surgical treatment and risk studying patients undergoing operations at low-volume centers without appropriate pancreatic surgical experience. Even though the conclusions of these studies are valid, one should keep in mind that this can lead to drawing flawed conclusions from skewed data resulting from inexperienced surgeons. Unfortunately, no prospective or multicenter studies comparing LPD with ODP were available.
In conclusion, experience with LPD is increasing worldwide, but more time is needed to draw a definite conclusion. Data available in the literature suggest that LPD is safe, feasible, and associated with similar postoperative and oncological outcomes to OPD. LPD should be implemented in high-volume centers with appropriately trained and experienced pancreatic surgeons to achieve the safest results. Randomized, controlled trials and multicenter studies are necessary to solidify the potential advantages of LPD. Even though it is clear that LPD is here to stay, its role still needs to be better defined. Time will shed more light on this issue, as more surgeons obtain experienced in the procedure, and improved studies are published. What is clear is that today, there is no role for a surgeon to embark in minimal access pancreatoduodenectomy, either laparoscopic or robotic assisted, without the appropriate training. There are now several centers in which these techniques are well established and the procedures are being done safely and efficiently.
References
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10(1):44–52. doi:10.1016/S1470-2045(08)70310-3
Uccella S, Bonzini M, Palomba S, Fanfani F, Malzoni M, Ceccaroni M, Seracchioli R, Ferrero A, Berretta R, Vizza E, Sturla D, Roviglione G, Monterossi G, Casadio P, Volpi E, Mautone D, Corrado G, Bruni F, Scambia G, Ghezzi F (2016) Laparoscopic vs. open treatment of endometrial cancer in the elderly and very elderly: an age-stratified multicenter study on 1606 women. Gynecol Oncol 141(2):211–217. doi:10.1016/j.ygyno.2016.02.029
Honda M, Hiki N, Kinoshita T, Yabusaki H, Abe T, Nunobe S, Terada M, Matsuki A, Sunagawa H, Aizawa M, Healy MA, Iwasaki M, Furukawa TA (2016) Long-term outcomes of laparoscopic versus open surgery for clinical stage I gastric cancer: the LOC-1 study. Ann Surg 264(2):214–222. doi:10.1097/SLA.0000000000001654
Cuschieri A (1994) Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 39(3):178–184
Gagner M, Pomp A (1994) Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 8(5):408–410
Asbun HJ, Stauffer JA (2012) Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 215(6):810–819. doi:10.1016/j.jamcollsurg.2012.08.006
Strasberg SM, Linehan DC, Hawkins WG (2009) The accordion severity grading system of surgical complications. Ann Surg 250(2):177–186. doi:10.1097/SLA.0b013e3181afde41
Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML (2014) Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 260(4):633–638. doi:10.1097/SLA.0000000000000937 (discussion 638–640)
Dokmak S, Fteriche FS, Aussilhou B, Bensafta Y, Levy P, Ruszniewski P, Belghiti J, Sauvanet A (2015) Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 220(5):831–838. doi:10.1016/j.jamcollsurg.2014.12.052
Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ (2015) Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann Surg 262(1):146–155. doi:10.1097/SLA.0000000000001079
Senthilnathan P, Srivatsan Gurumurthy S, Gul SI, Sabnis S, Natesan AV, Palanisamy NV, Praveen Raj P, Subbiah R, Ramakrishnan P, Palanivelu C (2015) Long-term results of laparoscopic pancreaticoduodenectomy for pancreatic and periampullary cancer-experience of 130 cases from a tertiary-care center in South India. J Laparoendosc Adv Surg Tech Part A 25(4):295–300. doi:10.1089/lap.2014.0502
Delitto D, Luckhurst CM, Black BS, Beck JL, George TJ Jr, Sarosi GA, Thomas RM, Trevino JG, Behrns KE, Hughes SJ (2016) Oncologic and perioperative outcomes following selective application of laparoscopic pancreaticoduodenectomy for periampullary malignancies. J Gastrointest Surg 20(7):1343–1349. doi:10.1007/s11605-016-3136-9
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
Paniccia A, Schulick RD, Edil BH (2015) Total laparoscopic pancreaticoduodenectomy: a single-institutional experience. Ann Surg Oncol 22(13):4380–4381. doi:10.1245/s10434-015-4450-2
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1):8–13
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Buchler MW (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142(5):761–768. doi:10.1016/j.surg.2007.05.005
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Buchler MW (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142(1):20–25. doi:10.1016/j.surg.2007.02.001
Kubota K (2011) Recent advances and limitations of surgical treatment for pancreatic cancer. World J Clin Oncol 2(5):225–228. doi:10.5306/wjco.v2.i5.225
Pisters PW, Lee JE, Vauthey JN, Charnsangavej C, Evans DB (2001) Laparoscopy in the staging of pancreatic cancer. Br J Surg 88(3):325–337. doi:10.1046/j.1365-2168.2001.01695.x
Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Rajapandian S, Madhankumar MV (2007) Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 205(2):222–230. doi:10.1016/j.jamcollsurg.2007.04.004
Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, Praveen Raj P (2009) Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 16(6):731–740. doi:10.1007/s00534-009-0157-8
Kendrick ML, Cusati D (2010) Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 145(1):19–23. doi:10.1001/archsurg.2009.243
Mesleh MG, Stauffer JA, Bowers SP, Asbun HJ (2013) Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc 27(12):4518–4523. doi:10.1007/s00464-013-3101-6
Kneuertz PJ, Patel SH, Chu CK, Maithel SK, Sarmiento JM, Delman KA, Staley CA 3rd, Kooby DA (2011) Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol 18(5):1327–1334. doi:10.1245/s10434-010-1476-3
Sun C, Wang Y, Yao HS, Hu ZQ (2015) Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg 13:102–110. doi:10.1016/j.ijsu.2014.11.044
Sutton JM, Kooby DA, Wilson GC, Squires MH 3rd, Hanseman DJ, Maithel SK, Bentrem DJ, Weber SM, Cho CS, Winslow ER, Scoggins CR, Martin RC 2nd, Kim HJ, Baker JJ, Merchant NB, Parikh AA, Abbott DE, Edwards MJ, Ahmad SA (2014) Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg 18(9):1575–1587. doi:10.1007/s11605-014-2567-4
Wang T, Luo L, Huang H, Yu J, Pan C, Cai X, Hu B, Yin X (2014) Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg 97(5):1827–1837. doi:10.1016/j.athoracsur.2013.12.044
Wehry J, Agle S, Philips P, Cannon R, Scoggins CR, Puffer L, McMasters KM, Martin RC (2015) Restrictive blood transfusion protocol in malignant upper gastrointestinal and pancreatic resections patients reduces blood transfusions with no increase in patient morbidity. Am J Surg 210(6):1197–1204. doi:10.1016/j.amjsurg.2015.08.013 (discussion 1204–1195)
Kwon AH, Matsui Y, Kamiyama Y (2001) Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer 91(4):771–778
Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M (2006) Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol) 18(1):60–66
Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML (2015) Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 19(1):189–194. doi:10.1007/s11605-014-2644-8 (discussion 194)
Palanisamy S, Deuri B, Naidu SB, Vaiyapurigoundar Palanisamy N, Natesan AV, Palanivelu PR, Parthasarathy R, Palanivelu C (2015) Major venous resection and reconstruction using a minimally invasive approach during laparoscopic pancreaticoduodenectomy: one step forward. Asian J Endosc Surg 8(4):468–472. doi:10.1111/ases.12208
Nussbaum DP, Adam MA, Youngwirth LM, Ganapathi AM, Roman SA, Tyler DS, Sosa JA, Blazer DG 3rd (2016) Minimally invasive pancreaticoduodenectomy does not improve use or time to initiation of adjuvant chemotherapy for patients with pancreatic adenocarcinoma. Ann Surg Oncol 23(3):1026–1033. doi:10.1245/s10434-015-4937-x
Stauffer JA, Coppola A, Villacreses D, Mody K, Johnson E, Asbun HJ (2016) Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc. doi:10.1007/s00464-016-5222-1
Sharpe SM, Talamonti MS, Wang CE, Prinz RA, Roggin KK, Bentrem DJ, Winchester DJ, Marsh RD, Stocker SJ, Baker MS (2015) Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the national cancer data base. J Am Coll Surg 221(1):175–184. doi:10.1016/j.jamcollsurg.2015.04.021
Cirocchi R, Partelli S, Trastulli S, Coratti A, Parisi A, Falconi M (2013) A systematic review on robotic pancreaticoduodenectomy. Surg Oncol 22(4):238–246. doi:10.1016/j.suronc.2013.08.003
Boggi U, Napoli N, Costa F, Kauffmann EF, Menonna F, Iacopi S, Vistoli F, Amorese G (2016) Robotic-assisted pancreatic resections. World J Surg. doi:10.1007/s00268-016-3565-3
Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, Weber SM, Abbott DE, Ahmad SA, Maithel SK, Hogg ME, Zenati M, Cho CS, Salem A, Xia B, Steve J, Nguyen TK, Keshava HB, Chalikonda S, Walsh RM, Talamonti MS, Stocker SJ, Bentrem DJ, Lumpkin S, Kim HJ, Zeh HJ 3rd, Kooby DA (2016) A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. doi:10.1097/SLA.0000000000001869
Adam MA, Choudhury K, Dinan MA, Reed SD, Scheri RP, Blazer DG 3rd, Roman SA, Sosa JA (2015) Minimally invasive versus open pancreaticoduodenectomy for cancer: practice patterns and short-term outcomes among 7061 patients. Ann Surg 262(2):372–377. doi:10.1097/SLA.0000000000001055
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. H.J. Asbun, Dr. J.A. Stauffer, and Dr. A. Coppola report no biomedical financial interests or potential conflicts of interest.
Ethical approval
There are no ethical conflicts with this manuscript.
Research involving human participants and/or animals
The article does not contain any research with human participants performed by the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Coppola, A., Stauffer, J.A. & Asbun, H.J. Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg 68, 217–224 (2016). https://doi.org/10.1007/s13304-016-0402-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-016-0402-z