Abstract
Hepatocellular carcinoma (HCC) and hepatic cholangiocarcinoma (CC) are the most aggressive malignancies with a poor prognosis in humans, and hepatic cholangiocarcinoma (CC) exhibits greater malignant behaviour. Yes-associated protein (YAP) is an important downstream target of the Hippo signalling pathway. As an oncogene, it plays a vital role in the occurrence and development of tumours. Our study focuses on the clinical significance of YAP protein expression in HCC and CC. Furthermore, we sought to explore the different survival rates between HCC and CC. A total of 137 patients with HCC and 122 with CC after resection were evaluated by immunohistochemistry for the expression of YAP. Our results showed that positive expression rates of YAP were more frequently noted in CC 67.2 % (82/122) than in HCC 56.9 % (78/137) (P = 0.024). High YAP expression in HCC and CC was significantly associated with tumour size (P < 0.001 and P = 0.019, respectively), liver cirrhosis (P = 0.002 and P = 0.009, respectively), vascular invasion (P = 0.047 and P = 0.018, respectively), multiplicity (P = 0.019 and P = 0.015, respectively), and intrahepatic metastasis (P = 0.015 and P = 0.047, respectively). Importantly, recurrence-free survival and disease-specific survival rates were lower in CC with high YAP expression than in HCC with high YAP expression (P < 0.001 and P < 0.001, respectively). Overall, high YAP expression was more frequently found in CC than in HCC, and YAP overexpression was associated with poor survival rates in patients with HCC and CC. Targeting YAP treatment requires further prospective investigations in larger patient populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer (PLC) ranks as the second most common cancer worldwide and the leading cause of cancer-related deaths, approximately 745,000 deaths occur annually [1]. Hepatocellular carcinoma (HCC) and hepatic cholangiocarcinoma (CC) are two important pathological types of primary liver cancer. A previous study showed that CC has a poorer prognosis than HCC [2, 3]. There are many determining risk factors for PLC, such as age and sex (male), hepatitis B and C virus, exposure to toxins (aflatoxin), chronic alcohol abuse, and cirrhosis [4]. Despite the identification of many prognostic markers of PLC, such as alpha fetoprotein(AFP), vascular endothelial growth factor, and transforming growth factor β, in large-scale clinical and basic research projects, it is still impossible unable to accurately predict the overall survival rates of patients with HCC [5, 6]. Therefore, it is very important and urgent to find an effective biomarker to identify PLC patients at high risk for recurrence or metastasis and provide personalized therapy according to the predicted risk of recurrence.
The Hippo pathway was originally identified in genetic mutant screens for tumour suppressors in drosophila melanogaster [7, 8]. In mammals, the core molecules of the Hippo pathway include STE20-like protein kinase 1 (MST1 and MST2), SAV1 (Sav homologue), large tumour suppressor 1/2 LATS1/2 (Wts homologue), MOB1A/B (Mats homologue), and YAP (Yki homologue). As a transcription co-activator, YAP interacts with the PPXY motif-containing protein [9, 10]. If YAP is not inhibited by the Hippo pathway and remains in the nucleus, it will interplay with TEADs and activate the expression of several genes, such as CTGF, ErbB-4, and ITGB2 [8–13]. Therefore, Yes-associated protein (YAP) can influence tissue homeostasis, organ size, and cancer development and play a pivotal role in regulating cell proliferation. In recent years, functional studies have elucidated that YAP is an important oncogene and functions at a key crossroads of a complex network of cancer-causing signal pathways [14]. It has been demonstrated that the YAP gene exists in various human cancers, including pancreatic ductal adenocarcinoma, cutaneous melanoma, cervical cancer, and other malignant tumours [14–16].
However, YAP’s clinical significance and expression patterns in HCC and CC have not been well explored. In the present study, we studied YAP expression in HCC and CC tissues through immunohistochemistry and explored the possibility of YAP as a prognostic factor for PLC.
Materials and methods
Patients and tissue specimens
In this study, all patient specimens were collected from July 2003 to July 2009 in the Second Hospital Affiliated to Chongqing Medical University. Eleven patients were excluded because their follow-up periods were interrupted for unclear reasons. In total, 259 PLC patients were analysed. The specimens included 137 HCC cases and 122 CC cases. All tissue samples were immediately processed after surgical removal, fixed with 4 % formalin (pH 7.0) and embedded in paraffin for no longer than 24 h. Finally, diagnoses of HCC and CC were identified histologically by two experienced pathologists in the Department of Pathology Archives of the Second Hospital Affiliated to Chongqing Medical University using haematoxylin and eosin (HE) staining. The complete clinical and prognostic data for each tumour tissue sample were recorded.
The diagnosis of PLC met the World Health Organization (WHO) criteria for the study of liver disease. The parameters of pathological analysis included age at diagnosis, sex, tumour size, distant metastases, cirrhosis, hepatitis B virus infection, and serum AFP levels (ng/ml), which were obtained from patient medical records. Before the deadline of May 2015, all patients with HCC and CC underwent follow-up. The follow-up time ranged from 6 to 142 months, and the median follow-up time was 40 months. The study was conducted in accordance with the protocol approved by the Declaration of Helsinki and the guidelines of the Ethics Review Committee of Second Hospital Affiliated with Chongqing Medical University.
Immunohistochemical staining
To determine YAP expression in HCC and CC, we used immunohistochemical staining to detect in the protein in paraffin-embedded sections (4 μm). Briefly, xylene was used to dewax the samples, which were rehydrated in a graded alcohol series. Antigen retrieval was performed in 10 mmol/L sodium citrate solution (PH 6.0) at 100 °C for 10 min, and the samples were cooled for 20 min. After rinsing in PBS (pH 7.2), endogenous peroxidase activity was inhibited with 3 % H2O2 for 15 min and closed with goat serum for 15 min at ambient temperature to avoid nonspecific protein binding. Thereafter, the slides were incubated with antihuman YAP rabbit monoclonal antibodies at a 1:100 dilution (rabbit monoclonal antibody, EP1647Y, 1:100, Abcam Inc., Cambridge, CA, USA) at 4 °C overnight. Next, the samples were washed three times in PBS (pH 7.2), incubated with biotinylated secondary antibody at 37 °C for 30 min and hatched with avidin horseradish enzyme at 37 °C for 20 min. Colour development was carried out with DAB (3, 3- diaminobenzidine) and running water for 15 min. The slides were counterstained with 1 % Mayer’s haematoxylin. A gradient ethanol series was used for dehydration, and the samples were sealed with neutral gum. Finally, the slides were cleaned and coverslipped.
Scoring systems for immunohistochemical staining
To evaluate the expression of YAP, all slides were assessed independently by two experienced pathologists with minimal interobserver variability, and we used a semi-quantitative assessment method of scoring. The scoring parameters included staining intensity (range 0–3: 0, negative; 1, weak; 2, moderate; and 3, strong) and the percentage of positive cells (range 0–4: 0, negative or <5 %; 1, 6 %–25 %; 2, 26 %–50 %; 3, 51 %–75 %; and 4, 76 %–100 %). We adopted the percentage of positive cells and the intensity to determine the final staining scores. Slides with a total score <4 were defined as having low YAP expression, while slides with a score ≥ 4 were defined as having high YAP expression.
Statistical analysis
All data were analysed using SPSS 17.0 software (version 17.0; SPSS Inc., Chicago, IL, USA). The relationships between YAP expression and clinicopathological parameters in HCC and CC were analysed using Fisher’s exact test or a χ2 test. Tumour recurrence-free survival (RFS) was recorded as the time from liver tumour resection as clean as possible to liver tumour recurrence. Disease-specific survival (DSS) was recorded as the time from cancer diagnosis to death from cancer or the follow-up deadline. The Kaplan–Meier method was used to assess RFS and DSS, and the log-rank test was used to analyse the differences between the curves. The prognostic meaning of YAP expression in HCC and CC was calculated by univariate and multivariate Cox regression analysis. The threshold for statistical significance was P < 0.05.
Results
Clinical pathological features of patients and the expression of YAP
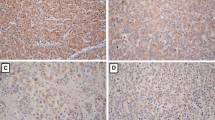
At the time of this analysis, 259 patients are with PLC, including 137 HCC and 122 CC. In HCC, there were 117 male patients (85.4 %) and 20 female patients (14.6 %). Sixty-seven patients < 45, 70 patients is ≥45 years old. Eight-five patients have a size over 5 cm while the others 52 ≤ 5 cm. In CC, 100 patients is male (82.0 %), 22 patients of female (18.0 %), 40 patients<45, 82 patients is ≥45. The details of clinicopathological characteristics of HCC and CC are summarized in (Table 1).YAP expression mainly showed an incomplete dyeing in nuclear and cytoplasmic compartments in HCC and CC tissues. As above mentioned, YAP expression levels were graded as negative/low and high. The result indicated that high YAP expression was observed in HCC is 56.9 % (78/137) (Fig. 1) and CC is 67.2 % (82/122) (Fig. 2).
Relationship between YAP expression and the clinicopathologic characteristics of patients with HCC and CC
In our study, we analysed the association between YAP expression and the clinicopathologic characteristics of patients with HCC and CC by χ2 (Table 1). High YAP expression in both HCC and CC had positive correlations with tumour size (P < 0.001 and P = 0.019, respectively), liver cirrhosis(P = 0.002 and P = 0.009, respectively), vascular invasion (P = 0.047 and P = 0.018, respectively), multiplicity (P = 0.019 and P = 0.015, respectively), and intrahepatic metastasis (P = 0.015 and P = 0.047, respectively). However, YAP status was not significantly associated with age (P = 0.960 and P = 0.716), patient gender (P = 0.857 and P = 0.915, respectively), HBV status (P = 0.991 and P = 0.934, respectively), and TNM stage (P = 0.422 and P = 0.622, respectively).
Association of YAP expression with recurrence-free survival in HCC and CC
The 1-year RFS rates in the low YAP expression groups with HCC and CC were 89.6 % and 63.9 %, respectively, and the 3-year RFS rates were 55.5 % and 41.2 %, respectively. However, the 1-year RFS rates in the high YAP expression groups with HCC and CC were 54.2 % and 37.3 %, respectively. The 3-year RFS rates were 31.3 % and 17.4 %, respectively. The results of the log-rank test showed statistically significant differences in RFS between the two groups (HCC, P = 0.001, CC: P = 0.010) (Fig. 3a, Fig. 4a).
When HCC and CC were split into two groups according to YAP expression, the recurrence-free survival rate was relatively lower in the high YAP expression group with CC than in the high YAP expression group with HCC (P < 0.001) (Fig. 5a).
Univariate analysis revealed that multiplicity (HR = 3.848, P<0.001), intrahepatic metastasis (HR = 2.844, P = 0.003), vascular invasion (HR = 3.295, P<0.001), and YAP expression (HR = 2.114, P = 0.006) were significantly associated with RFS in HCC (Table 2). Liver cirrhosis (HR = 1.809, P = 0.018), tumour size (HR = 1.919, P = 0.032), multiplicity (HR = 2.545, P = 0.041), intrahepatic metastasis (HR = 2.534, P = 0.028), vascular invasion (HR = 2.365, P = 0.010), lymph node metastases (HR = 2.978, P = 0.002), and YAP expression (HR = 1.800, P = 0.033) were adverse prognostic factors that affected RFS in CC after resection (Table 3).
In multivariate analysis, alpha fetoprotein (AFP), liver cirrhosis, and hepatitis B virus infection were found to be essential parameters. Therefore, we used three different models for multivariate analysis (Table 2, Table 3). For model 1, the parameters included alpha fetoprotein (AFP), liver cirrhosis, and hepatitis B virus infection, and the results showed that alpha fetoprotein (AFP), liver cirrhosis, and hepatitis B virus infection were not prognostic factors for RFS in patients with HCC. Liver cirrhosis (HR = 1.581, P = 0.048) was an independent prognostic factor for RFS in patients with CC. For model 2, the parameters included alpha fetoprotein (AFP), liver cirrhosis, hepatitis B virus infection, and YAP expression, and the results indicated that YAP expression (HR = 2.303, P = 0.001) was an independent prognostic factor for RFS in patients with HCC. Liver cirrhosis (HR = 1.679, P = 0.026) and YAP expression (HR = 2.161, P = 0.003) were independent prognostic factors for RFS in patients with CC. Finally, alpha fetoprotein (AFP), liver cirrhosis, hepatitis B virus infection, YAP expression, intrahepatic metastasis, multiplicity, lymph node metastases, and vascular invasion were essential parameters in model 3. Multivariable analysis indicated that multiplicity (HR = 3.893, P < 0.001), intrahepatic metastasis (HR = 2.994, P = 0.001), vascular invasion (HR = 3.083, P < 0.001), and YAP expression (HR = 2.055, P = 0.008) were independent predictors for RFS in patients with HCC; liver cirrhosis (HR = 1.750, P = 0.018), multiplicity (HR = 2.460, P = 0.041), vascular invasion (HR = 2.148, P = 0.021), lymph node metastases (HR = 4.021, P < 0.001), and YAP expression (HR = 1.824, P = 0.026) were independent predictors for RFS in patients with CC.
Association of YAP expression with disease-specific survival in HCC and CC
The 1-year DSS rates in the low YAP expression group with HCC and CC were 94.9 % and 60.4 %, respectively, and the 3-year DSS rates were 66.0 % and 44.7 %, respectively. However, the 1-year DSS rates in the high YAP expression group with HCC and CC were 65.3 % and 32.1 %, respectively. The 3-year DSS rates were 40.0 % and 18.2 %, respectively. In the log-rank test, the results were significantly different between the two groups for DSS (HCC: P = 0.001, CC: P = 0.013) (Fig. 3b, Fig. 4b).
When HCC and CC were split into two groups according to YAP expression, the disease-specific survival rate was relatively lower in the high YAP expression group with CC than in the high YAP expression group with HCC (P < 0.001) (Fig. 5b).
Univariate analysis revealed that multiplicity (HR = 4.045, P < 0.001), intrahepatic metastasis (HR = 3.050, P = 0.001), vascular invasion (HR = 3.281, P < 0.001), and YAP expression (HR = 1.902, P = 0.017) were significantly associated with DSS in HCC (Table 4). Liver cirrhosis (HR = 1.662, P = 0.034), tumour size (HR = 1.964, P = 0.022), multiplicity (HR = 2.478, P = 0.037), intrahepatic metastasis (HR = 2.323, P = 0.024), vascular invasion (HR = 2.265, P = 0.015), lymph node metastases (HR = 2.845, P = 0.005), and YAP expression (HR = 1.998, P = 0.010) were adverse prognostic factors affecting DSS in CC after resection (Table 5).
In multivariate analysis, we still used three different Cox models to analyse the significance of YAP for DSS in HCC and CC (Table 4, Table 5). For model 1, the parameters included alpha fetoprotein (AFP), liver cirrhosis, and hepatitis B virus infection, and the results showed that alpha fetoprotein (AFP), liver cirrhosis, and hepatitis B virus infection were not prognostic factors for DSS in patients with HCC. Liver cirrhosis (HR = 1.576, P = 0.049) was an independent prognostic factor for DSS in patients with CC. For model 2, the parameters included alpha fetoprotein (AFP), liver cirrhosis, hepatitis B virus infection, and YAP expression, and the results indicated that YAP expression (HR = 2.236, P = 0.001) was an independent prognostic factor for DSS in patients with HCC. Liver cirrhosis (HR = 1.677, P = 0.027) and YAP expression (HR = 2.134, P = 0.003) were independent prognostic factors for DSS in patients with CC. Finally, alpha fetoprotein (AFP), liver cirrhosis, hepatitis B virus infection, intrahepatic metastasis, multiplicity, vascular invasion, lymph node metastases, and YAP expression were essential model 3 parameters. Multivariable analysis indicated that multiplicity (HR = 4.042, P < 0.001), intrahepatic metastasis (HR = 3.179, P = 0.001), vascular invasion (HR = 3.089, P < 0.001), and YAP expression (HR = 1.823, P = 0.024) were independent predictors of DSS in patients with HCC; liver cirrhosis (HR = 1.657, P = 0.031), multiplicity (HR = 2.326, P = 0.046), intrahepatic metastasis (HR = 2.135, P = 0.035), vascular invasion (HR = 2.247, P = 0.015), lymph node metastases (HR = 3.106, P = 0.026), and YAP expression (HR = 1.897, P = 0.016) were independent predictors of DSS in patients with CC.
Discussion
Recent findings have shown that YAP is a crucial oncogene protein in a number of human primary tumours including breast cancer, lung cancer, gastric cancer, and colon cancer [17–20]. YAP plays an important role in tumour development and metastasis, and high YAP expression is closely related to poor clinicopathologic characteristics and prognosis. As one of the important potent oncogenic transcriptional co-activators in the Hippo pathway, YAP activation represses numerous target genes, including tumour-suppressor genes, such as DDIT4 (DNA-damage-inducible transcript 4) and Trail (TNF-related apoptosis-inducing ligand), which leads to changes in cell behaviour, such as decreased proliferation and invasiveness, and stimulation of angiogenesis [21].
A previous study that included 139 cases of colorectal cancer tissues showed that 52.5 % (73/139) of cases exhibited detectable nuclear staining for YAP [22]. Since then, advanced YAP and nuclear localization have been observed in human HCC. A study reported that 54 % of 115 HCC patients showed YAP overexpression in a tissue microarray, while the majority of normal liver tissues demonstrated very weak dyeing [23]. According to an analysis that enrolled 177 pairs of HCC patients and matched normal samples with complete clinical records, Xu et al. suggested that YAP was an independent prognostic indicator of the survival and disease-free survival of HCC patients and was clinicopathologically associated with serum AFP levels [24]. Our study agreed with their conclusion. Importantly, we demonstrated that CC shows higher positive YAP expression rates than HCC. Moreover, recurrence-free survival and disease-specific survival were significantly different between patients with CC with high YAP expression and patients with HCC with high YAP expression, which had not been shown before. These data suggested that YAP is important for tumour deterioration and plays a crucial role in tumour recurrence after hepatectomy and survival time.
In the present study, we examined YAP expression in 259 PLC patients, including 137 HCC patients and 122 CC patients, using immunohistochemical methods. We also analysed the relationship between YAP expression and clinical features and prognosis. We found that the positive YAP expression rate in PTC was 61.8 %, similar to other reports in China [25]. In HCC and CC, the positive YAP expression rates were 56.9 % and 67.2 %, respectively. Moreover, we found that high YAP expression in HCC and CC was significantly associated with the tumour size, AFP, liver cirrhosis, vascular invasion, multiplicity, intrahepatic metastasis and lymph node metastases; however, there was no clear relationship between YAP expression, age, patient gender, HBV status, and TNM stage. According to the survival analysis of YAP expression in HCC and CC, we found that the high YAP expression group had lower 1-year and 3-year RFS and DSS rates. Further survival analysis showed that CC with high YAP expression had lower 1-year and 3-year RFS and DSS rates than HCC with high YAP expression. These data strongly imply that YAP participates in tumour progression in HCC and CC. High YAP expression was closely correlated with poor patient prognosis in HCC and CC. This may facilitate targeted therapy for elevated YAP in HCC and CC.
The crucial characteristic of malignant tumours is unrestricted cell division, which leads to cancer progression and tight connections with genetic alterations linked to the regulation of proliferation, the cell cycle, apoptosis, and genetic stability [26]. YAP is a potent oncogenic transcriptional co-activator that is opposed by the Hippo tumour suppressor pathway [27]. As a downstream effector gene of the Hippo pathway, in collaboration with the TEAD transcription factor, the overexpression of YAP represents the aberrant activation a group of target genes (CTGF, CCND1, ITGB2, and BCL2L1) responsible for cell proliferation, anti-apoptosis, survival, and migration. In particular, TEAD1 and TEAD4 are most often associated with proliferation and cancer development [27–30]. YAP promoted the transcription of many genes that are normally associated with hepatocyte proliferation, such as Ki67, c-myc, SOX4, H19, and AFP. In addition, it also induced the expression of several negative regulators of apoptosis, such as the IAP family members BIRC5/survivin and BIRC2/cIAP1 and the BCL2 family gene MCL1 [27]. Remarkably, our results show that patients with high YAP expression exhibit increases in tumour volume, intrahepatic metastasis, and vascular invasion. In particular, CC, which is more malignant and invasive, exhibits high YAP expression and lower survival rates than HCC, which implies that YAP contributes to tumour cell proliferation and the metastasis of HCC and CC by promoting cell cycle progression, anti-apoptosis, and migration.
In conclusion, this is the first study of YAP expression in the comparison of HCC and CC. Our results suggested that YAP was more highly expressed in CC than in HCC. Moreover, YAP expression increased in HCC and CC tissues, which was strongly correlated with tumour size, liver cirrhosis, vascular invasion, multiplicity and lymph node metastases . Survival analysis results demonstrated that positive YAP expression was a poor prognostic factor for RFS and DSS. Additionally, high YAP expression in CC represents poorer survival than that in HCC. Our study showed that YAP increased the degree of malignancy and decreased patient survival. Of course, our research is limited, further studies should explore the specific mechanism of YAP’s influence on the occurrence of PLC and the development of targeted therapy for PLC.
References
Media centre. Title of subordinate document. In: Cancer. 2015. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 18 Feb 2015.
Koh KC, Lee H, Choi MS, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120–5.
Wang J, Wang F, Kessinger A. Outcome of combined hepatocellular and cholangiocarcinoma of the liver. Journal of Oncology Volume. 2010. doi:10.1155/2010/917356.
Bosetti C, Turati F, Vecchia CL. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753–70.
Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, et al. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121–31.
Wang XM, Yang LY, Guo L, Fan C, Wu F. p53-induced RING-H2 protein, a novel marker for poor survival in hepatocellular carcinoma after hepatic resection. Cancer. 2009;115:4554–63.
Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2013;13:63–79.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Huang J, Wu S, Barrera J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122(3):421–34.
Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124(2):267–73.
Kim JE, Finlay GJ, Baguley BC. The role of the hippo pathway in melanocytes and melanoma. Front Oncol. 2013;3:123.
Komuro A, Nagai M, Navin NE, et al. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278(35):33334–41.
Nallet-Staub F, Marsaud V, Li L, et al. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J Invest Dermatol. 2014;134:123–32.
Morvaridi S, Dhall D, Greene M, et al. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Scientific Reports. 2015;5:16759.
Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67.
He C, Mao D, Hua G, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Molecular Medicine. 2015;7(11):1426–49.
Maugeri-Saccà M, Barba M, Pizzuti L, et al. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med. 2015. doi:10.1017/erm.2015.12.
Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yesassociated protein contributes to progression and poor prognosis of nonsmall-cell lung cancer. Cancer Sci. 2010;101:1279–85.
Kang W, Tong JH, Chan AW. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130–9.
Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle. 2012;11:1090–6.
Kim M, Kim T, Johnson RL, et al. Transcriptional Co-repressor function of the Hippo pathway transducers YAP and TAZ. Cell Rep. 2015;11(2):270–82.
Wang Y, Xie C, Li Q, et al. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumor Biol. 2013;34:2169–74.
Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–85.
Li H, Wang S, Wang G, et al. Yes-associated protein expression is a predictive marker for recurrence of hepatocellular carcinoma after liver transplantation. Dig Surg. 2014;31(6):468–78.
Yang X, Xu T. Molecular mechanism of size control in development and human diseases. Cell Res. 2011;21:715–29.
Dong J, Feldmann G, Huang J, Comerford SA, et al. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell. 2007;130(6):1120–33.
Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71.
Hen L, Loh PG, Song H. Structural and functional insights into the TEAD-YAP complex in the hippo signaling pathway. Protein Cell. 2010;1:1073–83.
Hao Y, Chun A, Cheung K, et al. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–509.
Acknowledgments
This research was funded by the National Natural Science Foundation of China, projects No 81272570 and the Health Bureau of Chongqing City, projects No 2015ZDXM012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Hao Wu and Yan Liu made equal contributions to this study.
Hao Wu and Yan Liu were the co-first authors.
Rights and permissions
About this article
Cite this article
Wu, H., Liu, Y., Jiang, XW. et al. Clinicopathological and prognostic significance of Yes-associated protein expression in hepatocellular carcinoma and hepatic cholangiocarcinoma. Tumor Biol. 37, 13499–13508 (2016). https://doi.org/10.1007/s13277-016-5211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5211-y