Abstract
Objectives
The purpose of this study was to study the expression of CCL15 in hepatocellular carcinoma (HCC) and explore its clinicopathological significance, and study relationships between expressions of CCL15 and malignant behaviors of HCC.
Methods
The SP immunohistochemical method was used to detect expression of CCL15 in routinely paraffin-embedded sections from 80 cases of HCC, 80 of adjacent cancerous specimens and 50 of normal liver tissue. In these patients with HCC, Kaplan–Meier was used to assess survival outcomes.
Results
The positive rates and scores of CCL15 were significantly higher in HCC than adjacent cancerous specimens and normal liver tissue (p < 0.05), but not significantly higher between adjacent cancerous specimens and normal liver tissue (p > 0.05). The expression of CCL15 was significantly correlated to tumor size, tumor thrombi in portal vein of HCC, capsule and TNM stage (p < 0.05), but not to sex, age, liver cirrhosis and the level of AFP so on (p > 0.05). Survival time of the patients with positive CCL15 expression was significantly decreased, and multivariate analysis indicated CCL15 expression was one of the independent predictors of survival (p = 0.042).
Conclusion
The expression of CCL15 was significantly correlated with malignant behaviors of HCC, and CCL15 might be important biological markers for reflecting the carcinogenesis, progression, biological behaviors and prognosis of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver carcinoma is one of the most common malignancies in the world. It is the fifth most frequently diagnosed cancer worldwide but the second most frequent cause of cancer death in males globally. Half of these cases and deaths have been estimated to occur in China [1]. It is also the second greatest cause of cancer-related deaths in China [1]. Early metastasis and recurrence have become two main determinants in patient survival. Therefore, the identification of the key molecules and signaling pathways that correlate with tumor invasion, size, stage, and metastasis has the potential to provide new targets and novel clues for diagnosing, treating, preventing, and controlling early recurrence and metastasis of hepatocellular carcinoma (HCC) and improving prognosis.
The poor prognosis of HCC is mainly attributed to recurrence and metastasis [2]. Recent studies have shown that chemokines and their receptors play important roles in the metastatic potential of cancer cells [3]. For example, the CXCL12/CXCR4 pair has been identified to mediate metastasis of breast cancer cells [4].
Results from our previous study showed that CCL15 was uniquely expressed in serum samples from HCC patients using the SELDI-TOF technique. Biological analysis suggested that CCL15 promoted HCC migration and invasion [5].
This study explores the expression of CCL15 in 80 cases of HCC, and discusses the correlation between changes in CCL15 expression in HCC samples with clinicopathological parameters such as tumor size, vascular tumor thrombus, TNM stage, and prognosis, etc. The aim of this study was to add to the existing knowledge of the mechanisms of HCC progression and development, with the hope of gaining significant insights that can be used to treat liver cancer targeting CCL15.
Materials and methods
Patients with HCC
From June 2006 to June 2008, paraffin-embedded specimens from primary HCC lesions were collected from 80 patients with histologically proven HCC who had undergone hepatectomy at Tianjin Medical University cancer institute and hospital. Fifty patients with normal liver were included in a normal control group. A complete clinical picture of each patient was collected, including sex, age, tumor size, levels of AFP, TNM stages, etc. Other pathological information, such as presence of liver cirrhosis, capsule and vascular thrombosis, was also collected for each patient. None of the patients were pretreated with any kind of adjuvant therapy, such as radiotherapy or chemotherapy.
Immunohistochemistry (IHC) assay
Normal (n = 50), adjacent liver tissues (n = 80) and HCC tissues (n = 80) were processed according to standard approaches. The anti-CCL15 serum (1:1600, Immunechem Pharmaceuticals Inc., China) was applied to the slides of three groups and incubated in a moist chamber at 4 °C overnight. A total of 0.01 ml PBS was used as the negative control in all experiments. Slides cut in parallel to the IHC-treated sections were stained by HE for better identification of the different tissue areas. To avoid interindividual bias of IHC staining differentiations, all slides were determined by an experienced pathologist.
Immunohistochemistry evaluation
Semi-quantitative immunohistochemical detection was used to determine the CCL15 protein levels. Cytoplasm immunoreactivity for the CCL15 protein was scored by evaluating the sum of positive tumor cells and the staining intensity over the total number of tumor cells. In brief, the percent of positive cells was scored as “0” (0 %), “1” (1–10 %), “2” (11–50 %), “3” (51–80 %), and “4” (81–100 %). Intensity was scored as “0” (no staining), “1” (weakly stained), “2” (moderately stained), and “3” (strongly stained). Both the scores were decided under double-blind conditions by three independent professionals. The final immunoreactive scores were determined by multiplying the intensity scores with the extent of positivity scores of stained cells. The scores were then classified as follows: “−” (score 0), “+” (score 1–4), “++” (score 5–8), and “+++” (score 9–12). For the purpose of statistical analysis, the cohort was grouped into a negative group (“−”, “+”) and a positive group (“++”, “+++”).
Statistical analysis
Statistical analyses were performed with the SPSS 13.0 software (SPSS, Chicago, IL, USA). The correlation between CCL15 expression and various clinical and pathological characteristics were evaluated by Paired t test. Kaplan–Meier analyses were used to analyze CCL15 as univariate in prediction of patients’ survival. Comparisons of survival distributions were done with log-rank test. The multivariate survival analyses were performed by a stepwise Cox proportional hazard model using the Wald statistics. p values of <0.05 were considered significant.
Results
Clinical and pathological data of 80 hepatocellular carcinoma patients
The age of 80 patients with HCC ranged from 21 to 76 years, of whom 55 patients were younger than 60 years of age, 25 patients 60 years of age or older, and the median age was 67 years. Fifty patients were males and 30 were females; the male to female ratio was 1.67:1. For preoperative blood serum a-fetoprotein (AFP), 28 patients had 20 µg/L or less and 52 had more than 20 µg/L. For tumor diameter, 38 patients had 5.0 cm or less and 42 had more than 5.0 cm. In accordance with histological differentiation, 15 cases were well differentiated HCC, 20 were moderately differentiated HCC and 45 poorly differentiated HCC. Twenty-nine patients were found to have vein tumor thrombus and 35 patients with liver cirrhosis. For the TNM stage (American Joint Committee on International Union against Cancer), 25 patients were stage I, 32 were stage II and 27 were stage III.
Expression of CCL15 in hepatocellular carcinoma and normal liver tissues
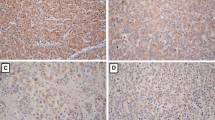
Among 80 tumor samples, CCL15 was detected in 64 tumor tissues and only in 16 tissues adjacent to tumor (Table 1). Only one out of 50 samples from healthy tissues showed positive staining with CCL15. The immunostaining expressions of CCL15 have been shown in HCC and adjacent cancer tissues using IHC (Fig. 1).
Relationship between clinic pathological characteristics and the CCL15 expression in HCC tissues
The expression of CCL15 was significantly correlated to tumor size, tumor thrombi in portal vein of HCC, capsule and TNM stages (p < 0.05), but not to sex, age, liver cirrhosis and the level of AFP, etc. (p > 0.05) (Table 2).
Association of CCL15 expression with survival in patients with HCC
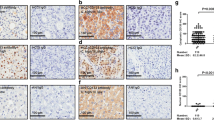
Hepatocellular carcinoma with patients' CCL15 expression was associated with a significantly lower overall survival rate than was HCC without CCL15 expression (p = 0.038, Fig. 2). In univariate analysis, tumor size (p = 0.036), vascular tumor thrombus (p = 0.047), tumor capsule (p = 0.038), TNM stage (p = 0.025) and CCL15 expression (p = 0.029) were important predictor for poor prognosis. Subsequently, multivariate analysis indicated CCL15 expression was one of the independent predictors of survival (p = 0.042), as were TNM stage (p = 0.045) (Table 3).
Discussion
CCL15/Leukotactin-1 (Lkn-1), a member of the CC chemokine family and expressed only in the gut and the liver, has been recently cloned and partially characterized [6–8]. CCL15 was demonstrated to have a chemoattractant role for monocytes, lymphocytes, neutrophils, eosinophils, and dendritic cells [8]. In addition, this molecule plays an important role in the development of inflammation and allergic inflammatory diseases. CCL15 has in vitro and in vivo angiogenic activity [9]. Moreover, desensitization studies have indicated that CCL15 acts mainly via the CC chemokine receptor, CCR1 [7, 10]. Accumulating evidence has suggested that CCL15 may have a crucial role in the progression of tumor cells by promoting leukocyte infiltration and modulating tumor cell motility [10–14].
Data from our previous study showed that CCL15 was uniquely expressed in serum samples from HCC patients using the SELDI-TOF technique. Biological analysis suggested that CCL15 promoted HCC migration and invasion [5]. Itatani et al. [14] found that in human CRC cells, loss of SMAD4 leads to up-regulation of CCL15 expression, and human liver metastases that express CCL15 contain higher numbers of CCR1(+) cells; patients with these metastases have shorter times of disease-free survival, and also showed that reagents designed to block CCL15 recruitment of CCR1(+) cells could prevent metastasis of CRC to liver.
This study shows that positive expression of CCL15 is significantly higher than, and different from, those in the adjacent cancer and normal tissues. CCL15 closely correlate with HCC tumor size, vascular tumor thrombus, capsule, TNM stage and patient survival time after surgery. Patients with positive CCL15 expression also have shorter survival times after surgery. Furthermore, the multivariable Cox proportional hazards model illustrated that positive expression of CCL15 was an independent prognostic variable, whereas the prognostic values of CCL15 expression in tumors and TNM stage were statistically significantly compromised by other clinical factors.
In conclusion, in HCC tumor samples, CCL15 is overexpressed. The overexpression significantly correlated with tumor size, tumor thrombi in portal veins of HCC, capsule and TNM stages. Postoperative survival time in the patients with CCL15 overexpression was decreased. A more in-depth study in CCL15 may offer a new target for biological therapy for malignancy.
References
Zhang Y, Fan Y, Mei Z. NGAL and NGALR overexpression in human hepatocellular carcinoma toward a molecular prognostic classification. Cancer Epidemiol. 2012;36(5):e294–9.
Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–46.
Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26:453–67.
Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6.
Li Y, Wu J, Zhang W, et al. Identification of serum CCL15 in hepatocellular carcinoma. Br J Cancer. 2013;108(1):99–106.
Youn BS, Zhang SM, Lee EK, et al. Molecular cloning of leukotactin-1: a novel human beta-chemokine, a chemoattractant for neutrophils, monocytes, and lymphocytes, and a potent agonist at CC chemokine receptors 1 and 3. J Immunol. 1997;159:5201–5.
Hwang J, Kim CW, Son KN, et al. Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett. 2004;570:47–51.
Pardigol A, Forssmann U, Zucht HD, et al. HCC-2, a human chemokine: gene structure, expression pattern, and biological activity. Proc Natl Acad Sci USA. 1998;95:6308–13.
Ko J, Kim IS, Jang SW, et al. Leukotactin-1/CCL15-induced chemotaxis signaling through CCR1 in HOS cells. FEBS Lett. 2002;515:159–64.
Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15.
Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391–8.
Kunz M, Hartmann A, Flory E, et al. Anoxia-induced up-regulation of interleukin-8 in human malignant melanoma. A potential mechanism for high tumor aggressiveness. Am J Pathol. 1999;155:753–63.
Desbaillets I, Diserens AC, Tribolet N, et al. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186:1201–12.
Itatani Y, Kawada K, Fujishita T, et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology. 2013;145(5):1064–75.
Acknowledgements
This work was supported by research grants from 863 program grants (#2011AA02A111) and the National Scientific Foundation of China (#81101754).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical standard
Human ethics approval was duly obtained from the Institutional Human Ethics Committee of Tianjin Medical University Cancer Institute and Hospital.
Informed consent
A written informed consent approved by the ethical committee was obtained from all participants involved in the study. Tissue specimens were obtained by diagnostic or therapeutic procedures from patients after obtaining the patients’ consent.
Rights and permissions
About this article
Cite this article
Li, Y., Yu, H.P. & Zhang, P. CCL15 overexpression predicts poor prognosis for hepatocellular carcinoma. Hepatol Int 10, 488–492 (2016). https://doi.org/10.1007/s12072-015-9683-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-015-9683-4