Abstract
Homeobox C8 (HOXC8) has been implicated in cell growth, migration, and metastasis of various cancers, yet its role in osteosarcoma remains to be explored. In the present study, resected osteosarcoma specimens from 50 patients were enrolled to evaluate the expression of HOXC8 protein by immunohistochemistry (IHC). In vitro and in vivo assays were used to determine the effect of HOXC8 on cell growth, migration, and tumor growth. HOXC8 expression was observed in 31 (62.0 %) of the 50 primary tumors and significantly associated with poorly or un-differentiated specimens (P = 0.031) and larger tumor size (P = 0.049). Survival analysis demonstrated that HOXC8 is a candidate predictive factor in predicting patients’ outcome and chemotherapeutic effect. HOXC8 knockdown led to inhibition of tumor cell proliferation and migration in vitro by inhibiting MMP-9 expression and tumor growth in vivo. Our results strongly suggest that HOXC8 is involved in the tumorigenesis of osteosarcoma and might serve as a novel predictor for patients’ outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS) is a highly aggressive bone tumor with a poor outcome in populations of children and young adolescents [1, 2]. The risk factors for OS involved certain rare and inherited syndromes, such as Li–Fraumeni syndrome, Bloom syndrome, hereditary retinoblastoma, and Werner syndrome [3]. The most effective methods for patients with OS are curative resection of the primary tumor with standard chemotherapy administered before and after surgery [4]. For those diagnosed at early stage, patients with OS might achieve a 5-year survival duration of 60–70 %. However, the median survival time reduced to about 20 months when the disease recurred or metastasized [5, 6]. Meanwhile, constitutive and acquired resistance to cytotoxic chemotherapy is always a common event [7]. Therefore, a deeper understanding of the underlying mechanism of tumor biology will help us in identifying promising diagnostic and prognostic biomarkers and/or therapeutic targets for patients with OS.
Homeobox C8 (HOXC8) belongs to the 39-member HOX family, which plays an important role in morphogenesis in all multicellular organisms [8]. HOXC8 is one of the several homeobox HOXC genes located in a cluster on chromosome 12 and plays a role in the regulation of cartilage differentiation [9]. Recent evidences focused the role of HOXC8 in the tumorigenesis of various cancer types [10–15]. HOXC8 was elevated in breast cancer, esophageal squamous cell carcinoma, hepatocellular carcinoma, etc. In breast tumor cell lines, HOXC8 is responsible for breast cancer cell growth, migration, and metastasis [12, 16]. However, the expression pattern of HOXC8 in OS is not well studied. It is of our interest to detect HOXC8 expression profiles in OS and to investigate its possible role for a tumorigenic event. In the present study, we revealed evaluated expression of HOXC8 in OS and determined the clinical value of HOXC8 in predicting patients’ outcome. Moreover, functional validation confirmed the pro-oncogenic role of HOXC8 on cell growth and invasiveness in vitro and in vivo.
Materials and methods
Cell culture and tissue samples
The osteosarcoma cell U2OS was purchased from the Cell Center of Chinese Academy of Sciences, Shanghai, China. U2OS was cultured in DMEM (Gibco, USA) with 10 % fetal bovine serum (Invitrogen Corp, Grand Island, NY) and grown in a 37 °C humidified atmosphere containing 5 % CO2.
The clinical samples for immunohistochemistry (IHC) were obtained from the Department of Pathology, Changzheng Hospital, Shanghai, China. Fifty patients with osteosarcoma between 2004 and 2008 were enrolled in this study. Detailed information was listed in Table 1. Forty-one patients received 2 or 4 cycles of preoperative chemotherapy (MTX-DDP-ADM). All these patients were available for follow-up by telephone or through Center for Disease Control and Prevention, China. The median follow-up for these patients was 22 months (range, 1–51 months). The Institutional Review Board of Changzheng Hospital approved the use of the tissues and clinical information. An informed consent was obtained from each patient or their guardians.
Immunohistochemistry and evaluation of the results
Four-micrometer-thick sections were prepared and performed for anti-HOXC8 (H55, Santa Cruz Biotechnology; dilution, 1:100) staining as described previously. An S-p (streptavidin-biotin) kit (#KIT-9720, MAIXIN, Fuzhou, China) was used to visualize the status of antibody binding to the tissues. Hematoxylin was used to counterstain the nucleus. The primary antibody was replaced by PBS as negative control.
Two pathologists evaluated the HOXC8 staining independently under an Olympus CX31 microscope (Olympus, Center Valley, PA) blinded to the clinical information. The mean percentage of positive tumor cells was ranged from 0 to 100 %. The staining intensity was defined as follows: negative, 0; weak, 1; moderate, 2; and intense, 3. At last, a weighted score (% × intensity of staining) was generated for each case ranging from 0 (0 × 0) to 3 (100 % × 3) [17].
Transfection of plasmids and cell proliferation assay
The HOXC8-short hairpin RNA (shRNA) plasmids were purchased from GECEM biotechnology, Shanghai, China. 1 × 105 U2OS cells were seeded in six-well plates and transfected with 5 ng HOXC8-shRNA and vector plasmids per well using Lipofectamine™ 2000 reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions. Sixteen hours after transfection, cells were digested and 5000 cells per well were seeded in 96-well plates. At 24 h and 48 h, CCK8 assay (Dojindo Kumamoto, Japan) was performed to measure the absorbance. Relative cell viability was calculated according to the ratio between HOXC8-shRNA group and control group.
Transwell assay
Tumor cells transfected with HOXC8-shRNA or empty vector were collected and re-suspended in serum-free media at a density of 1 × 105 cells/ml. Two hundred microliter cell suspension was placed into the top chamber of the transwell, and 0.5 ml DMEM with 5%FBS was placed into the lower chamber. Sixteen hours after incubation, the filters were taken out, rinsed with PBS, fixed with methanol, and stained with 0.5 % crystal violet reagent for 15 min. Only migrated cells on the lower side of the filter were calculated under an Olympus CX31 microscope.
Western blot analysis
Whole-cell lysates (P0013B, Beyotime, Shanghai, China) were collected and prepared from HOXC8-siRNA or empty vector transfected cells. The lysates were resolved by SDS/PAGE and transferred electrophoretically to PVDF membrane (Bio-Rad Lab., Hercules, CA, USA). The membranes were probed with anti-HOXC8 (H55, Santa Cruz Biotechnology; dilution, 1:1000), which were detected using an enhanced chemiluminescence (ECL) kit (Santa Cruz, CA, USA).
Animal models
Subcutaneous tumor xenograft models were used to assess the inhibitory effect of HOXC8 knockdown. Generally, HOXC8 knockdown and control tumor cells was digested and suspended in PBS (1 × 106 cells in 0.1 mL PBS). Then these cells were injected subcutaneously into the right flank of 4-week-old male Balb/c nude mice, respectively (n = 5). At 15 days, all animals were sacrificed and the tumors collected. Tumor volume (mm3) was calculated as V = 0.52 (length × width × depth). All animal experiments were approved by the Animal Ethics Committee of the Second Military Medical University.
Statistical analysis
Statistical analysis was performed using the SPSS 16.0 statistical software program for Microsoft Windows. χ 2 statistics tests were used for the analysis of correlation between HOXC8 expression and clinicopathological variables. The Kaplan–Meier method was used to estimate survival rates of OS patients. The significance of the in vitro or in vivo results was determined by using the Student t test (two tailed). A two-sided P < 0.05 was defined as statistically significant.
Results
HOXC8 expression in patients with osteosarcoma
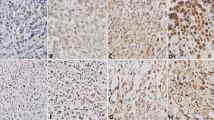
Firstly, we detected HOXC8 expression in a panel of 50 osteosarcoma specimens and matched non-cancerous tissues using IHC. HOXC8 was preferentially cytoplasmic localized. The non-cancerous tissues were negatively for HOXC8 staining (Fig. 1a). However, HOXC8 was upregulated in tumor cells analyzed by Student t test (t = 5.705, P < 0.001) (Fig. 1b–f). Overexpression of HOXC8 expression was observed in 62.0 % (31/50) OS samples.
Analysis of HOXC8 expression in human osteosarcoma and non-cancerous tissues. a Normal tissues showed negative staining of HOXC8. b Negative staining of HOXC8 in cancer cells. c Weak staining of HOXC8 in cancer cells. d Moderate staining of HOXC8 in cancer cells. e Strong staining of HOXC8 in cancer cells. f Graphical representation of the intensity of HOXC8 staining in tumor (T) and normal (N) tissues. Original magnification 200×
Association between HOXC8 expression and clinicopathologic characteristics of osteosarcoma
Next, we analyzed the correlation between HOXC8 expression and clinicopathological variables of osteosarcoma. Table 1 revealed no significant relationship between HOXC8 expression and gender, age, tumor location, and disease stage. However, HOXC8 overexpression was significantly associated with tumor size and tissue differentiation. HOXC8 was more often expressed in larger tumors (75.0 %) than in smaller ones (47.6 %, P = 0.049) (Table 1). Meanwhile, high-/moderate-differentiated tumor cells had lower expression level of HOXC8 than poor-/un-differentiated tumors (52.8 vs. 85.7 %, P = 0.031) (Fig.2a, b). Even in the same samples, HOXC8 was preferentially localized in poorly differentiated area (Fig.2c–e).
Expression of HOXC8 in different differentiated tumors. a Weak staining of HOXC8 in well-differentiated cancer cells. b Strong staining of HOXC8 in poorly differentiated cancer cells. c Heterogeneity of HOXC8 expression in the same specimen. d Positive staining of HOXC8 in poorly differentiated area from c specimen. e Negative staining of HOXC8 in moderate-differentiated area from c specimen. Original magnification for a, b, c 100×. Original magnification for d, e 200 ×
Relationship of HOXC8 expression with patients’ outcome in osteosarcoma
We then analyzed the factors associated with overall survival in this cohort of 50 patients with OS. Among those clinicopathological variables, only TNM was related with patients’ outcome (36 months for I/II stage vs. 22 months for III stage; χ 2 = 9.211, P = 0.002). Patients with HOXC8-positive staining tumors had a shorter median survival duration than those with HOXC8-negative staining tumors (14 months vs. 36 months; χ 2 = 5.744, P = 0.017) (Fig.3).
Twenty-three of the 41 patients who received preoperative chemotherapy showed positive staining of HOXC8. In the 23 cases, 14 cases showed sharply reduced expression of HOXC8 in the resected tumor specimens after chemotherapy than that in biopsy specimens before chemotherapy (t = 3.135, P = 0.0057). However, the other nine cases did not. Survival analysis revealed that the 14 cases had a longer survival duration that the other nine cases (26 months vs. 9 months; χ 2 = 6.950, P = 0.008) (Fig.4).
Analysis of HOXC8 expression in osteosarcoma specimens before and after adjuvant chemotherapy. a Strong staining of HOXC8 in cancer specimens before chemotherapy. b Weak staining of HOXC8 in cancer specimens after chemotherapy. Original magnification 100×. c Graphical representation of the intensity of HOXC8 staining in cancer specimens before and after chemotherapy. d Kaplan–Meier curves of overall survival in patients with osteosarcoma according to the altered expression of HOXC8 before and after chemotherapy
Silencing HOXC8 inhibits proliferation and migration of U20S cells
The functional significance of U2OS cells was further investigated by silencing HOXC8 using HOXC8-shRNA. Silencing HOXC8 led to reduced cell viability and the cellular trans-migration ability of U2OS cells compared with controls (Fig. 5a–c). In addition, HOXC8 knockdown significantly reduced tumor growth as compared with the control groups (Fig. 5d). We also observed that the level of MMP9 was reduced when HOXC8 was silenced (Fig.5a).
The effect of HOXC8 knockdown on the proliferation and migration ability of OS cells in vitro and in vivo. a Western blot analysis showing the protein level of HOXC8 and MMP-9 in U2OS cells 48 h after transfection. b The rate of proliferation of U2OS cells after transfection with HOXC8-shRNA. c Representative pictures of cell migration assay (upper) and graphical presentation of the migration numbers of cells transfected with HOXC8-shRNA and the control plasmid (lower). d Representative pictures of tumors resected from subcutaneous tumor xenograft models (upper) and tumor volume (lower). *P < 0.05, **P < 0.01, compared with the control group
Discussion
In the present study, we provided the first evidences that HOXC8 was elevated in osteosarcoma and its overexpression is significant associated with (1) poor-/un-differentiation of tumor cells, (2) tumor size, (3) drug resistance, and (4) shorter overall survival durations than those without HOXC8 expression. Moreover, silencing HOXC8 in cancer cells inhibited cell proliferation and migration in vitro and tumor growth in vivo by downregulating the level of MMP-9 protein. The above findings strongly suggest that HOXC8 plays a crucial role in osteosarcoma development and progression.
Numerous genomic and molecular alterations contributed to the development and progression of OS [6]. These include gene mutations, amplifications and deletions, overexpression, and activation, which induced cell proliferation and tumor growth, drug resistance, and disease progression [18]. The alteration of certain molecules or pathways (including PTEN, PI3K/Akt, CXCR4, and MMP9) has been proved to be responsible for the development of OS [19, 20]. In addition to these well-studied biomarkers, we here introduced a relatively novel biomarker, HOXC8. The key role of HOXC8 in tumor has been implicated by the fact that HOXC8 is dysregulated in various tumors including cervical, prostate, and breast cancer [12, 21, 22]. In the present study, HOXC8 was highly expressed in tumor cells but none in non-cancerous tissues. The high level of HOXC8 expression is significantly associated with poorly differentiated status. There is a tendency that HOXC8 expression is associated with larger tumor size. In line with previous reports, these findings confirmed the pro-oncogenic role of HOXC8 in tumorigenesis.
Pilot studies have tried to explore biomarkers for OS. No traditional markers were suitable for OS. It seems that only distant metastasis and histologic response to adjuvant chemotherapy (i.e., the extent of necrosis) are the widely accepted predictors of patients’ outcome. Recently, researchers have tried to introduce biomarkers into the survival model of OS. Numerous biomarkers (including HER2, CD44V6, MMP9, STAT3, etc.) have been found to be potential biomarkers for predicting survival of OS and even chemotherapy response [20, 23, 24]. In the present study, we further analyzed whether aberrant expression of HOXC8 was associated with survival and chemotherapy response of OS. Evidently, high expression of HOXC8 was associated with poor survival of OS. Moreover, those patients with significantly decreased expression of HOXC8 after chemotherapy had a better survival than those with not significant changes of HOXC8 expression after chemotherapy compared with before chemotherapy. The above results are in line with the previous study in hepatocellular carcinoma indicated that HOXC8 might serve as a novel predictor for chemotherapeutic effect and overall survival for patient with OS [11]. However, this study only enrolled a relative small cohort and the Cox regression model is unable to be carried out. A larger cohort needs to prove the predictive and prognostic value of HOXC8 in OS.
Given the elevated expression of HOXC8 in OS, we speculated that HOXC8 might impact OS tumorigenesis. Evidently, silencing HOXC8 impaired the ability of cell proliferation and migration of OS cells in vitro. Similarly, the ability of tumor growth was also inhibited by HOXC8 knockdown. These results were supported by a pilot study in breast cancer that HOXC8 could facilitate breast cancer cell migration by regulating the expression of genes essential for cell adhesion, migration, and tumorigenesis [12, 16]. Mechanistically, in OS cells, knockdown of expression of HOXC8 decreased the expression of MMP9, a molecule potentially related to tumor invasion [20]. The above results supported a crucial role for HOXC8 in tumor development and progression.
In summary, we confirmed HOXC8 as a novel predictive factor in human osteosarcoma, frequently upregulated in tumor cells. Increased expression of HOXC8 is a potential predictor for chemotherapeutic response and overall survival. Our findings further demonstrate silencing HOXC8 suppresses tumor cell proliferation, invasion, and tumor growth, suggesting a novel mechanism underlying the progression of osteosarcoma.
References
Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125:555–81.
Vander Griend RA. Osteosarcoma and its variants. Orthop Clin North Am. 1996;27:575–81.
Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. 2009;152:15–32.
Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–85.
Fagioli F, Aglietta M, Tienghi A, Ferrari S, Brach del Prever A, Vassallo E, Palmero A, Biasin E, Bacci G, Picci P, et al. High-dose chemotherapy in the treatment of relapsed osteosarcoma: an Italian sarcoma group study. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20:2150–6.
Anderson ME. Update on Survival in Osteosarcoma. Orthop Clin North Am. 2016;47:283–92.
Li S, Sun W, Wang H, Zuo D, Hua Y, Cai Z. Research progress on the multidrug resistance mechanisms of osteosarcoma chemotherapy and reversal. Tumour Biol. 2015;36:1329–38.
Gehring WJ, Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147–73.
Yueh YG, Gardner DP, Kappen C. Evidence for regulation of cartilage differentiation by the homeobox gene Hoxc-8. Proc Natl Acad Sci U S A. 1998;95:9956–61.
Lu S, Liu R, Su M, Wei Y, Yang S, He S, Wang X, Qiang F, Chen C, Zhao S et al. Overexpression of HOXC8 is associated with poor prognosis in epithelial ovarian cancer. Reprod Sci. 2016;23:944–54.
Xu P, Zhang X, Ni W, Fan H, Xu J, Chen Y, Zhu J, Gu X, Yang L, Ni R, et al. Upregulated HOXC8 expression is associated with poor prognosis and oxaliplatin resistance in hepatocellular carcinoma. Dig Dis Sci. 2015;60:3351–63.
Li Y, Chao F, Huang B, Liu D, Kim J, Huang S. HOXC8 promotes breast tumorigenesis by transcriptionally facilitating cadherin-11 expression. Oncotarget. 2014;5:2596–607.
YB D, Dong B, Shen LY, Yan WP, Dai L, Xiong HC, Liang Z, Kang XZ, Qin B, Chen KN. The survival predictive significance of HOXC6 and HOXC8 in esophageal squamous cell carcinoma. J Surg Res. 2014;188:442–50.
Adwan H, Zhivkova-Galunska M, Georges R, Eyol E, Kleeff J, Giese NA, Friess H, Bergmann F, Berger MR. Expression of HOXC8 is inversely related to the progression and metastasis of pancreatic ductal adenocarcinoma. Br J Cancer. 2011;105:288–95.
Manohar CF, Salwen HR, Furtado MR, Cohn SL. Up-regulation of HOXC6, HOXD1, and HOXD8 homeobox gene expression in human neuroblastoma cells following chemical induction of differentiation. Tumour Biol. 1996;17:34–47.
Li Y, Guo Z, Chen H, Dong Z, Pan ZK, Ding H, Su SB, Huang S. HOXC8-dependent cadherin 11 expression facilitates breast cancer cell migration through trio and Rac. Genes cancer. 2011;2:880–8.
Yu G, Fang W, Xia T, Chen Y, Gao Y, Jiao X, Huang S, Wang J, Li Z, Xie K. Metformin potentiates rapamycin and cisplatin in gastric cancer in mice. Oncotarget. 2015;6:12748–62.
Poos K, Smida J, Nathrath M, Maugg D, Baumhoer D, Neumann A, Korsching E. Structuring osteosarcoma knowledge: an osteosarcoma-gene association database based on literature mining and manual annotation. Database (Oxford). 2014. doi:10.1093/database/bau042.
Xi Y, Chen Y. Oncogenic and therapeutic targeting of PTEN loss in bone malignancies. J Cell Biochem. 2015;116:1837–47.
Ren Z, Liang S, Yang J, Han X, Shan L, Wang B, Mu T, Zhang Y, Yang X, Xiong S, et al. Coexpression of CXCR4 and MMP9 predicts lung metastasis and poor prognosis in resected osteosarcoma. Tumour Biol. 2015;37:5089–96.
Alami Y, Castronovo V, Belotti D, Flagiello D, Clausse N. HOXC5 and HOXC8 expression are selectively turned on in human cervical cancer cells compared to normal keratinocytes. Biochem Biophys Res Commun. 1999;257:738–45.
Waltregny D, Alami Y, Clausse N, de Leval J, Castronovo V. Overexpression of the homeobox gene HOXC8 in human prostate cancer correlates with loss of tumor differentiation. Prostate. 2002;50:162–9.
Ma Q, Zhou Y, Ma B, Chen X, Wen Y, Liu Y, Fan Q, Qiu X. The clinical value of CXCR4, HER2 and CD44 in human osteosarcoma: a pilot study. Oncol Lett. 2012;3:797–801.
Salas S, Jiguet-Jiglaire C, Campion L, Bartoli C, Frassineti F, Deville JL, Maues De Paula A, Forest F, Jezequel P, Gentet JC, et al. Correlation between ERK1 and STAT3 expression and chemoresistance in patients with conventional osteosarcoma. BMC Cancer. 2014;14:606.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board of Changzheng Hospital approved the use of the tissues and clinical information. An informed consent was obtained from each patient or their guardians. All animal experiments were approved by the Animal Ethics Committee of the Second Military Medical University.
Conflict of interest
We declare no conflicts of interest with any other person or units.
Rights and permissions
About this article
Cite this article
Cheng, L., Wei, X., Zhao, K. et al. The predictive potential and oncogenic effects of HOXC8 expression on osteosarcoma. Tumor Biol. 37, 14961–14967 (2016). https://doi.org/10.1007/s13277-016-5384-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5384-4