Abstract
Introduction
Second-generation basal insulin analogues have been shown to reduce hypoglycemia in several trials and observational studies of select populations; however, it remains unclear whether these results persist in real-world settings. Using self-reported hypoglycemia events, we assessed whether second-generation basal insulin analogues reduce rates of hypoglycemia events (non-severe/severe; overall/daytime/nocturnal) compared to earlier intermediate/basal insulin analogues among people with insulin-treated type 1 or 2 diabetes.
Methods
We used prospectively collected data from the Investigating Novel Predictions of Hypoglycemia Occurrence Using Real-World Models (iNPHORM) panel survey. This US-wide, 1-year internet-based survey assessed hypoglycemia experiences and related sociodemographic and clinical characteristics of people with diabetes (February 2020−March 2021). We estimated population-average rate ratios for hypoglycemia comparing second-generation to earlier intermediate/basal insulin analogues using negative binomial regression, adjusting for confounders. Within-person variability of repeated observations was addressed with generalized estimating equations.
Results
Among iNPHORM participants with complete data, N = 413 used an intermediate/basal insulin analogue for ≥ 1 month during follow-up. After adjusting for baseline and time-updated confounders, average second-generation basal insulin analogue users experienced a 19% (95% CI 3–32%, p = 0.02) lower rate of overall non-severe hypoglycemia and 43% (95% CI 26–56%, p < 0.001) a lower rate of nocturnal non-severe hypoglycemia compared to earlier intermediate/basal insulin users. Overall severe hypoglycemia rates were similar among second-generation and earlier intermediate/basal insulin users (p = 0.35); however, the rate of severe nocturnal hypoglycemia was reduced by 44% (95% CI 10–65%, p = 0.02) among second-generation insulin users compared to earlier intermediate/basal insulin users.
Conclusion
Our real-world results suggest second-generation basal insulin analogues reduce rates of hypoglycemia, especially nocturnal non-severe and severe events. Whenever possible and feasible, clinicians should prioritize prescribing these agents over first-generation basal or intermediate insulin in people with type 1 and 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Hypoglycemia remains a major adverse event of basal insulin, a commonly prescribed therapy to treat diabetes. Based on trial evidence, second-generation basal insulins confer lower risks of hypoglycemia than earlier intermediate/basal insulins, but this effect has been inadequately assessed in real-world settings |
This study evaluates the impact of second-generation insulins (insulin degludec and glargine U-300) versus earlier intermediate and basal insulins (NPH, insulin glargine U-100 and detemir, premixed and fixed-ratio [FRC] insulins) on rates of overall, daytime, and nocturnal non-severe and severe hypoglycemia |
What was learned from the study? |
The most salient effects were observed for nocturnal hypoglycemia. Overall, second-generation insulin versus earlier intermediate/basal insulin users reported a 43% reduction in non-severe nocturnal hypoglycemia (p < 0.001) and a 44% reduction in severe nocturnal hypoglycemia (p = 0.02). These trends persisted across diabetes types |
Among patients with either type 1 or 2 diabetes mellitus on basal insulin (with or without bolus), the use of second-generation basal insulins over earlier formulations should be prioritized whenever possible |
Introduction
Hypoglycemia remains the most deleterious side effect of insulin [1] and a barrier to diabetes control [2]. To avoid events, many people with type 1 and 2 diabetes mellitus (T1DM, T2DM) will deliberately reduce or skip their insulin dose [3]. Clinicians may also delay insulin initiation or fail to effectively up-titrate when necessary [4, 5].
Recent drug trials demonstrate promising reductions in hypoglycemia for second-generation basal insulin analogues (i.e., insulin Degludec [first available in 2015] and glargine U-300 [first available in 2013]) versus earlier formulations (risk reduction range 24–39%) [6,7,8,9,10,11,12]. However, whether this effect persists in general diabetes populations in the USA remains largely unaddressed in the literature.
The BEGIN [11, 12] and EDITION [6,7,8,9,10, 13] phase 3 trials excluded individuals at risk of severe hypoglycemia: those with tight glycemic control, clinically important diabetes complications, or, as in the SWITCH 2 trial [14, 15], history of combined insulin and secretagogue use [16]. According to Mauricio et al., these sample bounds can exclude up to 83% of the total T2DM population [17]. The DELIVER trial [18] enrolled a slightly broader cohort, but—similar to the few extant pharmacoepidemiologic studies on second-generation basal insulins [18,19,20]—restricted hypoglycemia assessment to the small [16, 21] percentage of events requiring healthcare.
The current analysis leverages prospective observational data collected between 2020 and 2021 to determine the effect of second-generation basal insulins, versus earlier intermediate/basal insulin analogues, on real-world rates of non-severe and Level 3 severe hypoglycemia. Our analysis supplies timely insight, given HEDIS’s (Healthcare Effectiveness Data and Information Set) newly sanctioned quality metric for hypoglycemia prevention in the US [22]. Real-world safety data favoring second-generation basal insulins could inspire their increased use—in the US, despite high dispensation rates for basal insulins in general [23, 24], prescribing of either insulin degludec and glargine U-300 remains relatively low [25].
Methods
Study Design
Data were analyzed from the Investigating Novel Predictions of Hypoglycemia Occurrence using Real-World Models (iNPHORM) study: a US-wide, 12-wave ambidirectional panel survey. Complete details on the design and conduct of iNPHORM are published elsewhere [26]. The present article complies with ‘The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies’ [27]; it addresses Objective 3 of the overall iNPHORM study (see protocol [26]).
Participants and Data Collection
Self-assessed data on hypoglycemia and potential non-clinical/clinical factors (intrinsic, extrinsic, non-modifiable, and modifiable) were captured across 14 closed questionnaires (screening, baseline, 12-monthly follow-ups) that were pretested and piloted prior to fielding.
Participants were recruited across two subpanels (A and B) from a probability-based internet panel (> 65,000 Americans with diabetes). After screening, eligible panelists were required to provide consent and complete a baseline questionnaire to enroll. Panelists who were (1) 18–90 years old, (2) living in the US (past year), (3) self-reporting T1DM or T2DM, and (4) using insulin, secretagogues, or both (past year) were eligible. Those diagnosed with gestational diabetes, participating in a concurrent trial, or pregnant (at screening or year prior) were ineligible.
Subpanel A (n = 1257) was conveniently sampled from a random subset of the internet panel. Those who failed to complete the first follow-up were withdrawn (n = 488) and refreshed with 437 new eligible participants conveniently sampled from a different random subset of the internet panel (subpanel B). Individuals in subpanel A who completed the first follow-up and all those in subpanel B comprised the iNPHORM Longitudinal Panel (N = 1206). Quota sampling was used to ensure minimum representation by diabetes type, sex, age, and medication regimen.
Enrollees were managed by Ipsos Interactive Services (IIS), a global leader in real-world survey conduct. In total, participants in the iNPHORM Longitudinal Panel were emailed 12-monthly follow-up questionnaires by IIS. Participants were given 7 days to submit each follow-up using any internet-enabled device (e.g., computer, tablet, smartphone). Responses were automatically stored on the IIS platform. Token cash incentives, in line with ethical guidelines [28], were distributed based on the number and timing of completed questionnaires.
Exposure and Confounding Assessment
At screening and all monthly follow-ups, participants were asked to self-report on their current use of second-generation (insulin degludec and insulin glargine U-300) and earlier intermediate/basal insulin analogues (insulin detemir, insulin glargine U-100, premixed insulin, fixed-ratio [FRC] insulin, and intermediate [insulin isophane {Neutral Protamine Hagedorn; NPH} insulin]). To improve self-reported accuracy, participants were encouraged to review their medication packaging before reporting, prompted by medication lists of generic and brand names, provided information about their last reported antihyperglycemic regimen, and allowed to complete the survey over multiple days, if needed.
The causal effect of second-generation versus earlier intermediate/basal insulin analogues on real-world rates of hypoglycemia is plausibly confounded by various clinical and non-clinical factors. To identify possible confounding variables, we constructed a directed acyclic graph (DAG) using the daggity plotting tool [29] for all severities and timings (daytime and nocturnal) of hypoglycemia (Supplementary Fig. 1 in the electronic supplementary material).
A single DAG was created to ensure internal comparability between effect estimates for hypoglycemia severities and timings. Based on the DAG, we identified the following minimally sufficient adjustment set: age, employment status, household income, diabetes type, diabetes duration, insulin therapy duration, bolus insulin use, and background secretagogue therapy.
All variables were self-reported and collected via iNPHORM questionnaires. Age was determined at screening based on each participant’s date of birth (month and year). Current employment status (i.e., working full or part time (including self-employment), retired, unemployed, or a student) was measured (single response option) at baseline and updated at 4-month intervals. Gross annual household income was assessed at baseline according to intervals of $15,000. Diabetes type (T1DM or T2DM) was self-reported at screener and verified at baseline. Diabetes duration was determined at baseline based on age of diabetes diagnosis. Insulin therapy duration was assessed at baseline by asking participants to self-report how long in years and months they had been taking any insulin, regardless of brand, type, or delivery method. Bolus insulin use (i.e., bolus/prandial insulin, including rapid- and short-acting insulin) and background secretagogue therapy (i.e., short-, intermediate-, or long-acting sulfonylurea; meglitinide; meglitinide and biguanide fixed-dose combination or sulfonylurea and biguanide fixed-dose combination; and/or some other secretagogue) were assessed (multi-response option) at baseline and updated at every follow-up.
Outcome Assessment
Frequencies of daytime and nocturnal non-severe and severe hypoglycemia were assessed at baseline and prospectively across 12 follow-ups. Definitions consistent with the International Hypoglycaemia Study Group and American Diabetes Association (ADA) Standards of Medical Care in Diabetes [30] were provided in all questionnaires. Specifically, non-severe hypoglycemia was defined as any event, identified via symptoms and/or blood glucose measurement, that the participant was physically able to self-treat. Severe hypoglycemia was defined as any Level 3 event that the participant was physically unable to self-treat and thus may have required external assistance for recovery (e.g., treatment by family/friend, ambulatory care, hospitalization) [31]. The response options “I recovered on my own without any kind of treatment,” “Other,” and “Unknown treatment” were provided. Participants reported whether their non-severe/severe hypoglycemia occurred while awake (daytime) or sleeping/attempting to sleep (nocturnal). Overall non-severe and overall severe hypoglycemia combined daytime and nocturnal events.
To ensure accurate responses and to prevent overlapping recall intervals, follow-up data on daytime and nocturnal non-severe hypoglycemia were captured “Within the past 30 days” (if the last scheduled questionnaire was not completed) or “Since the last time an iNPHORM survey was completed” (if the last scheduled questionnaire was completed). Conversely, given its relative infrequency and saliency, daytime and nocturnal severe hypoglycemia was captured “Since the last time an iNPHORM survey was completed.”
Statistical Analysis
Analyses were restricted to T1DM or T2DM participants who reported taking intermediate/basal insulin (with or without a bolus regimen) at any time over follow-up and who responded to at least one follow-up questionnaire. Sample characteristics were summarized using frequencies and percentages for categorical variables and means and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables. Incidence proportions of daytime and nocturnal non-severe and severe hypoglycemia were quantified over the duration of follow-up using Wilson’s confidence interval for binomial proportions. To account for over-dispersion, annualized rates were modeled using negative binomial regression with follow-up durations included as offsets.
We estimated the population-average rate ratio of hypoglycemia events comparing use of second-generation with earlier intermediate/basal insulin analogues using multivariable negative binomial regression with generalized estimating equations (GEEs). Models were adjusted for confounding variables identified by our DAG. Repeated and time-varying outcome and covariate measures were analyzed in our final models. We selected an exchangeable covariance structure to ensure all repeated observations within a participant had the same correlation over time. We estimated separate effects by severity (non-severe and severe), timing (overall, daytime, and nocturnal), and diabetes type. Finally, a sensitivity analysis was performed to test the potential time-varying confounding effect of hypoglycemia frequency on subsequent medication regimens (e.g., high hypoglycemia rates may have encouraged participants to initiate second-generation basal insulin analogue use).
All statistical tests were two-sided with a significance level of α = 0.05. Per convention, complete case analyses were performed if the proportion of missingness was low. All analyses were conducted using Stata version 15.1.
Compliance with Ethical Guidelines
Before recruitment, we obtained ethics approval from the Western University Health Sciences Research Ethics Board (Project ID: 112,986; December 17, 2019). The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. Consent to participate was obtained at screening and could be revoked by not completing future questionnaires. Consent to publication was obtained at screening.
Results
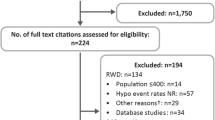
Among the iNPHORM Longitudinal Panel, 438 of 1206 (36%) were treated using a second-generation or earlier intermediate/basal insulin analogue for at least 1 month during follow-up. Of these participants, 25 (5.7%) reported missing baseline or follow-up information and were excluded. Complete case analyses were performed on the remaining 413 respondents (Fig. 1). On average, participants completed 9 (minimum: 1; maximum: 12) follow-up questionnaires.
Sample Characteristics
Table 1 presents the baseline clinical and sociodemographic characteristics of our final cohort. Roughly 20% (n = 81) of participants had T1DM, while the median diabetes duration across the sample was 15 years (IQR: 16 years). Individuals were on average 53.0 (SD: 13.6) years old, and 55% (n = 226) were female. Mean body mass index (BMI) was 33.0 (SD: 9.6) kg/m2, and 86% (n = 356) reported having one or more comorbidities. Roughly two-thirds (n = 271; 65.6%) of participants reported HbA1c values < 8%.
Overall, 23% (n = 93) reported using a second-generation basal insulin analogue, 64% (n = 264) a concomitant bolus insulin, and 22% (n = 92) a secretagogue therapy. Private insurance was more common among second-generation versus earlier intermediate/basal insulin users (44% vs. 36%; p = 0.07); contrariwise, public insurance (e.g., via government assistance plans) was more common among earlier versus second-generation insulin users (42% vs. 31%; p = 0.03). Participants treated with earlier intermediate/basal insulin analogues were also more likely to report annual gross household incomes < $25,000 (though not statistically significantly; 23% vs. 18%; p = 0.15) compared to second-generation insulin analogue users who more frequently reported incomes between $55,000 and $84,999 (28% vs. 19%; p = 0.03).
Hypoglycemia Incidence
For non-severe and severe hypoglycemia, respectively, a total of 311.7 and 287.7 person-years were observed, with a mean of 274.8 and 253.6 days observed per participant and 30.5 and 28.1 days observed per questionnaire. Tables 2 and 3 summarize hypoglycemia incidence proportions and rates among second-generation and earlier intermediate/basal insulin users, by type of diabetes, event severity, and timing. Most participants reported at least one overall non-severe hypoglycemia event over follow-up (83.5%, 95% confidence interval [CI]: 79.7−86.8%), while the rate of overall non-severe hypoglycemia was 27.0 events per person-year (EPPY) (95% CI 23.5−30.9). Participants reported a rate of 2.9 overall severe hypoglycemia EPPY (95% CI 2.2−3.8 EPPY); 31.2% experienced one or more overall severe hypoglycemia events (95% CI 27.0−35.9%). Overall, daytime events were more frequent than nocturnal.
The rate of non-severe hypoglycemia was higher among T1DM versus T2DM participants (63.5 vs. 17.9 EPPY, p < 0.001); however, for severe hypoglycemia, rates were statistically comparable (3.3 [T1DM] vs. 2.8 [T2DM] EPPY, p = 0.55). Second-generation basal insulin users reported lower crude rates of daytime and nocturnal non-severe and severe hypoglycemia compared to earlier intermediate/basal insulin users. Notably, the rate of non-severe nocturnal hypoglycemia among second-generation insulin users was nearly half that reported by earlier intermediate/basal insulin users (5.2 vs. 8.6 EPPY, p = 0.04); we observed a similar trend for severe nocturnal events (0.4 vs. 0.9 EPPY, p = 0.14).
Effect of Insulin Analogue Type on Hypoglycemia Rates
Table 4 reports population-average adjusted hypoglycemia rate ratios for second-generation versus intermediate/basal insulin use (by type of diabetes, event severity, and timing). Supplementary Table 1 in the Supplementary Material summarizes all parameter estimates.
The estimated population-average nocturnal non-severe hypoglycemia rate was statistically significantly lower among second-generation versus earlier intermediate/basal insulin analogue users (rate ratio [RR]: 0.57 [95% CI 0.44−0.74], p < 0.001); this trend was statistically non-significant for the estimated population-average daytime non-severe hypoglycemia rate (RR: 0.91 [95% CI 0.76−1.10], p = 0.32). Participants on second-generation versus earlier intermediate/basal insulin analogues also experienced statistically significantly fewer severe nocturnal events (RR: 0.56 [95% CI 0.35−0.90], p = 0.02); this association was more salient among participants with T1DM (RR: 0.23 [95% CI 0.06−0.93], p = 0.04) compared to those with T2DM (RR: 0.63 [95% CI 0.38–1.06], p = 0.08). Other estimated effects of second-generation compared to earlier intermediate/basal insulin analogue use on hypoglycemia rates were consistent between T1DM and T2DM participants as well as across event severities and timings (Table 4).
Sensitivity analyses revealed a low risk of time-varying confounding introduced by participants switching between second-generation and earlier intermediate/basal insulins. When we restricted our sample to participants treated with the same intermediate/basal insulin type over the entirety of follow-up (n = 385, 93%), similar rate ratios were estimated (Supplementary Table 2 in the Supplementary Material). Thus, the impact of confounding due to medication switching on our final effect estimates was minimal.
Discussion
Regardless of diabetes type, hypoglycemia was common across all iNPHORM participants, but particularly among those not on a second-generation basal insulin over follow-up. Most participants experienced one or more non-severe hypoglycemia event(s) (83.5% [95% CI 79.7–86.8]); however, crude rates of non-severe hypoglycemia were decreased among second-generation compared to earlier intermediate/basal insulin users (23.1 [95% CI 18.1–29.5] EPPY]; 28.3 [95% CI 24.2–33.1] EPPY, respectively). This difference was especially pronounced for nocturnal events, with second-generation basal insulin users reporting rates of non-severe and severe hypoglycemia roughly half those of earlier intermediate/basal insulin users (5.2 vs. 8.6 EPPY, 0.4 vs. 0.9 EPPY, respectively). These patterns were consistent among T1DM and T2DM intermediate/basal insulin users.
Consistent with previous trial [6,7,8,9,10,11,12] and observational [18,19,20] data, hypoglycemia incidences were lower among second-generation basal insulin users versus earlier intermediate/basal insulins—especially for nocturnal events. Specifically, second-generation basal insulin use effected a statistically significant relative rate reduction of 43% (RR: 0.57 [95% CI 0.44−0.74, p < 0.001]) for nocturnal non-severe hypoglycemia. This effect was consistent among our T1DM and T2DM second-generation basal insulin cohorts who experienced 48% (RR: 0.52 [95% CI 0.34–0.81, p = 0.003]) and 37% (RR: 0.63 [95% CI 0.46–0.86, p = 0.004]) fewer nocturnal non-severe events than T1DM and T2DM respondents on earlier intermediate/basal agents, respectively.
According to research by Brod and colleagues [32, 33], nocturnal non-severe hypoglycemia causes significant disruption and distress in the majority of people with T1DM and insulin-treated T2DM with negative impacts on quality of life; daily living, sleep, and other routines; physical functioning; as well as emotional and social well-being [32, 34]. Events can also intensify next-day glycemic variability and feelings of sleep deprivation and low energy [35]. These long-lasting ramifications can compromise work productivity and efficiency and lead to higher rates of absenteeism and other economic costs [36, 37]. Moreover, compared to daytime events, nocturnal hypoglycemia is typically more prolonged (by 3–5 h) [35], aggravating the risk of impaired awareness and hypoglycemia-associated autonomic failure.[36] Impaired versus intact counter-regulation has been associated with 3–20 times the probability of severe hypoglycemia [38,39,40,41,42].
Indeed, we also revealed a rate reduction in severe hypoglycemia, particularly for nocturnal severe events, among second-generation basal insulin versus earlier intermediate/basal insulin users (RR: 0.56 [95% CI 0.35−0.90, p = 0.02]). This protective trend was more salient among participants with T1DM compared to T2DM (T1DM RR: 0.23 [95% CI: 0.06−0.93; p = 0.04]; T2DM RR: 0.63 [95% CI 0.38−1.06; p = 0.08]). Our comparison of nocturnal severe hypoglycemia rates between second-generation compared to earlier intermediate/basal insulin users only possessed 59% power determined post hoc to detect a significant difference [43]. We identified one observational study that reported an independent protective effect for second-generation insulins on severe hypoglycemia [44], however, only for the < 5% of events resulting in healthcare [21, 45].
Regardless, the promising potential for second-generation basal insulins to prevent severe nocturnal hypoglycemia cannot be understated. Preferential use of these agents may enhance diabetes clinical outcomes, including quality of life, direct and indirect costs of care [46,47,48], and, furthermore, patient self-management capacity. Because people with diabetes often consider nocturnal hypoglycemia less predictable and, thus, scarier than daytime events, many will maintain their blood glucose levels higher than clinically recommended before bedtime. Such over-compensatory avoidance of hypoglycemia, compounded by other life changes including defensive eating, can exacerbate the onset of diabetes complications over time [3].
Overall, our population-based results support the use of second-generation basal insulins over earlier intermediate/basal insulins as a real-world solution to achieving better glycemic outcomes with enhanced safety. Indeed, our results demonstrate reduced nocturnal hypoglycemia associated with second-generation basal insulin use among both T1DM and T2DM intermediate/basal insulin users, in agreement with previous explanatory trials [6,7,8,9,10,11,12]. Our recommendation is backdropped by the more stable, ultra-long duration of action, and injection flexibility of second-generation basal insulins—features that can foster and sustain increased patient adherence. Nevertheless, despite the ubiquity of basal insulins to treat diabetes, current research indicates that second-generation basal insulins represent only a quarter of all insulin-naïve and non-naïve insulin prescriptions in T1DM [49] and T2DM [25].
Treatment inertia is a well-identified deterrent to basal insulin initiation and intensification that requires careful support and education to mitigate; newer insulin agents may be a key facilitator to this end. As an effective and safer insulin option, second-generation basal insulins can reduce fear of hypoglycemia and, in turn, other avoidable consequences of sub-optimal glycemic control [50,51,52,53,54,55]. This benefit is likely to persist across the wide spectrum of disease stages, dosing regimens, and pharmacotherapeutic requirements (e.g., bolus regimens) [56,57,58]. The specific yet potent effect of second-generation basal insulins on nocturnal hypoglycemia demands increased clinical appreciation of these events, in terms of both their frequency and burden in diabetes and related sequelae [54].
Notwithstanding, patient-level economic barriers in accessing newer antihyperglycemics must be addressed [59, 60]. Compared to second-generation insulin users in our study, earlier intermediate/basal insulin users were more likely to rely on public insurance and less likely to report a gross household income between $55,000 and $84,999 (42% vs. 31%, p = 0.03; 19% vs. 28%, p = 0.03). In 2023, the US National Committee for Quality Assurance amended their Healthcare Effectiveness Data and Information Set (HEDIS) tool to include hypoglycemia prevention as a key clinical performance measure [61]. We, thus, supply timely evidence in line with this change that, moreover, emphasizes the importance of government and healthcare insurance plans to improve access to and coverage of second-generation basal insulins in the US.
Strengths and Limitations
Prior to iNPHORM, no studies had assessed the real-world effect of second-generation versus earlier intermediate/basal insulin types on rates of hypoglycemia. Instead, earlier evidence stemmed from poorly generalizable trials that excluded individuals at greatest risk of hypoglycemia [62] and health administrative databases [44, 63, 64] prone to severe outcome ascertainment bias [16, 65].
Addressing this gap, we administered a screener, baseline, and 12-monthly questionnaires to an online panel reflective of the US public with diabetes. Primary self-reported data collection optimized complete and honest hypoglycemia reporting [21]; it also allowed us to capture information on potential confounding variables often unavailable or poorly documented in routine health records (e.g., employment, household income).
The use of internet panel surveys enabled us to reach participants with T1DM and T2DM across the US while ensuring feasibility and strong participant retention. Quota sampling ensured sufficient representation of key groups (i.e., people with T1DM and T2DM, older people, people assigned female sex at birth, and people treated with insulin, secretagogues, and their combination). Nonetheless, despite efforts to ensure sample representativeness, iNPHORM participants were often insured and unemployed, retired, or in school.
Prospective collection of hypoglycemia events at monthly intervals maximized participant recall while minimizing burden of data collection on participants. Needless to say, we could not assess events undetected or unreported by participants. For example, perception bias may have caused some individuals treated with second-generation insulins to under-estimate their hypoglycemia frequencies—particularly non-severe events. Consequent measurement error could have attenuated self-reported non-severe event frequencies among second-generation versus earlier intermediate/basal insulin users, resulting in a conservative estimate of the corresponding hypoglycemia rate reduction. Severe hypoglycemia was less likely affected by perception bias; nevertheless, we did not corroborate the percentage of events resulting in healthcare (e.g., using health service records).
Compared with glycemic measurements obtained by real-time/continuous glucose monitoring (rt-C/FGM), self-reported hypoglycemia events must be recalled by participants—risking measurement error when events are unable to be recalled (e.g., nocturnal events are less likely to be recalled if the participant was asleep while experiencing the event). While guideline-defined severe hypoglycemia does not stipulate a glycemic threshold[30], non-severe hypoglycemia detection could be improved using rt-C/FGM. However, rt-C/FGM use remains low: in our sample only 11.6% reported using a rt-C/FGM device overall and 10.5% among participants with T2DM. Simlarly, the Behavioral Risk Factor Surveillance System observed low adoption of rt-C/FGM (4.1%) among Americans with diabetes in 2020 [66]. Relying on self-report ensured data collection among a large sample was feasible and mitigated the risk of selection bias due to rt-C/FGM inaccessibility.
The broad range of self-reported participant characteristics allowed us to control for confounding factors unmeasured in earlier observational studies. Adjustments for confounding balanced feasibility with granularity; however, residual confounding remains possible. We also did not account for multiple comparisons, which may have increased type I error. Instead, we based inferences on the estimated directions and magnitudes of effect and confidence intervals.
Finally, owing to the design of our questionnaire, we were unable to isolate the effect of second- versus first-generation or intermediate insulins specifically. Additionally, we could not ascertain potential differences between glargine U-300 and insulin degludec on rates of hypoglycemia, despite some research suggesting that their effects vary [6, 7, 9, 10, 13,14,15, 63].
Conclusion
Real-world evidence from the iNPHORM study confirms that second-generation basal insulins are associated with lower hypoglycemia rates than earlier intermediate/basal insulins, particularly regarding reducing non-severe and severe nocturnal hypoglycemia in people with T1DM or insulin-treated T2DM. By mitigating hypoglycemia occurrence—the most feared and debilitating side effect of insulin—second-generation basal insulins provide an unignorable conduit to allaying the high human and economic costs of diabetes.
References
Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014. https://doi.org/10.1038/nrendo.2014.170.
Hsu WC. Consequences of delaying progression to optimal therapy in patients with type 2 diabetes not achieving glycemic goals. South Med J. 2009;102:67–76.
Leiter L, Yale J, Chiasson J, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemic management. Can J Diabetes. 2005;29:186–92.
Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in Type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21:220–6.
Cuddihy RM, Philis-Tsimikas A, Nazeri A. Type 2 diabetes care and insulin intensification: is a more multidisciplinary approach needed? Results from the MODIFY survey. Diabetes Educ. 2011;37:111–23.
Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755–62.
Home PD, Bergenstal RM, Bolli GB, et al. New Insulin Glargine 300 Units/mL Versus Glargine 100 Units/mL in People With Type 1 Diabetes: A Randomized, Phase 3a, Open-Label Clinical Trial (EDITION 4). Diabetes Care. 2015;38:2217–25.
Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–67.
Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–9.
Matsuhisa M, Koyama M, Cheng X, et al. Sustained glycaemic control and less nocturnal hypoglycaemia with insulin glargine 300U/mL compared with glargine 100U/mL in Japanese adults with type 1 diabetes (EDITION JP 1 randomised 12-month trial including 6-month extension). Diabetes Res Clin Pract. 2016;122:133–40.
Rodbard HW, Gough S, Lane W, Korsholm L, Bretler DM, Handelsman Y. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2014;20:285–92.
Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet Lond Engl. 2012;379:1498–507.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–94.
Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA. 2017;318:45–56.
Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA. 2017;318:33–44.
Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: Results of the InHypo-DM Study, Canada. BMJ Open Diabetes Res Care. 2018;6:503.
Mauricio D, Westerbacka J, Nicholls C, Wu J, Gupta R, Eliasson B. How many people with type 2 diabetes fulfil the eligibility criteria for randomized, controlled trials of insulin glargine 300 U/mL in a real-world setting? Diabetes Obes Metab. 2021;23:838–43.
Sullivan SD, Bailey TS, Roussel R, et al. Clinical outcomes in real-world patients with type 2 diabetes switching from first- to second-generation basal insulin analogues: Comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study. Diabetes Obes Metab. 2018;20:2148.
Bailey T, Zhou L, Gupta R, et al. E13: Glycemic goal attainment and hypoglycemia risk outcomes in patients with T2D initiating insulin glargine 300 U/mL versus 100 U/mL in real-world clinical practice. In: Poster J. Manag. Care Spec. Pharm. Conf. 2018.
Zhou FL, Ye F, Gupta V. LB SUN 81: lower risk of hypoglycemia after switch to insulin glargine 300 U/mL (Gla-300) vs other basal insulins in patients with type 2 diabetes (T2D) on basal insulin in real-world clinical settings (Deliver 2 study). In: Endocr. Soc. 2017 Annu. Meet. 2017.
Karter AJ, Moffet HH, Liu JY, Lipska KJ. Surveillance of hypoglycemia-limitations of emergency department and hospital utilization data. JAMA Intern Med. 2018;178:987–8.
Healthcare Effectiveness Data and Information Set. Emergency Department visits for hypoglycemia in older adults with diabetes (EDH): summary of changes to HEDIS. National Committee for Quality Assurance. 2023.
Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–47.
Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639–43.
Dankers M, Hek K, Nelissen-Vrancken M, Houweling ST, Mantel-Teeuwisse A, van Dijk L. Newer long-acting insulin prescriptions for patients with type 2 diabetes: prevalence and practice variation in a retrospective cohort study. Br J Gen Pract J R Coll Gen Pract. 2022;72:e430–6.
Ratzki-Leewing A, Ryan BL, Zou G, et al. Predicting real-world hypoglycemia risk in American adults with type 1 or 2 diabetes mellitus prescribed insulin and/or secretagogues: Protocol for a prospective, 12-wave internet-based panel survey with email support (the iNPHORM study, USA). JMIR Res Protoc. 2022. https://doi.org/10.2196/33726.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiol Camb Mass. 2007;18:800–4.
Kalfs N, Evert H van. Nonresponse and travel surveys. In: Transport survey quality and innovation. Elsevier; 2003.
Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiol Camb Mass. 2011;22:745.
Johnson EL, Feldman H, Butts A, et al. Standards of medical care in diabetes—2019 abridged for primary care providers. Clin Diabetes Publ Am Diabetes Assoc. 2019;37:11.
International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–7.
Brod M, Pohlman B, Wolden M, Christensen T. Non-severe nocturnal hypoglycemic events: experience and impacts on patient functioning and well-being. Qual Life Res. 2013;22:997–1004.
Gruenberger JB, Bader G, Benford M, Pike J. PDB54 HRQoL and clinical impact of mild patient-reported hypoglycaemic episodes in five european countries: extent of agreement between physician- and patient-reported hypoglycaemic episodes. Value Health. 2011;14:A481.
Allen KV, Frier BM. Nocturnal hypoglycemia: clinical manifestations and therapeutic strategies toward prevention. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2003;9:530–43.
Pedersen-Bjergaard MJU. Self-reported frequency and impact of non-severe hypoglycemic events in insulin-treated diabetic patients in Denmark. Diabetes Manag. 2015;5:66–77.
Edelman SV, Blose JS. The impact of nocturnal hypoglycemia on clinical and cost-related issues in patients with type 1 and type 2 diabetes. Diabetes Educ. 2014;40:269–79.
Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health J Int Soc Pharmacoecon Outcomes Res. 2011;14:665–71.
Pedersen-Bjergaard U, Pramming S, Thorsteinsson B. Recall of severe hypoglycaemia and self-estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev. 2003;19:232–40.
Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20:479–86.
Høi-Hansen T, Pedersen-Bjergaard U, Thorsteinsson B. Classification of hypoglycemia awareness in people with type 1 diabetes in clinical practice. J Diabetes Complic. 2010;24:392–7.
Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697–703.
Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–22.
Zhu H, Lakkis H. Sample size calculation for comparing two negative binomial rates. Stat Med. 2014;33:376–87.
Landstedt-Hallin L. Changes in HbA1c, insulin dose and incidence of hypoglycemia in patients with type 1 diabetes after switching to insulin degludec in an outpatient setting: an observational study. Curr Med Res Opin. 2015;31:1487–93.
Ratzki-Leewing A, Harris S, Ryan B, Zou G. Real-world estimates of severe hypoglycaemia and associated healthcare utilisation in the US: baseline results of the iNPHORM study. EASD. 2020; (Abstract 750).
Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10:617–21.
Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14:646–55.
Lawton J, Rankin D, Elliott J, et al. Experiences, views, and support needs of family members of people with hypoglycemia unawareness: interview study. Diabetes Care. 2014;37:109–15.
Renard E, Ikegami H, Daher Vianna AG, et al. The SAGE study: Global observational analysis of glycaemic control, hypoglycaemia and diabetes management in T1DM. Diabetes Metab Res Rev. 2021;37: e3430.
Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673–9.
Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ. 2009;35:1014–22.
Dailey G, Aurand L, Stewart J, Ameer B, Zhou R. Comparison of three algorithms for initiation and titration of insulin glargine in insulin-naive patients with type 2 diabetes mellitus. J Diabetes. 2014;6:176–83.
Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–34.
Gabriely I, Shamoon H. Hypoglycemia in diabetes: common, often unrecognized. Cleve Clin J Med. 2004;71:335–42.
Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19:1155–64.
Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12.
Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE (2005) Psychosocial problems and barriers to improved diabetes management: results of the cross-national diabetes attitudes, wishes and needs (DAWN) study. Diabet Med. 2005;22:1379–1385.
Centers for Disease Control and Prevention. Overweight and obesity: data and statistics. Atlanta (GA): Centers for Disease Control and Prevention; 2020.
Kozyrskyj A, Raymond C, Racher A. Characterizing early prescribers of newly marketed drugs in Canada: a population-based study. Eur J Clin Pharmacol. 2007;63:597–604.
Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. 2014;14:469.
HEDIS measurement year 2023 volume 2: technical specifications for health plans. National Committee for Quality Assurance. 2022.
Elliott L, Fidler C. Hypoglycemia event rates: a comparison between real-world data and randomized controlled trial populations in insulin-treated diabetes. Diabet Ther. 2016;7:45–60
Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–32.
Zhou FL, Ye F, Gupta V, et al. 986-P: older adults with type 2 diabetes (T2D) experience less hypoglycemia when switching to insulin glargine 300 U/mL (Gla- 300) vs other basal insulins (DELIVER 3 Study). Poster Am. Diabetes Assoc. Conf. 2017.
Sarkar U, Karter AJ, Liu JY, Moffet HH, Adler NE, Schillinger D. Hypoglycemia is more common among type 2 diabetes patients with limited health literacy: the Diabetes Study of Northern California (DISTANCE). J Gen Intern Med. 2010;25:962–8.
Sherrill CH, Lee S. Prevalence, characteristics, and health-related quality of life of continuous glucose monitoring use according to the Behavioral Risk Factor Surveillance System 2014–2020. J Manag Care Spec Pharm. 2023;29:541–9.
Acknowledgements
We thank Dr. Amy Metcalfe and Dr. Darren Brenner for their methodologic guidance on this work.
Funding
The iNPHORM study and related publishing fees, including the rapid service fee, were funded through an investigator-initiated grant from Sanofi Global. Neither Sanofi Global nor Sanofi Canada was involved in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the paper for publication. All authors confirm their independence from funders and that they had full access to the study data (including statistical reports and tables). They take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions
Jason E. Black, Stewart B. Harris, and Alexandria Ratzki-Leewing devised the research hypothesis. Jason E. Black, Guangyong Zou, and Alexandria Ratzki-Leewing developed the statistical analysis plan. Jason E. Black conducted all data analysis and drafted the manuscript. All authors critically reviewed, edited, and approved the manuscript.
Disclosures
Jason E. Black, Bridget L. Ryan, and Guangyong Zou have nothing to disclosure. Stewart B. Harris: Sanofi: grant, member advisory board, consultant; Eli Lilly: grant, member advisory board, consultant, clinical studies; Novo Nordisk: grant, member advisory board, consultant, clinical studies; Janssen: grant, member advisory board, consultant; AstraZeneca: grant, member advisory board, consultant, clinical studies; Abbott: grant, member advisory board, consultant; Boehringer Ingelheim: grant, member advisory board, consultant, clinical studies; JDRF: grant; Lawson: grant; Canadian Institutes of Health and Research: grants. Alexandria Ratzki-Leewing: Sanofi: grant; Eli Lilly: consultant; fees paid for presentations; Novo Nordisk: consultant.
Compliance with Ethical Guidelines
Before recruitment, we obtained ethics approval from the Western University Health Sciences Research Ethics Board (Project ID: 112,986; December 17, 2019). The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. Consent to participate was obtained at screening and could be revoked by not completing future questionnaires. Consent to publication was obtained at screening.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due limitations in data sharing of participant information.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Black, J.E., Harris, S.B., Ryan, B.L. et al. Real-World Effects of Second-Generation Versus Earlier Intermediate/Basal Insulin Analogues on Rates of Hypoglycemia in Adults with Type 1 and 2 Diabetes (iNPHORM, US). Diabetes Ther 14, 1299–1317 (2023). https://doi.org/10.1007/s13300-023-01423-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01423-3