Abstract
HCRP1 has been reported to have tumor suppressive function. However, its expression pattern and function in human non-small cell lung cancer (NSCLC) remain obscure. This study aims to explore clinical significance of HCRP1 in NSCLC. Immunohistochemical results showed high HCRP1 protein in normal bronchial epithelial tissue and downregulated HCRP1 expression in 47/98 lung cancer specimens. HCRP1 downregulation correlated with clinical stage (p = 0.0203), nodal status (p = 0.0168), and poor patient prognosis (log-rank, p = 0.0076). Univariate analysis showed that TNM stage (p < 0.0001) and HCRP1 (p = 0.0098) were significant prognostic factors; Cox regression model showed that TNM stage serves as an independent prognostic factor (p = 0.0011). We also found that HCRP1 was downregulated in lung cancer cells compared with normal HBE cells. HCRP1 plasmid transfection in H1299 cells inhibited proliferation, cell cycle progression, and invasion. HCRP1 depletion in A549 cells showed the opposite biological effects. In addition, we found that HCRP1 could inhibit MAPK and AKT signaling with downregulation of ERK and AKT phosphorylation, cyclin proteins, Bcl2 and MMP9, while HCRP1 depletion activated ERK and AKT signaling. The level of EGFR phosphorylation was also inhibited by HCRP1. In addition, we found that HCRP1 depletion confers multidrug resistance in H1299 cells. We employed paclitaxel and cisplatin in A549 cells with HCRP1 depletion. HCRP1 depletion decreased the effect of paclitaxel and cisplatin in A549 cells. Treatment with EGFR inhibitor AG1478 and AKT inhibitor LY249004 abolished the effect of HCRP1 depletion on drug resistance. In conclusion, the present study demonstrate that HCRP1 is downregulated in NSCLC and regulates proliferation, invasion, and drug resistance through modulation of EGFR signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide and the incidence of non-small cell lung cancer (NSCLC) is increasing [1–3]. Overall, the 5-year survival rate has remained at 15 % for the past two decades. Although targeted therapies have been established, genetic mutations causing activation of these gene products are identified only in a limited number of cancers [4–7]. On the other hand, a variety of complex genetic, epigenetic, and microenvironmental factors play important roles in the regulation of cancer cell survival and invasion. Hence, it is necessary to identify specific molecular mechanisms involved in the regulation of lung cancer cell aggressiveness.
Several studies have shown that HCRP1 was downregulated in human cancers, suggesting involvement of HCRP1 in carcinogenesis. HCRP1 (hepatocellular carcinoma related protein 1), is a member protein of endosomal sorting complexes required for transport (ESCRT)-I, which functions as membrane trafficking complex, mediating internalization of ubiquitinated membrane proteins [8]. It has been reported that HCRP1 was downregulated in hepatocellular carcinoma, oral cancer, breast cancer, and ovarian cancer [9–12]. HCRP1 downregulation could result in cetuximab resistance in cancer cell lines [12]. To data, the expression pattern and biological role of HCRP1 in human lung cancer remain unclear.
In the present study, we investigated the expression pattern of HCRP1 protein in 98 NSCLC samples using immunohistochemistry. We also examined the biological functions of HCRP1 in several lung cancer cell lines using plasmid transfection and siRNA knockdown. In addition, we explore the possible mechanisms of HCRP1 induced effect on cancer cells.
Materials and methods
Tissue samples
The study was approved by the Local Ethics Committee of Jinzhou Medical University. Ninety-eight NSCLC patients with surgical resection in The First Affiliated Hospital of Liaoning Medical University between 2005 and 2015 were included for study. Written informed consent was obtained. Tumor classification was obtained according to WHO classification (2004).
Immunohistochemistry
For immunohistochemistry, the expression of HCRP1 was then detected using EliVision plus kit (Maixin, Fuzhou, China). Tissue sections (3 μm) were incubated with rabbit anti-HCRP1 antibody (1:200, Sigma, USA) overnight at 4 °C. Diaminobenzidine visualization (Maixin, Fuzhou, China) was then performed and the slides were counterstained with hematoxylin.
Two independent pathologist examined all tumor slides randomly. Immunostaining of HCRP1 was scored following a scoring system by evaluating both intensity and percentage of staining. The intensity of HCRP1 was classified as 0 (none), 1 (weak), and 2 (strong). The percentages of HCRP1 positive cells were classified as 0 (0 %), 1 (1–25 %), 2 (26–50 %), 3 (51–75 %), and 4 (76–100 %). Each score was multiplied to get a final score from 0 to 8. HCRP1 status was indicated as HCRP1 low expression (score ≤ 4) and HCRP1 high expression (score > 4).
Cell culture and transfection
Human NSCLC cell lines A549, H1299, H157, H460, and normal bronchial epithelial cell line HBE were obtained from ATCC. RPMI-1640 (Gibco, USA) with 10 % fetal bovine serum was used as culture medium. pCMV6-HCRP1 plasmid and the pCMV6 empty vector were obtained from Origene company (Origene, MD, USA). Plasmid transfection was performed using Lipofectamine 3000 reagent (Invitrogen, USA) according to manufacturer’s instructions. HCRP1 siGENOME siRNA pool (M-016,816-00-0005), which was composed of 4 siRNA sequence, was obtained from Dharmacon (GE Healthcare, USA). siGENOME Non-Targeting siRNA Pool (D-001206-13-05) was used as negative control. Dharmafect1 (GE Healthcare, USA) reagent was used for siRNA transfection.
Western blotting
Total proteins from cells were extracted in lysis buffer and quantified using the Bradford method. Protein (30 μg) was separated by SDS-PAGE. Samples were transferred to PVDF membranes (Millipore, Billerica, MA, USA) and incubated overnight at 4 °C with antibody against HCRP1 (1:800, Sigma, USA), cyclin D1, MMP2, MMP9 (1:1000, Cell Signaling, USA), and GAPDH (1:500, Santa Cruz, USA). After incubation with HRP-coupled anti-mouse or rabbit IgG antibody (1:1000 dilution, Cell Signaling Technology, USA) at 37 °C for 2 h. Target proteins on PVDF membrane were visualized using Pierce ECL kit and captured using a DNR BioImaging System (DNR, Jerusalem, Israel).
After isolation of cell proteins, which were quantified using the Bradford method, electrophoresis was performed and samples were transferred to PVDF membranes (Millipore, MA, USA). Membranes were incubated with primary antibodies including cyclin D1, cyclin E, MMP9, GAPDH (Santa Cruz Biotechnology), HCRP1 (Sigma), p-AKT, p-ERK, p-EGFR, AKT, ERK, and EGFR (Cell Signaling Technology). Proteins were visualized using ECL and images were captured using DNR BioImaging System (DNR, Jerusalem, Israel).
Cell viability
For cell viability, 3-(4,5 dimethylthiazol-2-yl)-2,5diphenyl-tetrazolimbromide assay (MTT) was carried out in triplicate. Briefly, after adding MTT solution and incubating for 4 h, culture medium was removed and remaining MTT formazan was dissolved in 150 μl of DMSO. Solution was measured at 490 nm.
Colony assay
For the clonogenic assay, cells were seeded in 6-cm plate and cultured for 2 weeks. Colonies arising from survival cells were fixed and stained with Giemsa and their numbers were calculated.
Matrigel invasion assay
Matrigel invasion assay was performed with 24-well transwell chamber from Costar. The inserts were coated with 20–25 μl Matrigel (1:6 dilution, BD, USA). Cells were transferred to the upper chamber and medium containing 15 % serum was added to the lower chamber. After 16–20 h, the invaded cells were stained using hematoxylin.
Statistical analysis
SPSS 12 for Windows was used for all statistical analyses. χ 2 test was used to compare immunohistochemical results. Survival curves were analyzed by Kaplan-Meier method and compared by the log-rank test. Cell experiment results were analyzed by means of Student’s t test. All p values are based on a two-sided statistical analysis, and p < 0.05 was considered to indicate statistical significance.
Results
HCRP1 expression and clinicopathological characteristics in NSCLC
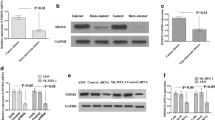
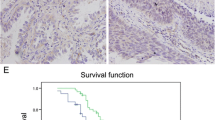
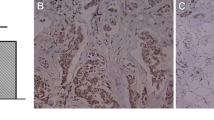
We examined HCRP1 protein in 98 NSCLC specimens and 10 adjacent normal lung tissue by immunohistochemistry. Representative staining figures of HCRP1 protein are listed in Fig. 1. Strong cytoplasmic HCRP1 immunostaining was found in normal bronchial epithelial cells. In alveolar cells, HCRP1 was weak compared to bronchial epithelia (Fig. 1a, b). Fifty-one cases of lung cancer tissues showed high HCRP1 staining and 47 cases (47.9 %) showed low HCRP1 staining (Fig. 1c–f). Patients’ clinicopathological characteristics and their relationship with HCRP1 status are listed in Table 1. The results showed that low HCRP1 status significantly correlated with higher TNM stage (stages III and II vs stage I, p = 0.0203) and nodal metastasis (p = 0.0168). HCRP1 status did not correlate with age, gender, histology, and differentiation (p > 0.05). In addition, Kaplan-Meier survival analysis showed patients with low HCRP1 have lower overall survival rate compared with those with high HCRP1 expression (p = 0.0076, log rank test; Fig. 2). Univariate analysis showed that TNM stage and HCRP1 were significant prognostic factors (TNM stage: hazard ratio, 2.011, p < 0.0001; HCRP1: hazard ratio, 0.476, p = 0.0098). Multivariate analysis with Cox regression model showed that TNM stage serves as an independent prognostic factor (TNM stage: hazard ratio, 1.864, p = 0.0011; Table 2).
Expression pattern of HCRP1 in non-small cell lung cancers. a Strong cytoplasmic HCRP1 staining in normal bronchial epithelial cells. b Weak HCRP1 staining in alveolar cells. c Negative HCRP1 staining in lung adenocarcinoma. d Negative HCRP1 staining in squamous cell carcinoma. e Positive cytoplasmic HCRP1 staining in lung adenocarcinoma. d Positive HCRP1 staining in squamous cell carcinoma (magnification ×400)
HCRP1 regulates lung cancer cell proliferation and invasion
We examined HCRP1 protein expression in normal bronchial cell line HBE and a panel of lung cancer cell lines including A549, H1299, H157, and H460. HCRP1 protein expression was lower in NSCLC cell lines compared with HBE, especially in H1299 and H157 cells (Fig. 3a). An HCRP1 expressing vector was used to transfect H1299 adenocarcinoma cell line. HCRP1 siRNA was employed in A549 cells to deplete its endogenous expression. Plasmid and siRNA transfection efficiency was examined by western blot analysis (Fig. 3b).
MTT assay showed that HCRP1 overexpression in H1299 cells inhibited proliferation rate and number of colony formation (Fig. 4a, b). HCRP1 depletion in A549 cells increased cell growth rate and colony number. To examine the effect of HCRP1 on cancer cell invasion, Matrigel invasion assay was performed in both cell lines. The results showed that HCRP1 transfection inhibited invading ability of H1299 cells while HCRP1 depletion increased invading ability (Fig. 4c).
HCRP1 inhibits proliferation and invasion in lung cancer cells. a MTT assay showed that in H1299 cells transfected with HCRP1 plasmid. The growth rate was lower compared with control. While HCRP1 siRNA upregulated cell growth in A549 cell line. b Colony formation showed that HCRP1 overexpression in H1299 cells decreased number of colony formation. HCRP1 siRNA increased the colony number of A549 cells. c Matrigel invasion assay showed that HCRP1 decreased cell invading ability while HCRP1 depletion increased invading cell number. *p < 0.05
To confirm the HCRP1 function, rescue experiment was performed using HCRP1 plasmid in A549 cells with HCRP1 depletion. As shown in Supplement Fig. 1, HCRP1 rescue could abolish the growth and invasion promoting effects of HCRP1 depletion in A549 cells.
HCRP1 inhibits cell cycle and regulates cyclin proteins, Bcl-2 and MMP9
The above results showed that HCRP1 inhibited lung cancer proliferation and invasion. To further explore the possible mechanism, we checked the change of cell cycle status after induction of siRNA and plasmid. As shown in Fig. 5, HCRP1 overexpression inhibited G1-S checkpoint transition in H1299 cell line, while in A549 cells HCRP1 depletion upregulated S phase percentage. We further checked a panel of cell cycle-related proteins and found that HCRP1 could inhibit cell cycle protein including cyclin D1 and cyclin E (Fig. 6). We also found that Bcl-2 expression was upregulated after HCRP1 depletion and downregulated after HCRP1 overexpression. In addition, the level of invasion related protein MMP9 was also downregulated by HCRP1.
HCRP1 regulates EGFR-ERK/AKT signaling cascade
Since HCRP1 regulates cyclin proteins and MMP9, we checked several related signaling pathways and found that HCRP1 transfection inhibited ERK and AKT phosphorylation while its depletion upregulated ERK and AKT phosphorylation. Previous reports indicated the association between HCRP1 and EGFR signaling, which functions upstream of both ERK and AKT signaling pathways [12]. Thus, we checked the level of EGFR phosphorylation. As shown in Fig. 6, HCRP1 inhibited p-EGFR expression in H1299 cells. These results indicated that HCRP1 inhibited lung cancer cell growth and invasion through EGFR-ERK signaling.
HCRP1 depletion mediates multidrug resistance in A549 cells
To determine the sensitivity of A549 cells to chemotherapy after HCRP1 depletion, the cells were treated with different concentration of cisplatin (DDP; 0, 10, 20, and 50 μmol/L) and paclitaxel (0, 10, 20, and 50 nmol/L) for 24 h. The survival rate was measured by MTT analysis. As shown in Fig. 7a, A569 survival rate was inversely correlated with drug dose. HCRP1 depletion increased the A549 survival rate with cisplatin and paclitaxel treatment. EGFR and AKT pathways have been reported to be involved in chemotherapy resistance. To assess the role of EGFR and AKT pathway in chemoresistance induced by HCRP1 depletion, we employed EGFR inhibitor AG1478 (100 nmol/L, 6 h) and AKT inhibitor LY294004 (5 μM, 6 h). As shown in Fig. 7b, blockage of either pathway could abolished the effect of HCRP1 in chemoresistance.
HCRP1 depletion mediates multidrug resistance in A549 cells. a Depletion of HCRP1 expression decreases A549 sensitivity to chemotherapeutic drugs including cisplatin (DDP) and paclitaxel. The absorbance rate of A549 cells treated with graded concentrations of cisplatin and paclitaxel for 24 h was measured by MTT assay. Cell survival rate was calculated by comparing absorbance of treated groups to that of untreated group. *p < 0.05 versus control siRNA group. b EGFR inhibitor AG1478 (100 nmol/L, 6 h) and AKT inhibitor LY294004 (5 μM, 6 h) were employed in A549 cells and cell survival rate was measured using MTT assay. In AG1478 and LY294004 group, the effect of HCRP1 on cell survival rate was not significant
Discussion
Downregulation of HCRP1 was first reported in hepatocellular carcinoma (HCC). HCRP1 was significantly reduced or undetected in HCC tissues and overexpression of HCRP1 significantly inhibited both anchorage-dependent and independent cell growth [11]. Low HCRP1 levels was reported in ovarian cancers with HER-2 overexpression and its downregulation led to increased EGFR and HER-2 in the SK-OV-3 and MDAH-2774 ovarian cell lines [12]. HCRP1 downregulation also correlated with poor prognosis in oral and breast cancers [9, 10]. However, there was no study concerning HCRP1 in non-small cell lung cancer.
In this study, immunohistochemical staining was applied to check HCRP1 expression in 98 lung cancer tissues. The results showed that 47 exhibited low HCRP1 levels. Low HCRP1 status significantly correlated with TNM stage and nodal metastasis, suggesting HCRP1 serve as a putative tumor suppressor. In accordance with previous researches, we found that patients with low HCRP1 had poor prognosis compared with those with high expression. Univariate analysis showed that TNM stage and HCRP1 were significant prognostic factors. Our results suggest that low HCRP1 status can be considered as an additional molecular marker for aggressive clinical course.
HCRP1 status was also examined in several lung cancer cell lines and we found low expression of lung cancer cells compared with HBE, which is in consistent with immunohistochemical results. By introducing HCRP1 plasmid and siRNA in lung cancer cells, we demonstrated that HCRP1 upregulation significantly decreased lung cancer proliferation, colony formation, and invasion. HCRP1 depletion in A549 cells significantly increased proliferation, colony formation, and invasion, which is in accordance with previous studies showing HCRP1 as a tumor suppressor [9, 10]. We further explore the effect of HCRP1 on cell cycle progression and related proteins. We found that HCRP1 overexpression inhibited S phase percentage and cyclin D1 and cyclin E expression, while HCRP1 depletion showed the opposite effects. Both cyclin D1 and cyclin E play pivotal roles during G1-S cell cycle progression and malignant proliferation of cancer cells. Many reports have showed that cyclin D1 and cyclin E were overexpressed in lung cancer and associated with malignant phenotype and poor prognosis [13, 14]. In addition, we found that HCRP1 inhibited lung cancer cell invasion with downregulation of MMP9 protein, which functions as an important mediator for invasion and metastasis. MMP9 also serves as prognostic indicator in lung cancer patients [15–18]. These results demonstrated that HCRP1 regulates proliferation and invasion through modulation of cell cycle and MMP9.
Further exploration of related signaling pathways revealed that HCRP1 overexpression inhibits ERK and AKT signaling. ERK pathway has been reported to activate cyclin and MMP9 transcription through AP-1 activation [15]. AKT has been shown to associate with malignant cell proliferation, cyclin activation, and cell survival [19, 20]. In addition, we showed HCRP1 overexpression suppressed EGFR phosphorylation while HCRP1 depletion activated EGFR phosphorylation. EGFR is the upstream regulator of both AKT and ERK pathways. These results were in consistence with the previous reports showing HCRP1 as an EGFR regulator.
To address the role of HCRP1 in drug resistance and explore the underlying mechanisms, two different types of chemotherapeutic drugs (cisplatin and paclitaxel) were used in A549 cells with HCRP1 depletion. EGFR inhibitor AG1478 and AKT inhibitor LY294004 were also used to assess the involvement of EGFR and AKT pathways during the development of drug resistance. With treatment of EGFR and AKT inhibitor, the effect of HCRP1 depletion on A549 cell survival was not significant. In addition, Bcl-2, an anti-apoptosis AKT target protein, was upregulated after HCRP1 depletion. AKT was reported to be involved in multidrug resistance in lung cancer [21–23]. EGFR activation was also related to cisplatin/paclitaxel resistance and EGFR inhibition sensitize tumor cells to chemotherapy [24–26]. Combination of EGFR inhibitor with cisplatin/paclitaxel offers a novel strategy for cancer treatment. Thus, these results revealed two new findings. First, the downregulation of HCRP1 decreased the sensitivity of NSCLC cells to chemotherapy in vitro. Second, the drug resistance by HCRP1 depletion was mediated, at least partly, through the EGFR-AKT pathway.
High levels of Bcl-2 has been associated with a more aggressive malignant phenotype and drug resistance to various chemotherapeutic agents in cancers. It has been reported that after long-term radiation and drug treatment, the Bcl2 protein level could be increased [27]. Pharmacologic agents targeting Bcl-2 could be considered when achieving better chemotherapy results. More and more agents with high specificity has been developed, providing approach to overcome cancer cell drug resistance [28]. Thus, we believe combination therapy with HCRP1 and Bcl-2 inhibitor could achieve better therapeutic results than HCRP1 alone.
In conclusion, the present study demonstrated that HCRP1 protein was downregulated in NSCLC and correlated with advanced TNM stage, nodal status, and poor prognosis. HCRP1 inhibits proliferation, invasion, and drug resistance through inhibition of EGFR/ERK/AKT signaling cascade. HCRP1 could serve as a new therapeutic target for lung cancer and further studies are needed to explore the significance of HCRP1 in lung cancers with EGFR activating mutation.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52.
Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8.
Dong QZ, Wang Y, Dong XJ, et al. CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann Surg Oncol. 2011;18:857–65.
Dong QZ, Zhao Y, Liu Y, et al. Overexpression of SCC-S2 correlates with lymph node metastasis and poor prognosis in patients with non-small-cell lung cancer. Cancer Sci. 2010;101:1562–9.
Fidler IJ, Kripke ML. Genomic analysis of primary tumors does not address the prevalence of metastatic cells in the population. Nat Genet. 2003;34:23 .author reply 25
van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
Bache KG, Slagsvold T, Cabezas A, Rosendal KR, Raiborg C, Stenmark H. The growth-regulatory protein HCRP1/hVps37A is a subunit of mammalian ESCRT-I and mediates receptor down-regulation. Mol Biol Cell. 2004;15:4337–46.
Xu J, Yang W, Wang Q, et al. Decreased HCRP1 expression is associated with poor prognosis in breast cancer patients. Int J Clin Exp Pathol. 2014;7:7915–22.
Perisanidis C, Savarese-Brenner B, Wurger T, et al. HCRP1 expression status is a significant prognostic marker in oral and oropharyngeal cancer. Oral Dis. 2013;19:206–11.
Xu Z, Liang L, Wang H, Li T, Zhao M. HCRP1, a novel gene that is downregulated in hepatocellular carcinoma, encodes a growth-inhibitory protein. Biochem Biophys Res Commun. 2003;311:1057–66.
Wittinger M, Vanhara P, El-Gazzar A, et al. hVps37A status affects prognosis and cetuximab sensitivity in ovarian cancer. Clin Cancer Res. 2011;17:7816–27.
Liu J, Liao Q, Zhang Y, Sun S, Zhong C, Liu X. Cyclin D1 G870 A polymorphism and lung cancer risk: a meta-analysis. Tumour Biol. 2012;33:1467–76.
Liu Y, Wang L, Lin XY, et al. The transcription factor DEC1 (BHLHE40/STRA13/SHARP-2) is negatively associated with TNM stage in non-small-cell lung cancer and inhibits the proliferation through cyclin D1 in A549 and BE1 cells. Tumour Biol. 2013;34:1641–50.
Dong QZ, Wang Y, Tang ZP, et al. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am J Pathol. 2013;182:954–64.
Jian H, Zhao Y, Liu B, Lu S. SEMA4b inhibits MMP9 to prevent metastasis of non-small cell lung cancer. Tumour Biol. 2014;35:11051–6.
Song H, Tian Z, Qin Y, Yao G, Fu S, Geng J. Astrocyte elevated gene-1 activates MMP9 to increase invasiveness of colorectal cancer. Tumour Biol. 2014;35:6679–85.
Feng X, Miao G, Han Y, Xu Y. CARMA3 is overexpressed in human glioma and promotes cell invasion through MMP9 regulation in A172 cell line. Tumour Biol. 2014;35:149–54.
Shimura T, Noma N, Oikawa T, et al. Activation of the AKT/cyclin D1/Cdk4 survival signaling pathway in radioresistant cancer stem cells. Oncogenesis. 2012;1:e12.
Kumar AP, Bhaskaran S, Ganapathy M, et al. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin Cancer Res. 2007;13:2784–94.
Fan DP, Zhang YM, Hu XC, Li JJ, Zhang W. Activation of AKT/ERK confers non-small cell lung cancer cells resistance to vinorelbine. Int J Clin Exp Pathol. 2014;7:134–43.
Wang M, Liu ZM, Li XC, Yao YT, Yin ZX. Activation of ERK1/2 and Akt is associated with cisplatin resistance in human lung cancer cells. J Chemother. 2013;25:162–9.
Li H, Schmid-Bindert G, Wang D, et al. Blocking the PI3K/AKT and MEK/ERK signaling pathways can overcome gefitinib-resistance in non-small cell lung cancer cell lines. Adv Med Sci. 2011;56:275–84.
Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1alpha in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia. 2015;17:190–200.
Chen S, Liu X, Gong W, et al. Combination therapy with VEGFR2 and EGFR siRNA enhances the antitumor effect of cisplatin in non-small cell lung cancer xenografts. Oncol Rep. 2013;29:260–8.
Hartmann JT, Kollmannsberger C, Cascorbi I, et al. A phase I pharmacokinetic study of matuzumab in combination with paclitaxel in patients with EGFR-expressing advanced non-small cell lung cancer. Investig New Drugs. 2013;31:661–8.
You S, Li R, Park D, et al. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther. 2014;13:606–16.
Han B, Park D, Li R, et al. Small-molecule Bcl2 BH4 antagonist for lung cancer therapy. Cancer Cell. 2015;27:852–63.
Acknowledgments
This study was supported by:1. Doctoral Promoter Project of Science and Technology Department of Liaoning Province of China (No. 20141135).2. Clinical Capability Construction Project for Liaoning Provincial Hospitals (NO.LNCCC-D29-2015).3. The President Fund of the Liaoning Medical University on the Clinical Medical Construction (NO.XZJJ 20130203).4. The Science and Technology Project of Science and Technology Department of Liaoning, Province of China (No. 2012225019).5. General Project of Education Department of Liaoning, Province of China (No. L2015323).6. General Project of Education Department of Liaoning, Province of China (No. L2012300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Yaming Du and Peng Wang are contribute equally to this work.
Electronic supplementary material
Figure 1
Rescue experiment. a Rescue experiment was performed by transfecting HCRP1 plasmid into HCRP1 depleted A549 cells (24 h after siRNA treatment). MTT assay showed that HCRP1 rescue could partly abolished the growth promoting effect of HCRP1 depletion. b Matrigel invasion assay showed that HCRP1 rescue could abolished the invasion promoting effect of HCRP1 depletion in A549 cells. (JPEG 29 kb)
Rights and permissions
About this article
Cite this article
Du, Y., Wang, P., Sun, H. et al. HCRP1 is downregulated in non-small cell lung cancer and regulates proliferation, invasion, and drug resistance. Tumor Biol. 37, 15893–15901 (2016). https://doi.org/10.1007/s13277-016-5416-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5416-0