Abstract
ApoE has been reported to be associated with tumorigenesis and tumor progression. In this study, we explored the potential diagnostic and prognostic role of serum ApoE in breast cancer patients. Subject cohorts consisted of 152 normal healthy controls female and 257 breast cancer cases. Serum levels of ApoE were determined with turbidimetric immunoassay. The serum levels of ApoE were significantly elevated in breast cancer patients compared with normal healthy controls (45.82 ± 13.96 mg/L vs. 33.61 ± 6.44 mg/L, respectively, P < 0.0001) and also significantly associated with TNM stage and lymph nodes status (all P < 0.05). Area under receiver operating characteristic curve for serum ApoE discriminate breast cancer patients from controls was 0.786 with specificity of 0.974 and sensitivity of 0.541, the cut-off value of ApoE was 43.15 mg/L. Kaplan-Meier log rank analysis showed that the high serum ApoE group (serum ApoE ≥ 43.15 mg/L) had a poorer progression-free survival and overall survival compared with low serum ApoE group (serum ApoE < 43.15 mg/L) (all P < 0.05). In addition, univariate and multivariate Cox regression analysis displayed serum ApoE as an independent risk factor of breast cancer patients prognosis (all P < 0.05). Serum ApoE played a role as serological biomarkers that indicated diagnostic and prognostic evaluation in breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an estimated 268,600 new cases and 69,500 deaths in 2015, breast cancer is the most frequent diagnosed cancer and the sixth cause of cancer-related mortality for women in China and leads to a considerable public health issue [1]. Although considerable improvements have been made in breast cancer diagnosis and comprehensive treatment recently, once recurrence and or relapse with distant metastasis, treatment is particularly invalid, and most of these patients eventually die from disseminated metastatic malignancy [2]. Recently, a series of breast cancer-related biomarkers have been identified and applied in early detection, personalized therapy, and prognosis prediction; the power of these biomarkers are still limited [3]. Indentifying novel biomarkers to diagnose early stage breast cancer will enable the doctors to choose less-aggressive treatments and improve clinical outcomes [4]. Therefore, there is a need to search for novel biomarkers for breast cancer early detection and prognosis prediction.
Apolipoprotein E (ApoE), approximately 34.5 kDa, with a 299-amino acid glycoprotein-rich arginine, is mainly synthesized in the liver, also in the lung, spleen, skin, kidney, brain, and macrophages [5, 6]. As an essential protein member of plasma lipoproteins family, ApoE play important roles in cholesterol transportation and metabolism by binding to members of the low-density lipoprotein (LDL) receptor family [7]. In addition, ApoE could play diverse roles in a number of biological process, such as cell growth, differentiation, immune stresses, macular degeneration, and survival [8]. Meanwhile, ApoE have been identified to promote lung adenocarcinoma proliferation and migration and was an independent factor for predicting prognosis in lung adenocarcinoma patients [6]. Recently, ApoE was found to associate with melanoma metastasis and angiogenesis [9]. As a secreted protein, elevated serum ApoE was associated with smoking, could be serve as a predictive biomarker for early squamous metaplasia in lung [10]. Liu et al. also found serum ApoE to be a potential biomarker for lung adenocarcinoma metastasis by combing proteomic and immunological analyses profiling the secretomes of lung adenocarcinoma cell lines, serum, and tissue of lung cancer patients [8]. Our previously study demonstrated that serum ApoE elevated in patients with non-small cell lung cancer (NSCLC), and was associated with lymph node metastasis and poor prognosis, might served as a useful serological biomarkers for predicting the prognosis of NSCLC [11].
Although it is well known that the expression level of the ApoE is elevated in several tumor types, its prognostic role in breast cancer is still unknown. In this study, we were aimed to examine the serum ApoE level in breast cancer patients, and the potential association with clinicopathological variables, overall and recurrence-free survivals, and evaluate the value of serum ApoE as a prognostic biomarker for breast cancer.
Materials and methods
Subjects
Subjects were divided into two groups, breast cancer patient group (n = 257), and normal healthy control group (n = 152). To avoid the difference of serum ApoE levels between gender, male patients were excluded. Breast cancer samples were collected from the First Affiliated Hospital of Sun Yat-sen University in the southern of China (Guangzhou, China) from January 2004 to December 2008. All the enrolled patients’ tumors were restricted to the breast, no clue of distant metastasis or skin involvement at the time of diagnosis. Most of the patients received modified radical mastectomy (92.6 %, 238/257); other patients underwent breast-conserving surgery (7.4 %, 19/257) with complete axillary lymph node dissection. Patients were given appropriate adjuvant chemotherapy, endocrine therapy, and adjuvant radiotherapy based on NCCN guidelines. Patients who had hyperlipidemia, liver disease, chronic kidney disease, previous malignancy, received any drug known to influence lipid metabolism, or received neoadjuvant chemotherapy, preoperative radiation therapy were excluded from study. All breast cancer samples were confirmed by pathological examination of tissue coming from biopsy or surgery resected specimens. The tumor stage at primary diagnosis was defined according to the American Joint Committee on Cancer and tumor-lymph node-metastasis classification system [12]. The normal healthy control group consisted of 152 age-matched and had no evidence of any cancer or family history cancer subjects who underwent a routine annual physical examination at the Centre of Physical Health Examination, the First Affiliated Hospital of Sun Yat-sen University. Exclusion criteria were the same as previously. All subjects enrolled in this study were Chinese and gave written informed consent form for participation in the study and for the use of their samples at the primary presented. This study was reviewed and approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University on the basis of the guidelines of Helsinki conventions.
Measurement of serum ApoE, CEA, and CA153 levels
All the blood samples were obtained at diagnosis before any surgery and adjuvant treatment were given. To minimize the influence of diet on measurement of ApoE levels, fasting was needed at least 8 h before blood collection from breast cancer patients or normal healthy controls by venipuncture in the morning between 7 a.m. and 10 a.m. for clinical tests, prior to treatment. Blood samples were clotted at room temperature and followed by centrifugation, and serum were stored immediately at −80 °C until being used. Hemolysis sample was removed from the cohort. All measurement was performed in the clinical laboratory of the First Affiliated Hospital of Sun Yat-sen University. Serum ApoE levels were tested by turbidimetric immunoassay commercial kit (Sekisui Medical Co. Ltd., Tokyo, Japan) with an automated analyzer (Beckman Coulter AU5800 Platform, Beckman Coulter Co. Ltd., CA, USA), according to the manufacturer’s instructions. Both the serum CEA and CA153 levels were determined by the ARCHITECT CEA assay and ARCHITECT CA153 assay (Abbott Diagnostics, Abbott Park, IL) with Abbott automated analyzer platform (ARCHITECT i2000SR, Abbott Park, IL) according to the manufacturer’s instructions. According to the manufacturers’ recommendation, the cut-off value of CEA and CA153 were 5.0 μg/L and 30 U/mL, respectively.
Data collection and follow-up
All patients’ clinical information was collected from medical records, including their age, tumor size, lymph node status, TNM stages, histopathological finding, medication history, and survival data. All subjects with breast cancer were followed up for 3–12 months (every 3 months for 2 years, then every 6 months for 3 years and every 12 months after 5 years) until June 2015. Appearance of a new disease in local position, contralateral breast, and distant organs metastasis was defined as disease progression.
Statistical analyses
Continuous variables and error bars were shown as mean ± standard deviation (SD). Categorical variables were presented as number and percentage. The relationships between biomarkers and clinicopathological variables were analyzed by the Mann-Whitney U test. Spearman’s correlation analysis was used to assess the correlations among biomarkers. Nonparametric receiver operating characteristic (ROC) curves were used to assess the sensitivity and specificity of serum ApoE as well as combined biomarkers to stratify patients’ risk of malignancy-related death. The distinguishing value of serum ApoE was evaluated by area under ROC curve (AUC) with 95 % confidence intervals (CI), sensitivity, and specificity. The cut-off value was obtained based on the score closet to the point under both the peak of sensitivity and specificity. Logistic regression analysis was applied to evaluate the sensitivity and specificity of the optimal combination of ApoE, CEA, and CA153. The overall survival (OS) and recurrence-free survival were calculated using the Kaplan-Meier method, and significant differences of survival were analyzed with the log-rank test. Univariate and multivariate analyses were performed by the Cox proportional hazards regression model. In all cases, P value of less than 0.05 indicated statistically significant, and all tests were calculated by two-sided. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics of patients
The characteristics of the total study subjects are presented in Table 1. Among the investigated subjects, a total of 257 breast cancer patients including 201 invasive ductal carcinoma (IDC), 54 ductal carcinoma in situ (DCIS), two other type of breast cancer, and 152 normal healthy controls were female. Clinical management and treatment of all patients was based on NCCN guidelines, including radiotherapy, chemotherapy, hormone therapy, and trastuzumab. Median age was 49.3 years (range 18–76 years) in breast cancer patients and 46.5 years (range 18–68 years) in controls. The median follow-up period was 91 months (8–120 months).
Serum ApoE levels in breast cancer patients and normal healthy controls
Though ApoE polymorphism significantly associated with tumorigenesis in breast cancer [13, 14], the biomarker role of serum ApoE played in this disease has remained unknown. To investigate the serum ApoE levels, we performed a turbidimetric immunoassay to analyze serum of 257 breast cancer patients and 152 normal healthy controls. The mean serum levels of ApoE were significantly higher in breast cancer group than in normal healthy controls group (Fig. 1a, P < 0.0001). For patients with breast cancer, the mean serum levels of ApoE was 45.82 ± 13.96 mg/L and 33.61 ± 6.44 mg/L for normal healthy controls (Table 2). In patients, we also analyzed the significance of the serum levels of ApoE based on different clinicopathological features. As shown in Table 2, the mean serum levels of ApoE was 41.55 ± 12.24 mg/L in stage I + II disease and 49.00 ± 14.61 mg/L in stage III. Based on TNM staging, data showed that the serum levels of ApoE elevated with disease progression (Table 2, P = 0.0030). We also found serum levels of ApoE significantly higher in breast cancer patients with lymph node metastases than those without (47.22 ± 14.33 mg/L and 43.73 ± 12.26 mg/L, respectively; Table 2, P = 0.0322). No significant difference of serum ApoE levels were observed in other clinicopathological features. After all, these data displayed that serum level of ApoE significantly elevated in breast cancer patients, and associated with the disease progression, which implied that serum levels of ApoE could be a potential biomarker for prognosis of patients with breast cancer.
Evaluation of the diagnostic performance of serum ApoE in breast cancer patients from normal healthy controls. a The mean concentration of serum ApoE for 257 breast cancer was 45.82 ± 13.96 mg/L, significantly higher than that of 152 normal healthy controls (33.61 ± 6.441 mg/L, P < 0.0001). b ROC analysis distinguished breast cancer patients from normal healthy controls (AUC = 0.786). The sensitivity was 0.541 at a specificity of 0.974
Diagnostic value of serum ApoE in breast cancer patients
Receiver operating characteristic/area under the curve (ROC/AUC) analysis was used to calculate the specific cut-off values of serum ApoE levels serve to discriminate between breast cancer patients and normal healthy controls. The serum levels of ApoE had an AUC of 0.786 with a specificity of 0.974 at a sensitivity of 0.541 for distinguishing breast cancer from normal healthy controls (the cut-off value was 43.15 mg/L, Fig. 1b). The sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) of serum ApoE, CEA, and CA153 in women with breast cancer and normal healthy controls were shown in Table 3. When used in discriminating breast cancer from normal healthy controls, serum ApoE had an apparently higher sensitivity (54.1 %) than both CEA (9.7 %) and CA153 (14.8 %). Even the specificity of ApoE (97.4 %) was close to that of CEA (98.7 %) and CA153 (98.0 %), the PPV of ApoE (97.2 %) was higher than that of CEA (92.6 %) and CA153 (92.7 %), and the NPV of ApoE (44.4 %) was lower than that of CEA (60.7 %) and CA153 (59.5 %).
Serum ApoE levels are an independent prognostic indicator for overall survival of breast cancer patients
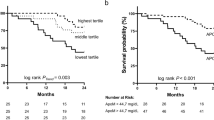
To evaluate the prognostic significance of serum ApoE in breast cancer patients, we analyzed 257 breast cancer patients with complete clinical follow-up by Kaplan-Meier analysis. After a median follow-up period of 91 months (range 11–120 months), 58 of 257 (22.57 %) patients relapsed and 48 of 257 (18.68 %) died of disease. Both progression-free survival (PFS) and overall survival (OS) in breast cancer patients with serum levels of ApoE over 43.15 mg/L were poorer than those of under 43.15 mg/L (P = 0.008, P = 0.013, respectively; Fig. 2a, b). Then, univariate and multivariate Cox regression analyses were performed to evaluate the hazard ratio (HR) of high ApoE serum levels in breast cancer patients’ disease-related death. As shown in Table 4, univariate analysis displayed that serum levels of ApoE were identified as a potential predictor of PFS and OS in breast cancer patients (HR = 2.39, 95 % CI 1.07–3.74, P = 0.01; HR = 2.96, 95 % CI 1.37–6.07, P = 0.003; respectively). Clinicopathological features such as menopausal status, ER status, HER2 status, tumor size, and lymph node metastasis also had independent prognostic power. In addition, significant variables observed in univariate analysis were reanalyzed by multivariate Cox regression analyses. However, only serum ApoE and lymph node status were confirmed to be independent risk factors of prognosis of breast cancer patients (Table 4).
Discussion
In the present study, we investigated the utility of the serum ApoE as a potential biomarker for the diagnosis and prognosis of breast cancer. We used a turbidimetric immunoassay to measure serum levels of ApoE. We found that serum ApoE levels in breast cancer patients were obviously higher than in normal healthy controls. Significant associations were found between serum ApoE and unfavorable clinicopathological features that represent poor prognosis in breast cancer patients. ROC analysis indicated that serum ApoE could be able to serve as a candidate biomarker to distinguish breast cancer patients from normal controls with AUC at 0.786 and a high specificity of 0.974. Then, we evaluated the diagnosis values of serum ApoE with established biomarkers, CEA and CA153. Although serum ApoE demonstrated similar specificity, positive predictive values (PPV), and negative predictive values (NPV) to CEA, CA153, but it showed higher sensitivity. Further, Kaplan-Meier and Cox regression analysis displayed that serum ApoE were associated with poor PFS and OS and it could be an independent prognostic variable for breast cancer.
ApoE, located on chromosome 19q13.2, is a class of apolipoprotein which involved in cholesterol transport, lipid metabolism, protein synthesis, tissue repair immune regulation, cell growth, and differentiation [15]. ApoE has three functionally distinct isoforms of the protein (E2, E3, and E4), which encoded by corresponding alleles ε2, ε3, and ε4. It was demonstrated that ApoE ε4 was a low-penetrant risk factor for development of breast cancer [14]. Recently, ApoE has been reported to associate with tumorigenesis and tumor progression. One recent report revealed that post-translational modifications of serum ApoE, including clustered methylation and dihydroxylation, may play a role in breast cancer [16]. Other study group found that ApoE polymorphism played a key role in breast cancer tumorigenesis, especially when correlated with higher serum triglyceride levels [13]. No study has reported ApoE polymorphism associated with serum ApoE levels in breast cancer. In fact, ApoE has been identified overexpressed in a variety of cancers, including breast cancer [13], bladder cancer [17], colorectal cancer [18], gastric cancer [19], lung cancer [11, 20], and ovarian cancer [21]. Therefore, we supposed that as a secreted protein, serum ApoE expression levels could associate with disease progression of breast cancer. However, to our knowledge, there is no previous study exploring the prognostic value of serum ApoE levels in breast cancer patients. In our study, we measured serum ApoE levels in 257 patients with primary breast cancer. According to the results of our study, serum ApoE displayed as a suitable serological biomarker for distinguishing breast cancer from normal healthy controls. At the same time, serum ApoE showed specificity almost as high as CEA, CA153 in breast cancer patients, with even higher sensitivity, which suggests that serum ApoE may contribute to clinical utility.
The function of ApoE in cancer cells seemed controversial as it promoted tumor cell growth and migration in ovarian cancer and lung cancer [6, 20] but served as an anti-angiogenic and metastasis-suppressive factor in melanoma [9]. Pencheva N et al. revealed cancer-secreted ApoE-inhibited cell invasion and metastatic through engaging cancer cell LRP1 and endothelial cell LRP8 receptors and final hindered endothelial recruitment in melanoma model. High expression of microRNA-199-5p/199a-3p/1908 cluster convergent down-regulated the ApoE expression, resulting in melanoma metastasis and angiogenesis, and the expression levels of microRNA-199-5p/199a-3p/1908 cluster and ApoE associated with clinical melanoma patients’ metastatic progression outcomes. However, the anti-angiogenic and metastasis-suppressive function of ApoE were derived from tumor tissue. Until now, few studies concerned the circulating ApoE in breast cancer. Meanwhile, for practical purposes, it is important to evaluate whether serum ApoE has any clinical value in predicting prognosis in breast cancer. In this study, Kaplan-Meier analysis was performed to study the association between serum ApoE and patient’s survival. We found that patients with elevated serum levels of ApoE displayed decreased progression-free survival and overall survival compared with patients with low serum levels of ApoE. At the same time, we calculated a univariate and multivariate analysis of survival by the Cox proportional hazards regression model and found serum ApoE to be an independent prognostic biomarker in breast cancer.
The major strengths of our study are as follows: (i) 257 cases breast cancer patients’ samples were collected strictly according to standard procedures, and the sample size was appropriate; (ii) the diagnostic power of the serum ApoE was evaluated comparing breast cancer patients with proper normal healthy controls; (iii) the cut-off value that served as an appropriate prognostic discriminator in breast cancer patients was not calculated in the cohort of breast cancer patients, was developed from the breast cancer patients with normal healthy controls as representing the threshold of disease in a whole population; (iv) overall survival analysis indicated that serum ApoE levels can be an independent prognostic indicator for prognosis of breast cancer patients. We are fully confident that these points make our work more convincible.
However, this study had several limitations that should be acknowledged. First, this is a single-center study in south of China although the study included a total of 257 subjects, which may restrict limit generalizablity of the conclusions to the whole Chinese population, a nationwide multicenter studies in China needed to conducted to more precisely assess the value of serum ApoE as a serological biomarker of breast cancer. Second, to our knowledge, the mechanism of serum ApoE for breast cancer’s tumorigenesis and disease progression was unclear. In addition, we did not know the reason of serum ApoE elevated in breast cancer patients, which result from cancer-secreted or by normal tissue in crowd with specific tumor susceptibility. Tumor-associated macrophages (TAM) are especially abundant and are present at tumor site on all stages of tumor progression and could serve as a multifunctional cell for potential diagnostic, prognostic, and therapy biomarkers in breast cancer [22]. ApoE could be synthesized by macrophages; therefore, TAM-secreted ApoE could be one of reasons of serum ApoE elevated in breast cancer patients.
Conclusions
In conclusion, we demonstrated that serum ApoE levels were obviously increased in the breast cancer patients compared with the normal healthy controls. The levels of serum ApoE also elevated with advanced stages and lymph node metastasis which represent that the serum ApoE may contribute to the tumor progression. In addition, breast cancer patients with high serum ApoE levels had worse prognosis, and serum ApoE could be an independent prognostic variable for breast cancer. Hence, our findings definitely show that serum ApoE may serve as a non-invasive serological biomarker for diagnostic and prognostic of breast cancer.
References
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Chi Y, Yao L, Hu X, Huang S, Huang N, Li S, Shao Z, Wu J: The bmp inhibitor dand5 in serum predicts poor survival in breast cancer. Oncotarget 2016
Rivenbark AG, O’Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol. 2013;183:1113–24.
Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat Rev Cancer. 2003;3:243–52.
Blue ML, Williams DL, Zucker S, Khan SA, Blum CB. Apolipoprotein e synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983;80:283–7.
Chen YC, Pohl G, Wang TL, Morin PJ, Risberg B, Kristensen GB, Yu A, Davidson B, Shih Ie M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65:331–7.
Herz J, Beffert U. Apolipoprotein e receptors: linking brain development and alzheimer’s disease. Nat Rev Neurosci. 2000;1:51–8.
Liu Z, Gao Y, Hao F, Lou X, Zhang X, Li Y, Wu D, Xiao T, Yang L, Li Q, Qiu X, Wang E. Secretomes are a potential source of molecular targets for cancer therapies and indicate that APOE is a candidate biomarker for lung adenocarcinoma metastasis. Mol Biol Rep. 2014;41:7507–23.
Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF. Convergent multi-miRNA targeting of ApoE drives LRp1/LRp8-dependent melanoma metastasis and angiogenesis. Cell. 2012;151:1068–82.
Rice SJ, Liu X, Miller B, Joshi M, Zhu J, Caruso C, Gilbert C, Toth J, Reed M, Rassaei N, Das A, Barochia A, El-Bayoumy K, Belani CP. Proteomic profiling of human plasma identifies apolipoprotein E as being associated with smoking and a marker for squamous metaplasia of the lung. Proteomics. 2015;15:3267–77.
Luo J, Song J, Feng P, Wang Y, Long W, Liu M, Li L: Elevated serum apolipoprotein e is associated with metastasis and poor prognosis of non-small cell lung cancer. Tumour Biol 2016
Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Staging system for breast cancer: revisions for the 6th edition of the ajcc cancer staging manual. Surg Clin North Am. 2003;83:803–19.
Cibeira GH, Giacomazzi J, Aguiar E, Schneider S, Ettrich B, De Souza CI, Camey S, Caleffi M, Weber B, Ashton-Prolla P, Moriguchi EH. Apolipoprotein e genetic polymorphism, serum lipoprotein levels and breast cancer risk: a case-control study. Molecular and clinical oncology. 2014;2:1009–15.
Saadat M. Apolipoprotein e (APOE) polymorphisms and susceptibility to breast cancer: a meta-analysis. Cancer research and treatment : official journal of Korean Cancer Association. 2012;44:121–6.
Mahley RW, Rall Jr SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37.
Uen YH, Liao CC, Lin JC, Pan YH, Liu YC, Chen YC, Chen WJ, Tai CC, Lee KW, Liu YR, Lin HT, Lin CY. Analysis of differentially expressed novel post-translational modifications of plasma apolipoprotein E in Taiwanese females with breast cancer. J Proteome. 2015;126:252–62.
Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS One. 2012;7:e47469.
Mrkonjic M, Chappell E, Pethe VV, Manno M, Daftary D, Greenwood CM, Gallinger S, Zanke BW, Knight JA, Bapat B. Association of apolipoprotein E polymorphisms and dietary factors in colorectal cancer. Br J Cancer. 2009;100:1966–74.
Oue N, Hamai Y, Mitani Y, Matsumura S, Oshimo Y, Aung PP, Kuraoka K, Nakayama H, Yasui W. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004;64:2397–405.
Su WP, Chen YT, Lai WW, Lin CC, Yan JJ, Su WC. Apolipoprotein E expression promotes lung adenocarcinoma proliferation and migration and as a potential survival marker in lung cancer. Lung Cancer. 2011;71:28–33.
Boylan KL, Andersen JD, Anderson LB, Higgins L, Skubitz AP. Quantitative proteomic analysis by iTRAQ(R) for the identification of candidate biomarkers in ovarian cancer serum. Proteome Sci. 2010;8:31.
Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332:3–10.
Acknowledgments
This work was supported by grants from the Specialized Research Fund for the Science and Technology Department of Guangdong Province (Grant No. 2014A020212477 to LSL, 2014A020212720 to JML), the Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030313035 to LSL), the Doctoral Program of Higher Education of China (Grant No. 20130171120069 to LSL), and the Science and Technology Department of Guangzhou City, China (Grant No. 201400000004-2 to ML). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Authors’ contributions
LSL and JML designed the experiment, interpreted the data, and prepared the manuscript. XDX, JXW, LJY, JHB, PNF, WQL, HH, PJL, YSC, ML, JML, and LSL conducted the experiment, collected the data, and helped to prepare the manuscript. All authors read and approved the final manuscript.
Additional information
Xiangdong Xu and Jianxin Wan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, X., Wan, J., Yuan, L. et al. Serum levels of apolipoprotein E correlates with disease progression and poor prognosis in breast cancer. Tumor Biol. 37, 15959–15966 (2016). https://doi.org/10.1007/s13277-016-5453-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5453-8