Abstract
Lemmings are a key component of tundra food webs and changes in their dynamics can affect the whole ecosystem. We present a comprehensive overview of lemming monitoring and research activities, and assess recent trends in lemming abundance across the circumpolar Arctic. Since 2000, lemmings have been monitored at 49 sites of which 38 are still active. The sites were not evenly distributed with notably Russia and high Arctic Canada underrepresented. Abundance was monitored at all sites, but methods and levels of precision varied greatly. Other important attributes such as health, genetic diversity and potential drivers of population change, were often not monitored. There was no evidence that lemming populations were decreasing in general, although a negative trend was detected for low arctic populations sympatric with voles. To keep the pace of arctic change, we recommend maintaining long-term programmes while harmonizing methods, improving spatial coverage and integrating an ecosystem perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lemmings are key herbivores in arctic tundra ecosystems where they play a major role both for the flow of energy from plants to avian and mammalian predators and the dynamics of the vertebrate food web (Gilg et al. 2003; Legagneux et al. 2012). Lemmings can consume more plant material than large herbivores (Batzli et al. 1980) and as prey, they constitute the main resource for many arctic predators (Krebs 2011; Schmidt et al. 2012). Lemmings are also well known for their population cycles with large periodic outbreaks (Stenseth and Ims 1993). These cycles create boom and bust dynamics, which influence the whole vertebrate tundra food web (Ims and Fuglei 2005). The fluctuations of furbearers such as arctic foxes resulting from these resource pulses have been known by hunters and trappers for centuries and eventually lead to the discovery of their persistent regularity—the 3–5-year lemming cycle (Elton 1942). In addition to plants and predators, which interact with lemmings directly, many ground nesting birds, such as geese and waders, are indirectly affected by the lemming cycles as alternative prey for predators (Bêty et al. 2002).
Arctic lemmings belong to two genera, collared lemmings (Dicrostonyx ssp.) and brown lemmings (Lemmus ssp.), which are represented by six and four geographic species in the Arctic, respectively (CAFF 2013). The two genera of lemmings appear to have co-evolved with the tundra biome since the beginning of the Pleistocene (Oksanen et al. 2008). In this cold environment with long winters, they have developed convergent adaptations to life under the snow, including growing large claws for digging (Dicrostonyx, and to a certain extent Norwegian lemmings) and developing robust teeth, strong jaws and large guts enabling them to survive on coarse food plants of low nutritive value. This last adaptation to harsh arctic conditions has been hypothesized to be at the cost of agility to escape predators, making lemmings particularly vulnerable to predation (Oksanen et al. 2008). In the high Arctic, lemmings are the only naturally occurring small rodent species. Dicrostonyx and Lemmus often occur together, but there are never more than one species per genus at a given locality. In the low Arctic and in mountain tundra areas further south (Oroarctic, Virtanen et al. 2016; Fig. 1), they usually occur in sympatry with voles (genus Microtus and/or Myodes), but persist primarily at higher altitudes, where vole densities are low (Ekerholm et al. 2001). The population dynamics of sympatric small rodent species are often synchronous, and both direct and indirect interactions mediated by shared predators have been hypothesized to occur between species (Oksanen 1993; Hanski and Henttonen 1996).
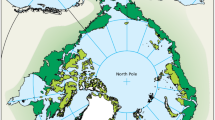
Maps showing the locations of lemming monitoring sites in (A) Circumpolar region and (B) Fennoscandia (delimited by the large grey square in panel A). Different shadings of green indicate the delimitations of bioclimatic subzones. The high Arctic, low Arctic and Subarctic are drawn according to CAFF (2013) and the Oroarctic, which is customarily pooled with the arctic tundra in global biome overviews and represents potential habitat for lemmings south of the Arctic proper, according to Virtanen et al. (2016). Numbers refer to the sites as listed in Table 1 and symbols indicate small rodent community composition. Time series of annual small rodent abundance estimates are presented for selected sites (graphs for the remaining sites are given in Fig S1). On the time series graphs, triangles represent lemmings (upwards and black for Lemmus, and downwards and white for Dicrostonyx), circles represent data not identified to species such as winter nests, qualitative indices or incidental observations, whereas grey squares represent voles (all species pooled). The colour of the y-axis indicates the data type: black refers to individuals caught per 100 trap-nights (6, 8, 41 and all Fennoscandia except 39), light blue to density in individuals per ha (13, 16, 17), green to winter nest density in nests per ha (11), orange to incidental observations (numbers seen per observer-day [1], or observer-hour [15]) and purple to qualitative indices (39, 44, 48, 49). Smoothed trend lines for the total abundance of lemmings are shown in light blue. 35H and 35 L refer to the highland and lowland series of Joatka (Table S3)
Extent of time during which lemmings have been monitored in the Arctic. Only sites which have still been collecting data after 2000 were included in this study. Sites are ordered according to site numbers listed in Table 1 and mapped in Fig. 1. Colours refer to geographic regions: brown—North America west of the Mackenzie River; red—mainland North America east of the Mackenzie River; orange—Canadian Arctic Archipelago; green—Greenland; light blue—Fennoscandia including the Kola Peninsula; blue—Russia east of the White Sea. Thick lines indicate sites from which data were included in the status and trends analysis, whereas thin lines refer to sites from which raw data were not contributed. The dotted vertical line shows 1993 and highlights the start of the last 25 years, which is the period for which trends were calculated
As small rodents specialized for life under the snow, lemmings are able to reproduce in winter (Dunaeva 1948; Millar 2001). In high arctic areas where summer predation is intense, the main population growth occurs in winter and density declines over the summer (Gilg 2002; Fauteux et al. 2015). Because winter reproduction appears to be a prerequisite for lemmings to reach peak densities, they are likely to be more sensitive to changing winter climate than northern voles (Ims et al. 2011). Unstable autumn and winter weather with warm spells and rain, leading to icing at the bottom of the snow pack, may prevent lemmings from moving in the subnivean space and thus limit their access to food plants (Berteaux et al. 2016). In recent decades, a fading out of lemming outbreaks associated with lower abundances has been reported from several regions, notably from high arctic Greenland (Gilg et al. 2009) and southern Fennoscandia (Kausrud et al. 2008). These changes in dynamics have been attributed to changes in winter climate. In eastern Greenland the collapse of the lemming cycles has had dramatic consequences for specialist predators such as snowy owls (Schmidt et al. 2012). Lemmings are therefore not only a key functional component of the tundra ecosystem, but also a key monitoring target for changes in the arctic tundra biome (Christensen et al. 2013). Detecting and understanding the multifaceted changes arctic ecosystems are experiencing in relation to global change requires well-designed ecosystem-based monitoring systems (CAFF 2013; Ims and Yoccoz 2017). Lemmings and other arctic small rodents have been identified as an important Focal Ecosystem Component in the Circumpolar Biodiversity Monitoring Program (CBMP), and a number of core attributes for their monitoring have been defined (Christensen et al. 2013).
Here we present a comprehensive overview of recent lemming monitoring and research activities in the Arctic, including oroarctic areas (Fig. 1). We assess spatial and temporal coverage of current monitoring efforts and the extent to which they address the core attributes for small mammals defined by the CBMP. We discuss the consequences of having gaps in monitoring effort and data availability, and identify ways (and limitations) to harmonize/standardize monitoring protocols. The second aim of this study is to collate the data from these monitoring initiatives in an attempt to assess the status and recent trends of lemming populations across the circumpolar Arctic. We assess geographic patterns in multiannual density fluctuations and examine temporal trends in lemming abundance. In particular we assess whether there is empirical evidence for a circumpolar decrease in lemming populations, which could be hypothesized to result from climate change. We also provide an overview of small rodent community composition.
MATERIALS AND METHODS
Inventory of lemming monitoring initiatives
For our overview of lemming monitoring, we collected information about recent small rodent monitoring and research activities throughout the Arctic, and south of the Arctic where lemmings are important. Temporally, we included only the programmes that have been collecting data after 2000 (Table S1). Contacts were made with relevant scientists from the Small Mammal Expert network of CAFF (https://www.caff.is/terrestrial/terrestrial-expert-networks/small-mammals). Scientists were asked to provide information about their monitoring/research activities and protocols used, which Focal Ecosystem Component attributes were addressed (abundance, health and phenology are regarded as essential, while demographics, spatial structure and genetic diversity are recommended; Christensen et al. 2013), and what other ecosystem components were monitored (Table S2). All scientists were asked to forward the request to other people having relevant data. For Russia, the rodent abundance overview of the International Breeding Conditions Survey on Arctic Birds (http://www.arcticbirds.net/) was also used to identify ongoing projects.
Assessment of status and trends
All data custodians were asked to share their data on small rodent abundances or indices to contribute to this circumpolar assessment. As many of the time series were shorter than required for rigorous statistical analyses (spectral or autoregressive analysis), we adopted a graphical approach: Annual time series for each lemming species at each site were plotted to assess population dynamic patterns (variability and cyclicity). To include as many localities as possible, we plotted and analysed data for all sites with at least 5 years of data. When the data consisted of a spring and a fall trapping session each year (Table S1), an average yearly abundance index was calculated, assuming that an average between a spring and a fall session is most comparable to data from a session in the middle of the summer as carried out in many high arctic sites. Where present, sympatric voles were also displayed on the plots.
The amplitude of multiannual density fluctuations was quantified by the standard deviation of the log-10 transformed annual estimates (s-index; Henttonen et al. 1985). Values of 0 were replaced with half of the smallest value which could have occurred in the series (i.e. half of one animal trapped or half of one nest found; Gruyer et al. 2008). In some sites, notably in Fennoscandia, lemmings appeared sporadically and were only registered in some years. Therefore, we also quantified the irregularity of lemming presence in each series as the overall proportion of years where trapping was carried out but no lemmings were caught.
For sites where both lemmings and voles occurred, average community composition was characterized by calculating the mean of the annual proportions of lemmings (both species together where two species were present) across all years. Similarly, the mean of the annual proportions of each species of lemmings was calculated relative to the total abundance of lemmings for sites where two species of lemmings occurred.
Trends of lemming abundance were assessed as the slope of a linear regression of abundance estimates against time. Abundance estimates were scaled to a mean of 0 and a standard deviation of 1. For the trend analysis, we used only time series with observations over at least 10 years. Moreover, we focused on data from the last 25 years (1993–2017) to compare trends over a specific time period. We tested for differences in the estimated trends (i.e. slope coefficients) among bioclimatic zones, species, geographical regions and sites with different community composition. For this analysis, the slopes were used as response variable in linear models with the respective categories as explanatory factors. To account for different lengths of the time series, length was included as a weighting parameter. Results for the different categories are presented as predicted means with 95% confidence intervals. For sites where lemmings co-occurred with voles, we also estimated linear trends in the annual proportion of lemmings (both Lemmus and Dicrostonyx if two lemming species were present) in the total abundance of rodents, and, where two lemming species were present, in the annual proportion of Dicrostonyx relative to total lemming abundance. All statistical analyses were carried out in R version 3.5.0 (R Development Core Team 2018).
Results
Inventory of monitoring projects
We obtained information from 49 sites, where lemmings were monitored after the year 2000 (Fig. 1, Table S1). The sites belong to all geographic regions of the Arctic: 15 in North America, three in Greenland, 21 in Fennoscandia including the Kola Peninsula (hereafter referred to as Fennoscandia) and ten in Russia east of the White Sea (hereafter referred to as Russia). Fennoscandia is thus clearly overrepresented relative to the geographical area it covers, whereas Russia is underrepresented. Considering only ongoing monitoring initiatives (78%), the geographic skew is even stronger with six ongoing programmes in Russia, 18 in Fennoscandia, three in Greenland and 12 in North America. The biased geographical coverage resulted also in a biased species coverage with an over-representation of the Norwegian lemming. Regarding bioclimatic zones (Fig. 1), 12 sites are located in the high Arctic, 16 sites in the low Arctic and 21 sites in the Oroarctic.
Temporally, the monitoring activities extended over periods ranging between 3 and 87 years (Fig. 2, Table S1). In eight sites, observations were lacking for some years. A majority of the ongoing monitoring activities started in the beginning of the 1990s or later, and only one programme in each of North America and Greenland, five in Fennoscandia and three in Russia began prior to 1990. There were, however, other monitoring programmes taking place in the 20th century, which are not included here as they stopped before 2000 (Kokorev and Kuksov 2002; Krebs et al. 2002; Pitelka and Batzli 2007).
Among the essential monitoring attributes of the CBMP, annual lemming abundance (mostly indices) were recorded at all sites (Fig. 3). The methodologies varied between sites and include snap-trapping, live-trapping, winter nest counts, systematically recorded incidental observations and qualitative indices (Fig. 4). Snap-trapping was the most used method overall, and live-trapping was mostly used in North America. Qualitative indices based on a general impression of lemming abundance in the field were used at least partly in half of the Russian sites. Among these methods, only live-trapping allows the estimation of true densities and all other methods provide relative abundance indices. At many sites (69%) several monitoring methods were used (Table S2).
Number of sites where the core attributes defined by CBMP for small mammals were monitored according to the frequency of data collection: data collected during “All”, “Most” or “Some” years, or “Never” (compiled from Table S2)
Methods used to assess lemming abundance. For each method the number of sites where it was used is plotted according to the frequency of data collection: data collected during “All”, “Most” or “Some” years, or “Never” (compiled from Table S2). Qualitative indices are integer index values based on a general impression of lemming abundance in the field, sometimes explicitly related to the number of lemmings seen per day
The two other essential attributes of the CBMP plan received limited attention. Health parameters (diseases, parasites) were only monitored systematically at a few sites. Phenology was regularly monitored only on Wrangel Island, where dates of first appearances on the snow, migration to summer habitat and observations of first juveniles were recorded (Fig. 3, Table S2). Of the recommended attributes, about half of the sites monitored some aspects of lemming demography and spatial structure regularly. Sex, age classes and sometimes reproductive status of trapped animals were recorded, but mortality was rarely determined. As trapping was often carried out in a design of replicated plots in different habitats, the data also provided some information about spatial structure (habitat use). Similar information can be obtained from winter nest counts. Genetic diversity was surveyed in some sites, but often only once (Fig. 3, Table S2). In addition to these attributes, a few programmes examined lemming diets (Table S2). Regarding other ecosystem parameters, the abundance and reproduction of lemming predators, as well as the availability of alternative prey for the predators was monitored at about half of the sites, but plant productivity and phenology received less attention (but see Björkman et al. 2019). Finally, less than half of the sites monitored abiotic conditions annually (Table S2). The size of the study area (Table S1) and the total effort used in monitoring (e.g. total number of trap-nights per session) also varied considerably between sites.
Status of lemming populations
Through our common effort and willingness to share data, we were able to assemble abundance time series for 43 sites (Fig. 1, Fig. S1), including 14 time series for Dicrostonyx, 18 for Lemmus lemmus, eight for other Lemmus species and nine for the combined abundance of Dicrostonyx and Lemmus (length ≥ 5 years). In addition, abundance indices for voles were recorded at 27 sites (63% of the sites; Table S1. Small rodent communities were composed of both voles and lemmings at all sites south of the high Arctic, except Walker Bay (Fig. 1). In Fennoscandia, voles were on average more abundant than lemmings in trapping data, whereas in North America several mixed communities occurred where lemmings were more abundant than voles (Fig. 1).
The available data present a very heterogeneous picture of temporal dynamics (Fig. 1, Fig. S1). Considerable multiannual fluctuations in abundance were recorded at all sites and for most species, but patterns of fluctuations differed considerably. Norwegian lemmings exhibited typical outbreak years at intervals of three to 6 years, but sometimes much longer periods occurred without outbreaks or even without lemming records at all (Fig. 1b). Vole peaks in Fennoscandia were often synchronous with lemming peaks, but sometimes vole peaks occurred without lemmings. Outside of Fennoscandia, heterogeneity in population dynamics was also large (Fig. 1a). Rather regular cycles with a period of 3–4 years were observed at some sites such as Bylot Island, but this pattern was not the general rule. Other sites exhibited multiannual fluctuations with a period of 4 or 5 years, but patterns were much less apparent because of large differences in abundance indices between different peak years (e.g. Utqiaġvik, Aulavik).
Amplitude, as estimated by the s-index, ranged from 0.26 to 0.91 (Table S3). The s-index was below the threshold defined for high amplitude population fluctuations (0.5; cf. Henttonen et al. 1985) in 11 time series (26%) and in most of these, lemmings occurred only rarely or at very low abundances (Kilpisjärvi, Daring Lake). On average, amplitude estimates were highest for Norwegian lemmings (Fig. 5). The observation method that recorded the highest amplitudes was incidental observations. Series based on winter nests and live-trapping exhibited high variation in s-index. The proportion of years with zero lemmings trapped had a lower median for Dicrostonyx than for the other species, but there were large differences among study sites, and between trapping methods (Fig. 5). Snap-trapping data had more zero observations than any other methods.
Boxplots of s-index values (see methods) and the proportion of years with abundance estimates of 0 according to species (DicroDicrostonyx spp.; LemLemmus spp. except L. lemmus; LlemL. lemmus and tot two lemming species together) and to observation method (live live-trapping; nests winter nest counts; obs incidental observations; snap snap-trapping). Boxes represent the middle 50% of the data with the median (thick line). Whiskers extend to the extreme values
Comparing lemming abundance between sites is difficult because of the various methods used. Indeed, statistically derived density estimates based on live-trapping were available only for four high arctic sites (Fig. 1, Fig. S1). Snap-trapping indices show large differences in the number of lemmings trapped in peak years with captures of up to 15 (Joatka) and even 30 (Finse) individuals per 100 trap-nights in Fennoscandia, whereas at some other sites there were less than 1 capture per 100 trap-nights in all years (Daring Lake). In addition to differences in densities between areas and species, these differences were likely related to differences in trapping design among studies (Fauteux et al. 2018), and possibly to differences in trapability of the different species.
Trends
Linear trends for scaled lemming abundance over the last 25 years (42 series, mean length 19.1 years) varied between − 0.87 (Chaun) and 0.82 (East Bay) standard deviations per decade (Table S3). The mean trend was − 0.011 (SE = 0.008), yielding no evidence of a panarctic increasing or decreasing trend for lemming populations over the last 25 years. There were no consistent differences in trends between species or bioclimatic zones, although estimates for the low Arctic were slightly negative (Fig. 6). Concerning geographical regions, there was an indication for a negative trend in Russia. For community composition, trends were on average not different from 0 for sites where only lemmings are present and for Fennoscandian sites with Norwegian lemmings and voles, but they were significantly negative for sites where arctic lemmings and voles co-occur (Fig. 6).
Predicted mean linear slopes of lemming abundance as estimated from linear models with 95% confidence intervals for time series scaled to 0 mean and standard deviation of 1. The y-axis is in units of standard deviation per year. Linear trends were analysed with respect to bioclimatic zone (High Arctic, Low Arctic and Oroarctic), species (DicroDicrostonyx spp.; LemLemmus spp. except L. lemmus; LlemL. lemmus and tot two lemming species together), geographic region (Fen Fennoscandia; Gre Greenland; NAm North America; Rus Russia east of the White Sea) and the community composition at each site (L only one or two species of lemmings; N + V = L. lemmus and several species of voles; A + V = arctic lemmings (all species except the Norwegian lemming) and one or several species of voles; see Table S1 for voles species at each site). Numbers above the x-axis indicate the number of time series in each category
Trends in the proportion of lemmings compared to voles and in the proportion of each species among lemmings were mostly weak and not significant (Table S3). A change in species composition was, however, recorded at two low arctic sites. In Churchill, Manitoba, no voles had been trapped in the 1990’s, but when trapping was resumed in 2010, meadow voles (Microtus pennsylvanicus) were caught. Similarly, M. middendorffii voles were observed in South-eastern Taimyr in 2013 and 2014 for the first time despite years of monitoring earlier (Golovnyuk 2017).
Discussion
Monitoring
The present inventory revealed a considerable effort to investigate and monitor lemming populations in the circumpolar Arctic. Monitoring/research initiatives are ongoing at 38 sites covering most regions of the Arctic, and in many sites data are available for 20 years or more. While in some programmes lemmings are the focus of specific research questions (Finse, Walker Bay), at other sites small rodent abundance is mostly surveyed to explain variation in other ecosystem components, such as breeding birds (south-eastern Taimyr, Karrak Lake), specialist predators (Børgefjell II, Helags) or vegetation dynamics (Joatka, Abisko). The objectives likely influence the choice of methods, temporal and spatial sampling design and resources allocated to this task. This fact, together with the inherent challenges of long-term fieldwork in remote arctic locations, may explain why methods used to monitor lemming abundance vary so much. Despite a substantial overall effort, biases in geographic distribution of sites and several short time series, some of them with gaps, limit the reliability of a general assessment of status and long-term trends of lemming populations in the Arctic.
The geographical distribution of monitoring sites is far from uniform (Fig. 1). Numerous sites in Scandinavia, where access is relatively easy by arctic standards and researcher density is high, lead to a good coverage for the Norwegian lemming, but less so for other more widespread species. In the Russian Arctic, there are only four ongoing monitoring initiatives with more than 5 years of data. The lack of data from Russia not only regarding lemmings, but for many important ecosystem components, has been highlighted in previous assessments (CAFF 2013) and hampers a circumpolar understanding of changes in the tundra ecosystems. Monitoring in the Canadian Arctic Archipelago is also sparse, mostly due to remoteness. Another gap in spatial coverage is south of the Arctic proper outside of Fennoscandia (Fig. 1), despite distributions of several lemming species extending into these areas (Stenseth and Ims 1993). This limits our ability to detect possible range shifts (Marcot et al. 2015).
Many of the longer series go back to the beginning of the 1990s, which represents 25 years or more of fieldwork, a substantial effort. However, this is also a period during which mean temperature in the Arctic increased consistently (Overland et al. 2015). Thus, the data do not allow a direct comparison to the period before recent arctic warming occurred. In this paper we focussed on ongoing monitoring and excluded data from older research programmes completed before 2000. Assessments of changes in lemming dynamics covering longer periods have, however, been published for specific regions and suggest a substantial amount of non-stationary dynamics (Angerbjörn et al. 2001; Aharon-Rotman et al. 2015).
Seasonally, lemming monitoring is usually carried out during one or two periods in summer. Analysing trends for specific seasons (spring or fall) may reveal changes important for trophic interactions (Ims and Fuglei 2005), but could not be done here because many data series consisted of one estimate per year. There are virtually no data on lemming abundance or activity during the long winter period, apart from counts of winter nests after snowmelt, although winter is likely to be critical to understand population dynamics (Krebs 2011; Domine et al. 2018) and the impact of climate change (Kausrud et al. 2008) in animals specialized for life under the snow. This knowledge gap has been stressed for a while (Stenseth and Ims 1993), but the challenges of studying lemmings under the snow in remote arctic locations are difficult to overcome. However, new technology is about to open up new possibilities through the development of camera tunnels for monitoring lemmings year-round, providing information about presence and timing of reproduction (Soininen et al. 2015).
Population density is a crucial parameter in ecology, but it is often difficult to estimate reliably. Most methods used to monitor lemmings provide abundance indices. Real density estimates are obtained only from live-trapping and subsequent mark-recapture analyses, which is a labour-intensive method usually carried out with a rather limited spatial extent. Several sites in North America moved from snap-trapping to live-trapping during the monitoring period (e.g. Bylot), improving data quality and providing additional information on vital rates such as survival. A recent study assessing how well different abundance indices are correlated with true density estimates concluded that systematic incidental observations and snap-trapping provided equally good proxies for lemmings in the high Arctic (Fauteux et al. 2018). Incidental observations are easy to implement, but they usually do not distinguish between species. Moreover, it is unclear whether they would work as well in the low Arctic where lush vegetation may hamper detection. Snap-trapping is the most commonly used method, but the sampling design used in Fennoscandia often does not target lemming habitat specifically, which results in series with many zero values and subsequent analytical challenges (Fig. 1).
The variety of methods used is a challenge when inferring large scale patterns (Berteaux et al. 2017). While all quantitative methods allow comparisons of trends and relative dynamic patterns, it can be difficult to compare abundance among sites, which may be the most important parameter to predict for instance trophic interactions (Henden et al. 2010). Moreover, the large heterogeneity in dynamics seen on Figs. 1 and S1 may be partly due differences in methods. For instance, incidental observation and winter nests series have considerably less 0-values than snap-trapping series, and qualitative index series tend to have more regular cycles than quantitative series. For long qualitative time series, there can also be an effect of shifting base-line when assessing abundance, making it difficult to infer long-term trends. Such considerations argue in favour of the standardization of monitoring methods across sites. However, adoption of new protocols in a long-term programme is challenging. Old and new protocols should be run simultaneously for a number of years to establish correction factors between time series. This may be difficult to implement logistically, but can result in well-assembled long time series, such as in Bylot or Karupelv (Gilg et al. 2006; Gauthier et al. 2013).
Parasites and diseases of lemmings, two indicators of health, have been studied at a few sites (Table S2), but are usually not included in regular monitoring protocols. This may be due to the tendency to study parasites separately from food web oriented ecosystem research (Lafferty et al. 2008), the latter of which most lemming monitoring programmes belong to. Genetic diversity is also usually addressed in snapshot studies looking at population structure or phylogeography (Ehrich et al. 2001), but is not investigated regularly. Tissue samples collected either occasionally or systematically from trapped animals could, however, be used for retrospective analyses of genetic structure or diseases/parasites. Phenology, in particular the timing of winter reproduction, is an important parameter to understand how changes in winter climate may be affecting lemming population dynamics. Unfortunately, present monitoring methods such as discrete trapping sessions or winter nest counts do not provide this information.
An ecosystem-based approach to monitoring, structured around explicit models for interactions between ecosystem components and drivers of change, is recommended by the CBMP (Christensen et al. 2013), and applied in some of the initiatives providing lemming data (e.g. Varanger, Zackenberg; Ims and Yoccoz 2017; Schmidt et al. 2017). Analysing trends of change as functions of ecological drivers is only possible if likely drivers of change are also measured at a relevant scale. To address how abiotic and biotic drivers influence lemming population dynamics and other lemming attributes, monitoring/research projects should take an ecosystem-based approach and collect data about a selection of other important state variables (Ims and Yoccoz 2017). Addressing the hypothesized importance of snow conditions for lemmings requires for instance some local data about snow such as snow physical properties, snow cover duration or depth (Domine et al. 2018). Remote sensing data and snow modelling can also provide useful information.
Status and trends
The large heterogeneity in patterns of lemming dynamics observed here is in accordance with previous work. The amplitude of lemming fluctuations often varies (CAFF 2013) and dynamics in some areas can look more like irregular outbreaks than typical population cycles (Ims et al. 2011). The longer series, at least for Norwegian lemmings, suggest non-stationary dynamics characterized by periods with regular cycles followed by periods with persistent low density over a decade or more (Finse, Joatka and Laplandsky, Fig. 1). Similar patterns have been documented over the last century based on diverse sources of evidence (Angerbjörn et al. 2001). This natural variability in lemming dynamics makes population trends inherently difficult to detect, in particular considering the modest length of most time series (McCain et al. 2016). This implies that our result of no overall trend in lemming populations across the circumpolar region needs to be considered cautiously. The exception are abrupt changes in dynamics such as in NE Greenland (Karupelv and Zackenberg; Gilg et al. 2009) or at Finse, where the disappearance of cycles in the late 1990s may be attributed to changes snow conditions (Kausrud et al. 2008). At Finse, however, a new outbreak occurred in 2014 (Fig. 1), indicating that also the more recent changes may be transient.
Regionally, our results indicate that lemming populations co-occurring with one or several species of voles outside Fennoscandia may be declining. This finding is in agreement with a predicted decline of specialized arctic species at the southern edge of their distribution (Loarie et al. 2009). At the same time, voles have recently appeared in two sites (Churchill and South-eastern Taimyr) indicating a northward advance of these boreal species. It has been hypothesized that Norwegian lemmings at low altitudes may be exposed to apparent competition from voles mediated by shared predators (Oksanen 1993). It is possible that a similar mechanism, together with an increase of generalist predators from adjacent boreal areas (Reid et al. 1995), contributed to the observed declines in lemming populations at these sites. In addition, climate change may lead to more frequent melt and freeze events in winter, which have been hypothesized to be detrimental to the subniveal life of lemmings (Kausrud et al. 2008; Ims et al. 2011; Berteaux et al. 2016). A climate-driven increased growth of meadows and tall shrubs (Myers-Smith et al. 2011) at the detriment of more typical moss-graminoid and dwarf shrub tundra, which is the preferred habitat of lemmings, may also contribute to vole expansion and lemming population decline.
The primarily oroarctic populations of the Norwegian lemming did not show a similar negative trend, although they always occur together with several species of voles. This could be because they live in mountainous areas, where they have the possibility to exploit altitudinal gradients in winter temperature, snow conditions and productivity (Oksanen 1993; Ims et al. 2011). Moreover, several of the monitored populations were characterized by a large proportion of years without lemming captures, which may indicate that they are not in optimal lemming habitats, but in areas which lemmings colonize only in some peak years. Their permanent habitats might be at higher altitudes (Kalela et al. 1971), where harsh winters provide good snow cover and scarce vegetation prevents voles from establishing.
Conclusions and recommendations
Overall, the available time series for lemmings in the Arctic did not show any consistent declining trend. Hence, although low precision of the data need to be kept in mind, our results do not support the contention that climate change has negatively affected lemmings at a global scale so far. However, given the large geographical variability in climate, snow physical properties and community composition across the circumpolar Arctic, this may not be surprising. Nonetheless, when the data were split according to different bioclimatic and community contexts, negative trends were detected in low arctic populations co-occurring with one or several species of voles. Voles also appeared for the first time in some of these areas during our study period, possibly connected to climate change in accordance with predictions of a northward displacement of arctic specialist species.
Considering the large environmental changes that will affect the Arctic in the near future and the critical importance of lemmings in the tundra food web, it is very important to continue and improve monitoring of this group. Based on the present review, we recommend harmonizing as far as possible the collection of abundance data across sites using well described quantitative methods and explicit spatial designs to obtain reliable estimates allowing comparison of patterns among sites. While the continuation of existing time series is a first priority, improving the spatial coverage of monitoring in underrepresented areas such as Russia and the Canadian Arctic Archipelago would be highly desirable. Considering that standardized incidental observations provide a comparable measure of abundance and can be easily implemented, this simple non-invasive method is recommended for sites with little resources, at least in areas where only lemmings are present. To monitor species-rich low arctic communities, live and snap-trapping should be used when possible. The development of new non-invasive methods such as camera tunnels, which have the potential to provide year-round information, should also be continued. In addition to abundance, the collection of data on potential drivers of change and on other attributes recommended by CBMP such as parasites/diseases and phenology should be improved.
Change history
11 October 2019
In the original published article, some of the symbols in figure 1A were modified incorrectly during the typesetting and publication process. The correct version of the figure is provided in this correction
References
Aharon-Rotman, Y., M. Soloviev, C. Minton, P. Tomkovich, C. Hassell, and M. Klaassen. 2015. Loss of periodicity in breeding success of waders links to changes in lemming cycles in Arctic ecosystems. Oikos 124: 861–870.
Angerbjörn, A., M. Tannerfeldt, and H. Lundberg. 2001. Geographical and temporal patterns of lemming population dynamics in Fennoscandia. Ecography 24: 298–308.
Batzli, G.O., R.G. White, S.F. MacLean, F.A. Pitelka, and B.D. Collier. 1980. The herbivore-based trophic system. In An Arctic Ecosystem: the Coastal Tundra at Barrow, Alaska, ed. J. Brown, P.C. Miller, L.L. Tieszen, and F.L. Bunnell. Stroudsburg, PA: Dowden, Hutchinson & Ross, Inc.
Berteaux, D., G. Gauthier, F. Domine, R.A. Ims, S.F. Lamoureux, E. Lévesque, and N. Yoccoz. 2016. Effects of changing permafrost and snow conditions on tundra wildlife: Critical places and times. Arctic Science 3: 65–90.
Berteaux, D., A.-M. Thierry, R. Alisauskas, A. Angerbjörn, E. Buchel, L. Doronina, D. Ehrich, N.E. Eide, et al. 2017. Harmonizing circumpolar monitoring of Arctic fox: Benefits, opportunities, challenges and recommendations. Polar Research 36: 2.
Bêty, J., G. Gauthier, E. Korpimäki, and J.F. Giroux. 2002. Shared predators and indirect trophic interactions: Lemming cycles and arctic-nesting geese. Journal of Animal Ecology 71: 88–98.
Björkman, A.D., M.G. Criado, I.H. Myers-Smith, V. Ravolainen, I.S., Jónsdóttir, K.B. Westergaard, J.P. Lawler, M. Aronsson, et al. 2019. Status and trends in Arctic vegetation: Evidence from experimental warming and long-term monitoring. In Terrestrial biodiversity in a rapidly changing arctic: Status and trends of monitoring and key species in Arctic terrestrial ecosystems, eds. N.M. Schmidt, and H. Jóhannesdóttir, Ambio vol. 49, Special Issue. https://doi.org/10.1007/s13280-019-01161-6.
CAFF. 2013. Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity. Akureyri: Conservation of Arctic Flora and Fauna.
Christensen, T.R., J. Payne, M. Doyle, G. Ibarguchi, J. Taylor, N.M. Schmidt, M. Gill, M. Svoboda, et al. 2013. The Arctic Terrestrial Biodiversity Monitoring Plan. Akureyri, Iceland: CAFF International Secretariat.
Domine, F., G. Gauthier, V. Vionnet, D. Fauteux, M. Dumont, and M. Barrere. 2018. Snow physical properties may be a significant determinant of lemming population dynamics in the high Arctic. Arctic Science 4: 813–826.
Dunaeva, T.N. 1948. Comparative ecology of the tundra voles of Yamal.
Ehrich, D., P.E. Jorde, C.J. Krebs, A.J. Kenney, J.E. Stacy, and N.C. Stenseth. 2001. Spatial structure of lemming populations (Dicrostonyx groenlandicus) fluctuating in density. Molecular Ecology 10: 481–495.
Ekerholm, P., L. Oksanen, and T. Oksanen. 2001. Long-term dynamics of voles and lemmings at the timberline and above the willow limit as a test of hypotheses on trophic interactions. Ecography 24: 555–568.
Elton, C.S. 1942. Voles, Mice and Lemmings: Problems in Population Dynamics. Oxford, UK: Clarendon Press.
Fauteux, D., D. Gauthier, M.J. Mazerolle, N. Coallier, J. Bêty, and D. Berteaux. 2018. Evaluation of invasive and non-invasive methods to monitor rodent abundance in the Arctic. Ecosphere 9: e02124.
Fauteux, D., G. Gauthier, and D. Berteaux. 2015. Seasonal demography of a cyclic lemming population in the Canadian Arctic. Journal of Animal Ecology 84: 1412–1422.
Gauthier, G., J. Bêty, M.C. Cadieux, P. Legagneux, M. Doiron, C. Chevallier, S. Lai, A. Tarroux, et al. 2013. Long-term monitoring at multiple trophic levels suggests heterogeneity in responses to climate change in the Canadian Arctic tundra. Philosophical Transactions of the Royal Society B-Biological Sciences 368: 20120482.
Gilg, O. 2002. The summer decline of the collared lemming, Dicrostonyx groenlandicus, in high arctic Greenland. Oikos 99: 499–510.
Gilg, O., I. Hanski, and B. Sittler. 2003. Cyclic dynamics in a simple vertebrate predator-prey community. Science 302: 866–868.
Gilg, O., B. Sittler, and I. Hanski. 2009. Climate change and cyclic predator-prey population dynamics in the high Arctic. Global Change Biology 15: 2634–2652.
Gilg, O., B. Sittler, B. Sabard, A. Hurstel, R. Sane, P. Delattre, and L. Hanski. 2006. Functional and numerical responses of four lemming predators in high arctic Greenland. Oikos 113: 193–216.
Golovnyuk, V.V. 2017. Records of some species of vertebrates at the edge of their distribution area in the lower reaches of the River Khatanga (south-eastern Taimyr). Fauna Urala i Sibiri 2: 48–61.
Gruyer, N., G. Gauthier, and D. Berteaux. 2008. Cyclic dynamics of sympatric lemming populations on Bylot Island, Nunavut, Canada. Canadian Journal of Zoology-Revue Canadienne De Zoologie 86: 910–917.
Hanski, I., and H. Henttonen. 1996. Predation on competing rodent species: A simple explanation of complex patterns. Journal of Animal Ecology 65: 220–232.
Henden, J.A., R.A. Ims, N.G. Yoccoz, P. Hellström, and A. Angerbjörn. 2010. Strength of asymmetric competition between predators in food webs ruled by fluctuating prey: The case of foxes in tundra. Oikos 119: 27–34.
Henttonen, H., A.D. McGuire, and L. Hansson. 1985. Comparisons of amplitudes and frequencies (spectral analyses) of density variations in long-term data sets of clethrionomys species. Annales Zoologici Fennici 22: 221–227.
Ims, R.A., and E. Fuglei. 2005. Trophic interaction cycles in tundra ecosystems and the impact of climate change. BioScience 55: 311–322.
Ims, R.A., and N.G. Yoccoz. 2017. Ecosystem-based monitoring in the age of rapid climate change and new technologies. Current Opinion in Environmental Sustainability 29: 170–176.
Ims, R.A., N.G. Yoccoz, and S.T. Killengreen. 2011. Determinants of lemming outbreaks. Proceedings of the National academy of Sciences of the United States of America 108: 1970–1974.
Kalela, O., L. Kilpelainen, T. Koponen, and J. Tast. 1971. Seasonal differences in habitats of the Norwegian lemming Lemmus lemmus (L.) in 1959 and 1960 at Kilpisjärvi, Finnish Lapland. Annales Academii Scientarum Fennici 178: 1–22.
Kausrud, K.L., A. Mysterud, H. Steen, J.O. Vik, E. Østbye, B. Cazelles, E. Framstad, A.M. Eikeset, et al. 2008. Linking climate change to lemming cycles. Nature 456: 93–U93.
Kokorev, Y., and V.A. Kuksov. 2002. Population dynamics of lemmings, Lemmus sibirica and Dicrostonyx torquatus, and Arctic Fox Alopex lagopus on the Taimyr peninsula, Siberia, 1960–2001. Ornis Svecica 12: 139–145.
Krebs, C.J. 2011. Of lemmings and snowshoe hares: the ecology of northern Canada. Proceedings of the Royal Society B-Biological Sciences 278: 481–489.
Krebs, C.J., A.J. Kenney, S. Gilbert, K. Danell, A. Angerbjörn, S. Erlinge, R.G. Bromley, C. Shank, et al. 2002. Synchrony in lemming and vole populations in the Canadian Arctic. Canadian Journal of Zoology-Revue Canadienne De Zoologie 80: 1323–1333.
Lafferty, K.D., D. Allesina, M. Arim, C.J. Briggs, G. De Leo, A.P. Dobson, J.A. Dunne, P.T.J. Johnson, et al. 2008. Parasites in food webs: the ultimate missing links. Ecology Letters 11: 533–546.
Legagneux, P., G. Gauthier, D. Berteaux, J. Bêty, M.C. Cadieux, F. Bilodeau, E. Bolduc, L. McKinnon, et al. 2012. Disentangling trophic relationships in a High Arctic tundra ecosystem through food web modeling. Ecology 93: 1707–1716.
Loarie, S.R., P.B. Duffy, H. Hamilton, G.P. Asner, C.B. Field, and D.D. Ackerly. 2009. The velocity of climate change. Nature 462: 1052.
Marcot, B.G., M.T. Jorgenson, J.P. Lawler, C.M. Handel, and A.R. DeGange. 2015. Projected changes in wildlife habitats in Arctic natural areas of northwest Alaska. Climatic Change 130: 145–154.
McCain, C., T. Szewczyk, and K.B. Knight. 2016. Population variability complicates the accurate detection of climate change responses. Global Change Biology 22: 2081–2093.
Millar, J.S. 2001. On reproduction in lemmings. Ecoscience 8: 145–150.
Myers-Smith, I.H., B.C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K.D. Tape, M. Macias-Fauria, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters 6: 045509.
Oksanen, T. 1993. Does predation prevent Norwegian lemmings from establishing permanent populations in lowland forests? In The Biology of Lemmings, ed. N.C. Stenseth and R.A. Ims. London, UK: Academic Press.
Oksanen, T., L. Oksanen, J. Dahlgren, and J. Ofsson. 2008. Arctic lemmings, Lemmus spp. and Dicrostonyx spp.: Integrating ecological and evolutionary perspectives. Evolutionary Ecology Research 10: 415–434.
Overland, J.E., I. Hanna, I. Hanssen-Bauer, S.-J. Kim, J.E. Walsh, M.Y. Wang, U.S. Bhatt, and R.L. Thoman. 2015. Surface Air Temperatures. Silver Spring: The National Oceanic and Atmospheric Administration.
Pitelka, F.A., and G.O. Batzli. 2007. Population cycles of lemmings near Barrow, Alaska: A historical review. Acta Theriologica 52: 323–336.
Reid, D.G., C.J. Krebs, and A. Kenney. 1995. Limitation of collared lemming population-growth at low-densities by predation mortality. Oikos 73: 387–398.
Schmidt, N.M., T.R. Christensen, and T. Roslin. 2017. A high arctic experience of uniting research and monitoring. Earth’s Future 5: 650–654.
Schmidt, N.M., R.A. Ims, T.T. Høye, O. Gilg, L.H. Hansen, J. Hansen, M. Lund, E. Fuglei, et al. 2012. Response of an arctic predator guild to collapsing lemming cycles. Proceedings of the Royal Society B-Biological Sciences 279: 4417–4422.
Soininen, E.M., I. Jensvoll, S.T. Killengreen, and R.A. Ims. 2015. Under the snow: a new camera trap opens the white box of subnivean ecology. Remote Sensing in Ecology and Conservation 1: 29–38.
Stenseth, N.C., and R.A. Ims. 1993. The Biology of Lemmings. London, UK: Academic Press.
Virtanen, R., L. Oksanen, T. Oksanen, J. Cohen, B.C. Forbes, B. Johansen, J. Kayhko, J. Olofsson, et al. 2016. Where do the treeless tundra areas of northern highlands fit in the global biome system: toward an ecologically natural subdivision of the tundra biome. Ecology and Evolution 6: 143–158.
Acknowledgements
Numerous funding agencies supported all the monitoring and research programmes included in this paper; they are listed in the appendix, and a large number of field workers were involved over the years in all sites. We thank Denver Holt for providing metadata for this study, and the Danish Environmental Protection Agency (NMS), the Norwegian Environmental Agency (DE), and the Natural Sciences and Engineering Research Council of Canada (GG) for supporting work with this review. We thank Greenland Ecosystem Monitoring programme for access to data. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Figure 1 was corrected.
Lead authors—Dorothée Ehrich, Niels M. Schmidt and Gilles Gauthier.
Dorothée Ehrich and Niels M. Schmidt share first authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ehrich, D., Schmidt, N.M., Gauthier, G. et al. Documenting lemming population change in the Arctic: Can we detect trends?. Ambio 49, 786–800 (2020). https://doi.org/10.1007/s13280-019-01198-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-019-01198-7