Abstract
The Lagoa do Peixe National Park is one of the most important wetlands in southern Brazil, serving as a contranuptial or breeding site for several species of birds, including the Chilean Flamingo. Despite being the only area where Chilean Flamingo can be seen all year round, the population dynamics of the species in the area is still poorly known. To fill this gap, we reunite populational data from three different sources: literature, fieldwork census, and citizen science data. We use Generalized Additive Models (GAMs) to detect population trends over the years and within the years. The most significant models reveal that the number of Chilean Flamingos in the park decreased from the 1970s until the early 2000s, stabilizing after 2010. During the year, the dynamic of Chilean Flamingo match the dynamics of other contranuptial colonies, with an increase in individuals during winter and early spring and a reduced number from December to May. We discuss how these trends can reflect general populational trends for the species across its distribution, but also changes in the conservation and management administration in the park. We also discuss how the demographic knowledge of Chilean Flamingos in the area can be affected by ongoing activities at Lagoa do Peixe, mainly the artificial opening of the lagoon, shrimp farming, fishing, and tourism, recommending further actions that consider the flamingos in the park and maintain a healthy relationship between human activities and bird conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coastal plain of the southernmost Brazilian state of Rio Grande do Sul is 630 km long and 100 km wide and contains many lagoons, the largest of which are the Lagoa dos Patos and Lagoa Mirim. These two huge lagoons are separated from the ocean by a sandbar, where smaller lakes and lagoons predominate (Villwock 1984). The Lagoa do Peixe on the state’s central coast is perhaps the most well-known of such smaller lagoons. This 35 km long and 2 km wide lagoon, with a mean depth of 60 cm, is one of the most critical areas for biodiversity conservation in south Brazil (Knak 1999). It connects to the sea almost annually, creating an ecotone with a high diversity of algae, crustaceans, and other invertebrates that supports many birds (Matsubara et al. 2008; Rolon et al. 2013).
The Lagoa de Peixe is inside a national park of the same name—Parque Nacional da Lagoa do Peixe (hereafter, “the park”)—, which was created in 1986 to protect nonbreeding habitats of Nearctic–Neotropical migrant birds. In 1990, the park was recognized as a Western Hemisphere Shorebird Reserve Network site of international importance and in 1993 as a Ramsar Site for its significance for wetlands conservation. More recently, in 1999, it became an outpost of the Atlantic Forest Biosphere Reserve in Rio Grande do Sul. (Knak 1999). Threatened bird species threatened protected under Brazil and Rio Grande do Sul laws in the park include Buff-breasted Sandpiper Calidris subruficollis, Dot-winged Crake Laterallus spiloterus, Black-and-white Monjita Heteroxolmis dominicanus, Cinereous Harrier Circus cinereus, Hudson’s Canastero Asthenes hudsoni, Bearded Tachuri Polystictus pectoralis, and Chestnut-bellied Seed-finch Sporophila angolensi (Rio Grande do Sul 2014; Pacheco et al. 2021; MMA 2022).
Besides being essential for migratory birds, the park is perhaps the only site in Brazil where flocks of Chilean Flamingos Phoenicopterus chilensis can be seen year-round, with the highest numbers from April to September (Belton 1985; Rezende and Leeuwenberg 1987; Delfino and Aldana-Ardila 2020). Birds in the park are supposed to be nonbreeding males from breeding colonies in northern and central Argentina (Belton 1985; Antas 1994). There, Chilean Flamingos breed during austral spring and summer, after which some move to Uruguay and southern Brazil (del Hoyo 1992; BirdLife International 2008). However, despite the park being a relevant site for Chilean Flamingos, no comprehensive study on the population number has yet been conducted. Furthermore, preliminary information from nearby communities suggests that the number of flamingos in the area has decreased in the last decades (HCD unpub. data). The park suffers from land property disputes that challenge conservation and planning strategies and is currently threatened by a downgrading of its protection status (Almudi and Kalikoski 2009). The area is also an important fishing and shrimp ground, having high economic importance for the subsistence of local communities despite the potentially high level of disturbance that these activities can cause to local fauna (Péron et al. 2013).
The Chilean Flamingo is the park’s official symbol and perhaps the most photographed natural beauty in the area. Nevertheless, its potential as a charismatic flagship species for conservation and public awareness of biodiversity in the area is underdeveloped (Delfino and Aldana-Ardila 2020). Therefore, efforts to increase ecological information about these birds are essential for proposing management and conservation strategies for the species in their most crucial area in Brazil, suggesting changes that can mitigate the effects of economic activities in the area without harming the survival of traditional communities. Accordingly, we gathered information on Chilean Flamingo numbers in the Lagoa do Peixe National Park to assess the populational variation over the years and within the years, verifying if it is possible to detect any trend that can impact management and conservation actions.
Methods
Data collection
To estimate the Chilean Flamingo population size and trend in the park, we combined three types of information: historical and scientific published data, fieldwork in the area, and citizen science data. The use of multiple data formats is known to enrich population estimations, helping to improve models that fit better in the reality of bird population dynamics (Freeman et al. 2007; Sæther et al. 2016; Barras et al. 2021; Brlík et al. 2021).
Historical and recently published data
We searched the Web of Science (Clarivate Analytics 2020), Scopus (Elsevier 2020), and Google (Google Inc. 2020) complete databases using the keywords “Phoenicopteriformes”, “Phoenicopteridae”, “Phoenicopterus chilensis”, “flamingo”, and “Chilean Flamingo” combined with “Lagoa do Peixe”, “Lagoa do Peixe National Park”, “Mostardas”, “Tavares”, and “Rio Grande do Sul” in three different languages: Portuguese, Spanish and English. Except for Google, we entered search terms in the Title, Abstract, and Keywords fields, separated by the Boolean operators “And” and “Or” to increase the efficiency of the search, meanwhile reducing redundancy and wrong results (Sayers 2008). After removing the duplicate results, we submitted the remaining documents to a filtration phase (Khan et al. 2003; Tawfik et al. 2019). We selected books, official papers, reports, articles, thesis, and dissertations containing data on the Chilean Flamingos in the Lagoa do Peixe National Park.
Fieldwork
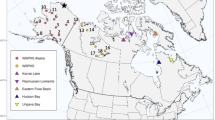
We included our fieldwork data from October 2005 to May 2006 (Fedrizzi and Carlos unpublished data) and October 2021 to May 2022 (Delfino and Carlos unpublished data). We actively searched for Chilean Flamingos flocks during both periods and estimated their numbers through line transects (Bibby et al. 2000; Raman 2002). We always conducted counts respecting 50–70 m from the flocks and walking from the beach to the posterior area of the lagoon, thus avoiding recounting and redundant samplings (Sutherland et al. 2004) (Fig. 1). We also did not consider flying birds to avoid overcounting (Bibby et al. 2000).
Map showing the Lagoa do Peixe National Park and the surrounding areas. The featured areas are the different main habitat types of that Chilean Flamingos and other waterbirds that occur within the park: the Talha-Mar and das Figueiras trail, the Barra, and the coastal beach. Map provided by Oscar Aldana-Ardila. Images by Henrique Cardoso Delfino
Citizen science data
We collected all available occurrence data of Chilean Flamingos in the park from two different citizen science platforms: The Global Biodiversity Information Facility (GBIF; https://www.gbif.org/) and eBird (https://ebird.org/home), both commonly used by birdwatchers and tourists to record their observations (Horns et al. 2018; Wijewardhana et al. 2020). We selected data only from the validated record, which underwent a review process to ensure the correct species identification, with precise geographic coordinates and the abundance of Chilean Flamingo within the region available (Bird et al. 2014; Horns et al. 2018). We did not include records that only show presence/absence for the study, despite recent studies assigning the count of one individual to these types of records (i.e., Hernández-Brito et al. 2022). We opted for this because flamingos are gregarious birds, and supposing that presence records are related to single individuals would inflate the populational number based on single individual observations, decreasing the test power (Bird et al. 2014). We gathered the abundance data and date of every observation from both platforms, combining them in a single list. To avoid redundancy, we excluded duplicated observations on different platforms.
Data analysis
We assembled the data from the three sources into a unified table containing the number of Chilean Flamingos in the Lagoa do Peixe National Park and the sampling date (Wijewardhana et al. 2020). From this initial source, we organized the data by year and month. Then, we assessed the temporal trend in the mean number of Chilean Flamingos over years and months using the Generalized Additive Models (GAM) (Fewster et al. 2000; Clarke et al. 2003). In this type of analysis, we used semi-parametric smooth functions (s) to fit the nonlinear relationship between abundance (dependent variable) and year/month (independent variable) (Fewster et al. 2000; Grego 2012). The degree of smoothness (s) of model terms is estimated as part of the fitting, intrinsic of the analysis (Wood 2017). The GAM is suitable for these types of data since it does not assume a priori any specific form of relationship among data and can be used to reveal and estimate nonlinear effects of the covariate on the dependent variable, primarily used in meta-analysis of multisource data (Grego 2012; Wijewardhana et al. 2020).
For comparison, we also constructed Generalized Linear Models (GLM) with the same year and monthly variation data (Walker and Taylor 2017). Finally, we analyzed the fit of the models to the data through the level of significance (p-value) and compared the GAM and GLM using the Akaike Information Criteria (AIC), where smaller AIC corresponds to better models to the fitted data (Johnson and Omland 2004). We performed all the analyzes using the package mgcv (Wood 2017) on the software R (version 4.1.2) (R Core Team 2022).
Results
Data selection
We retrieved 123 articles and other documents from the three databases. After eliminating duplicate results, we were left with 61 papers. First, we reviewed the abstract of the articles according to the criteria described in the methods, resulting in 22 papers on the ecology, distribution, and occurrence of flamingos. Then, we assessed the full text of the remaining studies and excluded another 13 articles about other flamingo species or that addressed areas other than the Lagoa do Peixe. Ultimately, we considered only five documents in our statistical analysis: Belton (1985), Rezende and Leeuwenberg (1987), Antas (1994), Delfino and Carlos (2021), and Aldana-Ardila and Carlos (2021).
From the fieldwork data, we retrieved abundance data of 16 different sampling occasions, totaling 1365 birds counted (a mean of 50.56 flamingos per sampling). Lastly, in the two citizen science platforms, we retrieved 321 different points of occurrence for Chilean Flamingo in the Lagoa do Peixe. However, from these points, only 241 had abundance data that we used in our population estimates, and after a verification check and elimination of duplicates, only 173 points were adequate for the analysis.
The final data we used for estimating the Chilean Flamingo populational number consisted of 222 different dates of abundance data, 33 from the literature, 16 from fieldwork, and 173 from citizen science databases (Online Resource 1). The data covers 50 years, from 1972 to 2022, with the oldest records being from Belton (1985), with the significant majority of data reported for the last decade (2012–2022), mainly in the eBird and GBIF platforms. The mean numbers of flamingos observed on all occasions were 40.16 (sd ± 35.31), with a higher value (n = 348) reported by Rezende and Leeuwenberg (1987) for September 1986. The lowest values vary from 0 to 5 flamingos observed, reported by different observers on different occasions in the three data sources.
Population size and trend
The GAM annual model better fits the multisource data used in the population estimates, providing significant results on the variation of Chilean Flamingo abundance in Lagoa do Peixe through the years (Table 1). Despite the Linear model also finding significant statistical results, the GAM model presented better AIC in comparison, a better prediction of historical population trends. The GAM model showed that the population varies from a mean of 167 individuals in the 1970s to 71 individuals during the 2020s. The GAM model also showed fluctuation in the Lagoa do Peixe population, slightly increasing during 1985–1990 and 2005–2011 (Fig. 2).
The inclusion of citizen science data provided a better adjustment to the model, reducing the possible confidence interval observed after 2010; meanwhile, the confidence interval for the previous decade encompasses a much higher variance due to the non-continuity of the data among the years and the different high values obtained from the various data sources, as expected.
Significant models reported important population trends for the Lagoa do Peixe National Park along the years, from 1974 to 2022. In blue, the model generated using Generalized Additive Model (GAM) with its respective confidence interval in gray, highlights fluctuations, whereas the Generalized Linear Model (GLM), in red, shows a slight decrease in Chilean Flamingo abundance in the Park. Gray points indicate the raw data used in the study
Related to the models of monthly variation, the GAM was the only model to explain the intra-annual variance of flamingo significantly, while the Linear model nonsignificant value (Table 1). The month GAM model predicted a population that varies from nearly 200 individuals during August and September to less than five individuals during January and February (Fig. 3). Due to the extensive monthly coverage of the data sources used in this study, the model could predict and estimate population trends with high confidence and lower variety in the confidence intervals. These trends matched the expected for the species, from low numbers during the summer, when Chilean Flamingos are in their breeding colonies, to increasing numbers during the fall, achieving a higher number during the end of the winter and the beginning of the spring, before the birds return to their colonies for the next breeding season. Nevertheless, the model confirmed the presence of Chilean Flamingos in the park year-round.
Discussion
The flamingos are a well-distributed group and present complex patterns of habitat occupation, with high variation related to time, areas, and populational trends (Delfino and Carlos 2022). The distribution and movements of Chilean Flamingos in South America remain a mystery in many aspects, mainly due the high mobility of the species, high number of different wetlands occupied and the logistical difficulties of movement ecology studies with these populations, but the species seems to adopt a nomadic behavior, moving among wetlands according to the resource availability, climate conditions, and water features (Caziani et al. 2007; Torres-Cristiani et al. 2020). Chilean Flamingos breed mainly in specific high Andean wetlands located in Chile and Argentina (e.g., Pozuelos, Surire, and Vilama lagoons), but the species can also breed in low wetlands almost at sea level, such as Mar Chiquita and Melincué lagoons (Bucher et al. 2000; Caziani et al. 2007; Romano et al. 2008). During the year, in the nonbreeding season, the species fly to other areas, with a very heterogeneous distribution pattern that usually changes within the year and from one year to another (Bucher 1992; Mascitti and Bonaventura 2002; Romano et al. 2008).
Another relevant characteristic of the movements of flamingos is the strong philopatry among a given population in specific areas. In Europe, genetic studies found that Greater Flamingos present high tendencies to return to the same breeding colonies or wintering areas, with a strong indication of despotic influence and wetland conditions in determining site fidelity (Balkiz et al. 2010). The constant movements between areas formed a cohesive metapopulation of Greater Flamingos around the Mediterranean Sea (Balkiz et al. 2007a, b; Boucheker et al. 2011). Likewise, recent genetic studies failed to detect any segmentation and genetic differentiation among the Chilean Flamingo population in South America (Torres et al. 2014). This finding supports the idea that the individuals fly out among wetlands very often and maintain the connectivity between these ecosystems through their displacement behaviors while also confirming the possible fidelity to wintering areas and the influence of climatic and environmental conditions on the distribution of the species on the continent (Caziani et al. 2007; Frias-Soler et al. 2022).
The Lagoa do Peixe is the more distant area to the west within the Chilean Flamingo distribution, where this species occurs all year round. (Antas 1994; Delfino and Aldana-Ardila 2020). Despite this no breeding behavior and nesting have been observed, the Chilean Flamingos return to the area annually, mainly during the austral winter and spring, with some individuals remaining in the Lagoa do Peixe National Park also during the summer (Belton 1985; Rezende and Leeuwenberg 1987; Delfino and Aldana-Ardila 2020). Our results showed patterns of demographic variation similar to other contranuptial areas used by flamingos, like the population of Greater Flamingo around the Mediterranean Sea (Geraci et al. 2012; Liordos et al. 2014), or the demographic variation of Chilean Flamingos populations in lowlands such as Mar Chiquita and Melincué lagoon (Caziani et al. 2007; Romano et al. 2008); these birds usually arrive at the end of the fall, achieving their high density in the late winter and early spring, right before leaving the area to return to the breeding colonies in south Africa or high Andean wetlands, respectively. Although there is no genetic or tracking information, the Chilean Flamingo patterns of occupation in Lagoa do Peixe suggest that the population that uses the area during the winter may come from the large breeding colonies in northeastern Argentina and Chile, covering about 2500 km, and probably use the wetlands of northwestern Argentina as a stopover until they finish this journey (Antas 1994; Delfino and Aldana-Ardila 2020).
Regarding the annual and historical demographic patterns of Chilean Flamingos at Lagoa do Peixe National Park, the decrease in the park occupation may reflect trends alongside the entire species population. During the late 80s and early 90s, the populations of South American Flamingos presented an accentuated decrease, mainly in the case of Andean and Puna flamingos, but included some Chilean Flamingo populations (Rocha 1994; Rocha and Quiroga 1997; Ugarte-Nuñez and Mosaurieta-Echegarey 2000). The rapid urbanization around wetlands, the continuous use of wetlands for agricultural and mining processes, and the high level of urban disturbances around these areas directly affected the reproductive capacity of these species, which are highly sensitive to stress and disturbances during the nesting and egg incubation phases (del Hoyo 1992; Rocha 1994; Stacey 2019). Although there was no complex census data from this period, del Hoyo (1992) estimated 200,000 animals in the Chilean Flamingo population in the mid-eighties. However, a census by Valqui et al. (2000) counted a maximum of 75,000 animals in the sampled lagoons between 1997 and 1998, indicating a substantial decline. These could reflect directly in the decrease of Chilean Flamingos observed at Lagoa do Peixe during this period, reported both by the quantitative analysis here presented but also by the nearby population of birdwatchers and fishermen (HCD, pers. observ.).
In the late 90s and early 2000, South American researchers made a great effort to include the three flamingo species in more active conservation and management plans by pressuring local and national politicians to protect wetlands where these species occur and developing research to document population dynamics and the relationship between these animals and humans’ activities in breeding and nonbreeding areas (BirdLife International 2008; Marconi et al. 2011). The activities of the High Andean Flamingos Conservation Group managed to raise attention and help restore many populations of Chilean flamingos along their distribution and could collaborate with the stabilization of Chilean Flamingos populations at the park, despite the non-inclusion of Lagoa do Peixe in the monitoring area of the Conservation Group. Marconi et al. (2011) estimated a population of 300,000 Chilean Flamingos, indicating a rapid recuperation of the population along their distribution (Marconi et al. 2011, 2020). Nevertheless, the end of the 90s was also marked by the creation of the first environmental management plan for Lagoa do Peixe National Park, in 1999, thirteen years after its creation (Knak 1999). This plan establishes rules for the park occupation and uses and determines conservation and management goals to preserve the area and all its associated species (Knak 1999). Despite the short citation of Chilean Flamingos in the park plan, the plan made official many conservation actions and allowed better monitoring of the area, reducing illegal activities, human disturbances, and stressful situations for flamingos in the area, potentially collaborating with the maintenance of the Chilean Flamingo population in the area during the 2000 and 2010 s. It is also important to acknowledge that the decrease in population within the Park can also reflect the limited availability of demographic data in the early years, while the higher number of data collected in 2010 highlights the substantial differences in sampling effort between these periods.
Nevertheless, the population of Chilean Flamingos at the National Park is still overlooked and poorly studied, with only recent efforts to study the ecology and ethology of the animals in the area toward a better understanding of their relationship with the community, the park ecosystem, and the activities nearby and within Lagoa do Peixe (Delfino and Aldana-Aldila 2020). The Barra of Lagoa do Peixe, an area where the lagoon meets the sea, is a rich ecotone where Chilean flamingos rest and feed due to a large number of invertebrates and algae (Loebmann and Vieira 2005; Schossler 2011). This area is also essential for artisanal shrimp fisheries and other small-scale fisheries performed only by local communities, under license, as well as one of the main areas to receive tourists and visitors, potentially benefiting important sectors of the communities around the park (Almudi and Kalikoski 2009; Delfino and Aldana-Ardila 2020). The Barra area is artificially opened to allow better fishing, changing the physiognomies of the entire area, with unknown ecological consequences to the local ecosystem dynamics (Schossler 2011; Crippa 2011). Human disturbances in the area can cause stressful situations for flamingos, often animals susceptible to human presence, making the group leave the area earlier and take time to return (Galicia and Baldassarre 1997; Yosef 2000). Furthermore, overfishing and predatory fishing can deplete directly or indirectly the primary food resources of Chilean Flamingos in the area, such as crustacean larvae, other invertebrates, and phytoplankton, forcing the animals to leave the Barra earlier (Crippa 2011, Aldana-Ardila and Carlos 2021).
Knowledge about the demography and population dynamics of Chilean Flamingos in Lagoa do Peixe is vital to developing an action plan that optimizes land planning for conservation, especially on a temporal scale, thus avoiding the disappearance of Chilean Flamingos from the area and helping to protect other birds’ species that use the area and that can also be threatened (Péron et al. 2013; Wang et al. 2018). In the last years, the Barra was opened during the winter, in August, a period when Chilean Flamingos are in higher concentrations in the area (HCD, pers. observ.). Nevertheless, in the last three years, when La Nina reduced the rainfall in the area, the early Barra opening caused a severe drought in Lagoa do Peixe, thus harming not only the animals but also the economic activities in the area (Grimm et al. 2000; Carvalho et al. 2020; Francisco and Netto 2020). Therefore, the area’s management needs to consider the bird populations that use the area during the winter and the climate to avoid droughts. Then, when necessary, the Barra opening can be made when the Chilean Flamingos are in lower numbers in the area, during the beginning or middle of austral spring, if the climatic previsions are favorable, not prejudicing the food availability in the area (Schossler 2011; Crippa 2011).
The same factors can be applied to the area’s fishing management and shrimp farming, especially during spring and summer, increasing monitoring to avoid furtive fishing that can disturb the birds. Other measures to consider are regulating the fishing intensity, the fishing calendar, and the proximity between the fishing areas and the feeding areas of the Chilean flamingos. (Adomilli 2006; Loebmann and Vieira 2006; Almudi and Kalikoski 2009). Better than that, environmental education actions could help to familiarize fishers with the animals and integrate their action in the conservation of the bird species in the park, not excluding them from conservation practices but putting them directly in an active and leading role inside their communities toward conservation of the area (Mazzochi and Carlos 2020; Sanchez 2021). Better communication between local authorities, scientists, and the community is essential to promote effective conservation and management actions (Sanchez 2021). Finally, conscious and regulated tourism activities inside the park, avoiding excessive disturbances in the foraging and resting areas of flamingos, and keeping a minimum distance toward the flamingos flocks, can become an economic activity that complements the earnings of local communities (Braga et al. 2005; Kaiser et al. 2022).
Demographic and populational studies are essential to understand the intrinsic dynamic between a species’ population and its environment, having a high potential to affect economic and conservation actions, such as described above. Therefore, continuous monitoring of the Chilean Flamingo population in the park is necessary to check the conservation of the area and the species. Also, important as a tool to regulate the activities and the park and the necessity of new actions to mitigate potential threats. Furthermore, more ecological and conservation studies are needed in the area to better assess the effect that the artificial management of the Barra, the fishing, and the tourism have in the area, creating management and conservation plans that effectively collaborate to avoid the decrease not only in the Chilean Flamingo population but also in other birds’ species that use the area.
Data Availability
The data used on the manuscript is available as Online Resource.
References
Adomilli GK (2006) Tempo e Espaço: Considerações sobre o modo de vida dos pescadores do Parque Nacional da Lagoa do Peixe - RS em um contexto de conflito ambiental. Iluminuras 7(15). https://doi.org/10.22456/1984-1191.9249
Aldana-Ardila O, Carlos CJ (2021) Feeding Ecology of the chilean Flamingo Phoenicopterus chilensis (Aves: Phoenicopteridae) in a coastal wetland in southern Brazil. J Nat Hist 55(41–42):2589–2603. https://doi.org/10.1080/00222933.2021.2003459
Almudi T, Kalikoski D (2009) Homem e “natureza” em um parque nacional do sul do Brasil: meios de vida e conflitos nos arredores da Lagoa do Peixe. Desenvolv e Meio Ambient 20:47–57. https://doi.org/10.5380/dma.v20i0.12291
Balkiz Ö, Béchet A, Rouan L, Choquet R, Germain C, Amat JA, Rendón-Martos M, Baccetti N, Nissardi S, Özesmi U, Pradel R (2010) Experience-dependent natal philopatry of breeding greater flamingos. J Anim Ecol 79(5):1045–1056. https://doi.org/10.1111/j.1365-2656.2010.01721.x
Balkız Ö, Béchet A, Rouan L, Choquet R, Germain C, Amat JA, Rendón-Martos M, Baccetti N, Özesmi U, Pradel R (2007a) Metapopulation dynamics of the Greater Flamingo Phoenicopterus roseus in the Mediterranean: implications for conservation. Flamingo, Bull IUCN-SSC/Wetlands Int Flamingo Spec Gr
Balkız Ö, Özesmi U, Pradel R, Germain C, Sıkı M, Amat JA, Rendón-Martos M, Baccetti N, Béchet A (2007b) Range of the Greater Flamingo, Phoenicopterus roseus, metapopulation in the Mediterranean: new insights from Turkey. J Ornithol 148(3):347–355. https://doi.org/10.1007/s10336-007-0136-2
Barras AG, Blache S, Schaub M, Arlettaz R (2021) Variation in demography and life-history strategies across the range of a declining Mountain Bird Species. Front Ecol Evol 9. https://doi.org/10.3389/fevo.2021.780706
Belton W (1985) Birds of Rio Grande do sul, Brazil. Part 1, Rheidae through Furnariidae. Bull Am Mus Nat Hist 178:371–631
Bibby CJ, Burgess ND, Hillis DM, Hill DA, Mustoe S (2000) Bird Census techniques. Elsevier Science, p 302
BirdLife International (2008) Memorandum of Understanding on the Conservation of High Andean Flamingos and their Habitats. Downloaded from http://www.birdlife.org on 22 ago 2022
Bird TJ, Bates AE, Lefcheck JS, Hill NA, Thomson RJ, Edgar GJ, Stuart-Smith RD, Wotherspoon S, Krkosek M, Stuart-Smith JF, Pecl GT, Barrett N, Frusher S (2014) Statistical solutions for error and bias in global citizen science datasets. Biol Conserv 173:144–154. https://doi.org/10.1016/j.biocon.2013.07.037
Boucheker A, Samraoui B, Prodon R, Amat JA, Rendón-Martos M, Baccetti N, Esquerre i, Nissardi S, Balkiz Ö, Germain C, Boulkhssaim M, Béchet A (2011) Connectivity between the algerian population of Greater Flamingo Phoenicopterus roseus and those of the Mediterranean basin. Ostrich 82(3):167–174. https://doi.org/10.2989/00306525.2011.607856
Braga PLS, Abadallah PR, de Oliveira CR (2005) Valoração Econômica do Parque Nacional Da Lagoa do Peixe, RS. Repositório Int Univ Fed rio Gd :17
Brlík V, Šilarová E, Škorpilová J et al (2021) Long-term and large-scale multispecies dataset tracking population changes of common european breeding birds. Sci Data 8(1):21. https://doi.org/10.1038/s41597-021-00804-2
Bucher EH (1992) Population and Conservation Status of Flamingos in Mar Chiquita, Cordoba, Argentina. Colon Waterbirds 15(2):179. https://doi.org/10.2307/1521451
Bucher EH, Echevarria AL, Juri MD, Chani JM (2000) Long-term survey of chilean Flamingo breeding colonies on Mar Chiquita Lake, Córdoba, Argentina. Waterbirds Int J Waterbird Biol 23:114. https://doi.org/10.2307/1522155
Carvalho BC, Dalbosco ALP, Guerra JV (2020) Shoreline position change and the relationship to annual and interannual meteo-oceanographic conditions in Southeastern Brazil. Estuar Coast Shelf Sci 235:106582. https://doi.org/10.1016/j.ecss.2020.106582
Caziani SM, Olivio OR, Ramírez ER, Romano M, Derlindati EJ, Tálamo A, Ricalde D, Quiroga C, Contreras JP, Valqui M, Sosa H (2007) Seasonal distribution, abundance, and nesting of Puna, Andean, and chilean flamingos. Condor 109(2):276–287. https://doi.org/10.1650/0010-5422(2007)109[276:SDAANO]2.0.CO;2
Clarivate Analytics (2020) Web of Science. Available at https://www.webofscience.com/wos/woscc/advanced-search. Accessed on 12 Sep 2022.
Clarke ED, Spear LB, Mccracken ML, Marques FFC, Borchers DL, Buckland ST, Ainley DG (2003) Validating the use of generalized additive models and at-sea surveys to estimate size and temporal trends of seabird populations. J Appl Ecol 40(2):278–292. https://doi.org/10.1046/j.1365-2664.2003.00802.x
Crippa LB (2011) O manejo de abertura da Barra influencia a comunidade de macroinvertebrados nas áreas úmidas do sul do Brasil? Um estudo de caso no Parque Nacional da Lagoa do Peixe. Dissertation, Universidade do Vale do Rio dos Sinos
de Antas P TZ (1994) Migration and other movements among the lower Paraná River valley wetlands, Argentina, and the south Brazil/Pantanal wetlands. Bird Conserv Int 4(2–3):181–190. https://doi.org/10.1017/S0959270900002768
Delfino HC, Aldana-Ardila O (2020) Comments on the population status of Chilean Flamingos at Lagoa do Peixe. Flamingo 3:21–26.
Delfino HC, Carlos CJ (2021) To be or not to be a migrant: the different movement behaviours of birds and insights into the migratory status of flamingos (Phoenicopteridae). Bull Br Ornithol Club 141(4). https://doi.org/10.25226/bboc.v141i4.2021.a5
Delfino HC, Carlos CJ (2022) Intra-annual variation in activity budgets of a wild chilean Flamingo (Phoenicopterus chilensis) population in Southern Brazil. Austral Ecol. https://doi.org/10.1111/aec.13180
del Hoyo J (1992) Phoenicopteridae (Flamingos). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world. Lynx Edicions, Barcelona, pp 508–526
Elsevier (2020) Scopus. Available at https://www.scopus.com/home.uri. Accessed on 12 Sep 2022.
Fewster RM, Buckland ST, Siriwardena GM, Baillie SR, Wilson JD (2000) Analysis of population trends for farmland birds using generalized additive models. Ecology 81(7):1970–1984. https://doi.org/10.1890/0012-9658(2000)081[1970:AOPTFF]2.0.CO;2
Francisco AS, Netto SA (2020) El Niño–Southern Oscillations and Pacific Decadal Oscillation as Drivers of the Decadal Dynamics of Benthic Macrofauna in two subtropical estuaries (Southern Brazil). Ecosystems 23(7):1380–1394. https://doi.org/10.1007/s10021-019-00475-6
Freeman SN, Noble DG, Newson SE, Baillie SR (2007) Modelling population changes using data from different surveys: the Common Birds Census and the breeding Bird Survey. Bird Study 54(1):61–72. https://doi.org/10.1080/00063650709461457
Frias-Soler RC, Bauer A, Grohme MA, Espinosa López G, Gutiérrez Costa M, Llanes-Quevedo A, Van Slobbe F, Frohme M, Wink M (2022) Phylogeny of the order Phoenicopteriformes and population genetics of the caribbean flamingo (Phoenicopterus ruber: Aves). Zool J Linn Soc. https://doi.org/10.1093/zoolinnean/zlac040
Galicia E, Baldassarre GA (1997) Effects of motorized tourboats on the behavior of nonbreeding american flamingos in Yucatan, Mexico. Conserv Biol 11(5):1159–1165. https://doi.org/10.1046/j.1523-1739.1997.96080.x
GBIF.org (2022) GBIF Occurrence Download. https://doi.org/10.15468/dl.3v3tum. Accessed on 10 Ago 2022.
Geraci J, Béchet A, Cézilly F, Ficheux S, Baccetti N, Samraoui B, Wattier R (2012) Greater flamingo colonies around the Mediterranean form a single interbreeding population and share a common history. J Avian Biol 43:341–354. https://doi.org/10.1111/j.1600-048X.2012.05549.x
Google Inc. (2020) Google Scholar. Available at https://www.scholar.google.com.br. Accessed 12 Sep 2022.
Grego JM (2012) Generalized additive models. Encyclopedia of environmetrics. Wiley
Grimm AM, Barros VR, Doyle ME (2000) Climate Variability in Southern South America Associated with El Niño and La Niña events. J Clim 13(1):35–58. https://doi.org/10.1175/1520-0442(2000)013%3C0035:CVISSA%3E2.0.CO;2
Hernández-Brito D, Carrete M, Tella JL (2022) Annual Censuses and Citizen Science Data Show Rapid Population increases and Range Expansion of Invasive Rose-Ringed and Monk parakeets in Seville, Spain. Animals 12(6):677. https://doi.org/10.3390/ani12060677
Horns JJ, Adler FR, Şekercioğlu ÇH (2018) Using opportunistic citizen science data to estimate avian population trends. Biol Conserv 221:151–159. https://doi.org/10.1016/j.biocon.2018.02.027
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19(2):101–108. https://doi.org/10.1016/j.tree.2003.10.013
Kaiser SM, Goncalves JM, dos Perelló A LFC (2022) Turismo de observação de aves no PN Lagoa do Peixe: oportunidades ou ameaças? Rev Bras Ecoturismo 15(1). https://doi.org/10.34024/rbecotur.2022.v15.11994
Khan KS, Kunz R, Kleijnen J, Antes G (2003) Five steps to conducting a systematic review. JRSM 96(3):118–121. https://doi.org/10.1258/jrsm.96.3.118
Knak RB (ed) (1999) (cord) Plano de Manejo do Parque Nacional da Lagoa do Peixe – Fase 2. Ministério do Meio Ambiente. https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/marinho/lista-de-ucs/parna-da-lagoa-do-peixe/copy_of_PM.pdf. Accessed on 22 Ago 2022
Liordos V, Pergantis F, Perganti I, Roussopoulos Y (2014) Long-term population trends reveal increasing importance of a Mediterranean wetland complex (Messolonghi lagoons, Greece) for wintering waterbirds. Zool Stud 53(1):12. https://doi.org/10.1186/1810-522X-53-12
Loebmann D, Vieira JP (2005) Distribuição espacial e abundância das assembleias de peixes no parque Nacional da Lagoa do Peixe, Rio Grande do sul, Brasil. Rev Bras Zool 22(3):667–675
Loebmann D, Vieira JP (2006) O impacto da pesca do camarão-rosa Farfantepenaeus paulensis (Perez-Farfante) (Decapoda, Penaeidae) nas assembleias de peixes e siris do Parque Nacional da Lagoa do Peixe, Rio Grande do sul, Brasil. Rev Bras Zool 23(4):1016–1028. https://doi.org/10.1590/S0101-81752006000400006
Marconi P, Arengo F, Castro A, Rocha O, Valqui M, Aguilar S, Barberis I, Castellino M, Castro L, Derlindati E, Michelluti M, Moschione F, Musmeci L, Ortiz E, Romano M, Sosa H, Sepulveda D, Sureda A (2020) Sixth International Simultaneous Census of three flamingo species in the Southern Cone of South America: Preliminary analysis. Flamingo 3:67–75.
Marconi P, Sureda AL, Arengo F, Aguilar MS, Amado N, Alza L, Rocha O, Torres R, Moschione F, Romano M, Sosa H, Derlindati E (2011) Fourth simultaneous flamingo census in South America: preliminary results. Flamingo 18:48–53
Mascitti V, Bonaventura SM (2002) Patterns of abundance, distribution and Habitat Use of Flamingos in the high Andes, South America. Waterbirds 25(3):358–365
Matsubara CP, Maltchik L (2008) Diversity and distribution of Algae in Wetlands of the Rio Grande do sul, Brazil Diversidade e distribuição de algas em áreas úmidas do Rio Grande do. Wetlands 3(april):21–27
Mazzochi MS, Carlos CJ (2020) Pescadores e aves marinhas: etnobiologia de uma comunidade pesqueira no sul do Brasil. Biotemas 33(2):1–16. https://doi.org/10.5007/2175-7925.2020.e71987
Ministério do Meio Ambiente (2022) Lista Oficial de Espécies Brasileira Ameaçadas de Extinção. Portaria MMA Nº 148 June 7, 2022. https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Portaria/2020/P_mma_148_2022_altera_anexos_P_mma_443_444_445_2014_atualiza_especies_ameacadas_extincao.pdf. Accessed on 10 Ago 2022
Pacheco JF, Silveira LF, Aleixo A et al (2021) Annotated checklist of the birds of Brazil by the brazilian Ornithological Records Committee—second edition. Ornithol Res 29(2):94–105. https://doi.org/10.1007/s43388-021-00058-x
Péron G, Ferrand Y, Leray G, Gimenez O (2013) Waterbird demography as indicator of wetland health: the french-wintering common snipe population. Biol Conserv 164:123–128. https://doi.org/10.1016/j.biocon.2013.04.015
Raman TRS (2002) Assessment of census techniques for interspecific comparisons of tropical rainforest bird densities: a field evaluation in the western ghats. India Ibis (Lond 1859) 145(1):9–21. https://doi.org/10.1046/j.1474-919X.2003.00105.x
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, AT.
Rezende SL, Leeuwenberg F (1987) Ecological studies of Lagoa do Peixe. Final report to World Wildlife Fund, Washington, DC
Rio Grande do Sul (2014) Espécies da Fauna Silvestre Ameaçadas de Extinção no Estado do Rio Grande do Sul. Decreto Nº 51797 September 8, 2014. http://www.al.rs.gov.br/filerepository/replegis/arquivos/dec%2051.797.pdf. Accessed on 10 Ago 2022
Rocha OO (1994) Contribución preliminar a la conservación y el conocimiento de la ecología de flamencos en la Reserva Nacional de Fauna Andina “Eduardo Avaroa”, Departamento Potosí, Bolivia. Academia Nacional de Ciencas de Bolivia, Museo Nacional de Historia Natural, La Paz
Rocha OO, Quiroga OC (1997) Primer censo simultáneo internacional de los flamencos Phoenicoparrus jamesi y Phoenicoparrus andinus en Argentina, Bolivia, Chile y Perú, con especial referencia y análisis al caso boliviano. Ecología en Bolivia 30:33–42
Rolon AS, Rocha O, Maltchik L (2013) Does the lagoa do peixe sandbar opening influence the macrophyte richness and composition in southern Brazil wetlands? Rev Biol Trop 61(1):409–417. https://doi.org/10.15517/rbt.v61i1.11137
Romano M, Barberis IM, Pagano F, Marconi P, Arengo F (2008) Winter monitoring of andean and chilean flamingos in lowland wetlands of central Argentina. Flamingo, Bull IUCN-SSC/Wetlands Int Flamingo Spec Group
Sanchez, L del PJ (2021) Comunidades tradicionais e educação ambiental: um estudo a partir de teses e dissertações brasileiras. Dissertation, Universidade Estadual Paulista “Júlio de Mesquita Filho”
Sayers A (2008) Tips and tricks in performing a systematic review. Br J Gen Pract 58(547):1361–1136. https://doi.org/10.3399/bjgp08X277168
Schossler V (2011) Morfodinâmica da Embocadura da Lagoa do Peixe e da Linha de Praia Adjacente. Dissertation, Universidade Federal do Rio Grande do Sul
Stacey JR (2019) Lithium mining in the High Puna of the Andes: an environmental blessing with some dark footprints? Levin Sources
Sæther B-E, Grøtan V, Engen S, Coulson T, Grant PR, Visser ME, Brommer JE, Rosemary Grant B, Gustafsson L, Hatchwell BJ, Jerstad K, Karell P, Pietiäinen H, Roulin A, Røstad OW, Weimerskirch H (2016) Demographic routes to variability and regulation in bird populations. Nat Commun 7(1):12001. https://doi.org/10.1038/ncomms12001
Sutherland WJ, Newton I, Green R (2004) Bird Ecology and Conservation: A Handbook of Techniques. OUP Oxford
Tawfik GM, Dila KAS, Mohamed MYF, Tam DNH, Kien ND, Ahmed AM, Huy NT (2019) A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health 47(1):46. https://doi.org/10.1186/s41182-019-0165-6
Torres-Cristiani L, Machkour-M’Rabet S, Calmé S, Weissenberger H, Escalona-Segura G (2020) Assessment of the american Flamingo distribution, trends, and important breeding areas. PLoS ONE 15(12):e0244117. https://doi.org/10.1371/journal.pone.0244117
Torres CR, Ogawa LM, Gillingham MA, Ferrari B, van Tuinen M (2014) A multi-locus inference of the evolutionary diversification of extant flamingos (Phoenicopteridae). BMC Evol Biol 14(1):36. https://doi.org/10.1186/1471-2148-14-36
Ugarte-Núñez J, Mosaurieta-Echegaray L (2000) Assessment of threats to flamingos at the Salinas and Aguada Blanca National Nature Reserve (Arequipa, Perú). Waterbirds 23(Special publication):134–140
Valqui M, Caziani SM, Rocha-O O, Rodriguez- RE (2000) Abundance and distribution of the South American Altiplano flamingos. Waterbirds 23(Special publication):110–113
Villwock JA (1984) Geology of the Coastal Province of Rio Grande do Sul, Southern Brazil. A Synthesis. Pesquisas 16:5–49.
Walker J, Taylor PD (2017) Using eBird data to model population change of migratory bird species. Avian Conserv Ecol 12(1):art4. https://doi.org/10.5751/ACE-00960-120104
Wang X, Kuang F, Tan K, Ma Z (2018) Population trends, threats, and conservation recommendations for waterbirds in China. Avian Res 9(1):14. https://doi.org/10.1186/s40657-018-0106-9
Wijewardhana UA, Meyer D, Jayawardana M (2020) Statistical models for the persistence of threatened birds using citizen science data: a systematic review. Glob Ecol Conserv 21:e00821. https://doi.org/10.1016/j.gecco.2019.e00821
Wood SN (2017) Generalized additive models. Chapman and Hall/CRC, p 496
Yosef R (2000) Individual distances among Greater Flamingos as indicators of tourism pressure. Waterbirds Int J Waterbird Biol 23:26. https://doi.org/10.2307/1522143
Acknowledgements
We thank the Lagoa do Peixe National Park for helping with logistics and accommodation during the field procedures describe in this study and for the support to conduct this research.
Funding
HCD and O A-A are supported by a PhD fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CJC was supported by a Postdoctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and CEF is an independente researcher.
Author information
Authors and Affiliations
Contributions
HCD, CJC, OA-A and CEF conceived the paper; HCD wrote the first draft and all authors discussed ideas and collaborated on subsequent drafts.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
. Table showing the complete data used to generate the demographic models for the Chilean Flamingos of Lagoa do Peixe National Park. For each abundance value, the month, year, and source of data are informed
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delfino, H.C., Aldana-Ardila, O., Fedrizzi, C.E. et al. Multisource data reveals relevant trends in a Chilean Flamingo Phoenicopterus chilensis population at an important coastal wetland of Southern Brazil: implications for conservation and planning. J Coast Conserv 27, 33 (2023). https://doi.org/10.1007/s11852-023-00963-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11852-023-00963-x