Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies because of its broad resistance to chemotherapy. Numerous evidence indicates that integrinβ1 is upregulated in some human cancers, and it is correlated with resistance to various therapies. However, the role of integrinβ1 in chemotherapy is not clear in pancreatic cancer. The present study evaluates the potential of integrinβ1 to predict chemoresistance and prognosis in patients and to modulate resistance to gemcitabine in PDAC cells. Primary drug-resistance (DR) cancer cells were isolated, and DR cells from MiaPaCa-2 and AsPC-1 parent cell lines (PCL) were selected. Integrinβ1 expression was determined using immunohistochemistry (IHC), quantitative real-time PCR (qRT-PCR) and Western blotting. Changes in drug response after knockdown of integrinβ1 via RNA interference (RNAi) were evaluated using the viability of cancer cells as colon formation, proliferation using Western blot of Ki-67 and apoptosis using cleaved caspase-3 immunofluorescence. qRT-PCR and Western blot also detected variations in the activities of cdc42 and AKT after integrinβ1 suppression. Patient survival and relative factors were assessed using Kaplan-Meier and Cox regression analyses. Integrinβ1 expression was upregulated in PDAC, which was significantly associated with intrinsic and acquired gemcitabine resistance and worse outcomes. The downregulation of integrinβ1 attenuated PDAC chemoresistance, and this attenuation partially correlated with reduced Cdc42 and AKT activity, which are target molecules of integrinβ1 in some human cancers. These findings identified integrinβ1 as a special marker of drug resistance and a serious prognosis, and they furthermore support the use of integrinβ1 as a novel potential therapeutic target to overcome chemotherapy resistance. The results also suggest a possible drug-resistant signalling pathway of integrinβ1 in PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a terrible human malignancy, and it is exhibits the lowest survival rate of all major cancers [1]. One factor that leads to poorer prognosis is the lack of early symptoms. Patients are diagnosed late with advanced stage and metastasis, and surgical resection does not improve survival. Unfortunately, although PDAC patients who undergo pancreatectomy followed by adjuvant chemotherapy receive some benefits from the standard cure, survival is not ideally prolonged because of the extensive resistance to gemcitabine, which is the first-line and essential drug that is administered alone or in combination with other compounds [2–4]. Cancer relapse always exhibits more aggressive features and an insensitivity to chemotherapy, which contributes to high lethality [5, 6]. Therefore, the identification of new markers to predict gemcitabine resistance and poor outcomes as well as to elucidate the molecular signalling pathways related to chemoresistance should be high research priorities for PDAC [5–7].
Chemoresistance in cancers is related to many factors, such as increased drug efflux, impairment of drug delivery, drug inactivation by detoxifying factors, increased damage repair, tolerance of damage, gene mutations, cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT) [8–10]. The tumour microenvironment may also act as a pharmacological barrier, which is suggested to underlie broad chemoresistance in various cancers [11]. Abundant stromal content and poor blood supply are key features of PDAC [11, 12].
Recent evidence demonstrated that integrins modulated tumour growth and cell survival and proliferation, which resulted in drug resistance [13–15]. Integrinβ1 also promoted tumour angiogenesis and interaction with the microenvironment; it especially withstood hypoxic conditions, which indicates that integrinβ1 plays major roles in tumour growth, metastases and invasion [16–18]. Integrinβ1 also confers resistance to treatment with some therapeutic agents [19–22]. These findings suggest that integrinβ1 may be a special marker of drug resistance and poor prognosis and a potential target for antitumour therapies in PDAC, which is characterised by an abundant extracellular matrix (ECM) and hypoxia [11, 23, 24].
The present study investigated the role of integrinβ1 in the drug-resistant phenotype of PDAC cells. We screened samples of resected tumours from patients and established GR-pPDAC cell lines, selected gemcitabine-resistant subpopulations of ASPC-1 and MiaPaCa-2 parent cell lines (PCLs) and established GR-PDAC cell lines. Integrinβ1 expression was analysed in tumour tissue and cell lines to explore the relationship between integrinβ1 and gemcitabine responses and outcomes in PDAC. We inhibited integrinβ1 using RNA silencing and examined changes in viability and apoptosis in DR cell lines after gemcitabine administration to confirm the contribution of integrinβ1 to the drug-resistant phenotype. We detected changes in the activities of downstream cdc42 and AKT after integrinβ1 knockdown.
Materials and methods
Patients
Human PDAC surgical specimens were obtained from continuous patients undergoing radical resection between September 2012 and September 2014 at Changzheng Hospital (Shanghai, China) where adjuvant gemcitabine therapy was prescribed. Informed consent was obtained from patients, and the Second Military Medical University Research Ethics Committee (Institutional Review Board), Shanghai, China, approved the protocols. Tissue for establishing primary cell lines was confirmed to exhibit a gemcitabine-resistance phenotype using a chemosensitivity test ex vivo. All PDAC sporadic resectable tumours were included in the study. These cancers were classified as a gemcitabine-resistant, median and sensitive types. The treating oncologist scheduled gemcitabine (1000 mg/kg) administration, and patients were followed up clinically until September 2015.
Ex vivo chemosensitivity test
Primary human PDAC cells were isolated, and a cellular ATP-based tumour chemosensitivity assay (ATP-TCA) was performed as described previously [25, 26]. Briefly, primary human cancer cells were seeded in 96-well microtiter plates with 10,000 cancer cells per well in a tumour cell-supporting growth medium. Gemcitabine (Eli Lily and Company, IN, USA) was added at test drug concentrations (TDCs) of 200, 100, 50, 25, 12.5 and 6.25 % (with 100 % TDC corresponding to peak plasma concentration). Cancer cells were lysed after 7 days of incubation, and the amount of cellular ATP was detected using a luciferase reaction to evaluate the number of viable cells. Cell preparation and ATP-TCA were performed using available reagents (TCA-100, DCS, Hamburg, Germany). Luminescence was measured using an LB953 luminometer (Berthold Technologies, Bad Wildbad, Germany). The IC50 (inhibitory concentration of 50 %) is the concentration at which cell growth and survival were inhibited by 50 %. This value was calculated following ATP-TCA test protocols using an interpolation of two neighbouring measurements. Test values below 50 % were identified as ‘sensitive’, values above 100 % were identified as ‘resistant’ and other values were identified as ‘median’. The tests were performed in triplicate.
DR cell selection, cultures, treatments and transfections
For primary cell isolation, PDAC tissues were obtained from core of samples, incubated in DMEM containing streptomycin, penicillin and amphotericin (Invitrogen, CA, USA) and washed in ice-cold PBS. Samples were cut into 1-mm3 pieces, which were put into a thermostatic water bath at 37 °C in a 50-fold tissue volume of trypsin (Invitrogen, CA, USA) and shaken once every 5 min for 30 min. Digested tissues were cleared via filtering through 100-mesh stainless steel mesh. The filtrate was centrifuged at 800 rpm for 3 min, and the supernatant was discarded. The retained cells were washed twice with D-Hanks solution. The primary drug-resistant pancreatic cancer (GR-pPDAC) cell lines from resistant tumours were cultured in DMEM containing 1.5 % serum (Invitrogen). Typical sensitive PDAC cells were cultured as controls (Ctrl-pPDAC). We isolated adjacent normal cells (most were acinar cells) from adjacent tissues using collagenaseII digestion, and these cells were cultured in DMEM/F12 with 20 % foetal bovine serum. AsPC-1 and Miapaca-2 cell lines were obtained from the Department of Cell Biology, Basic Research Institute, Second Military Medical University (Shanghai, China). DR cells were induced by continuous treatment of PCLs with 0.1 μM of gemcitabine for 7 days (medium replaced every 72 h) followed by a normal medium for 15 days. Resistant clones were pooled, amplified and cultured by performing a 24-h pulse of 1 μM of gemcitabine every other week to obtain drug-resistant pancreatic cancer (GR-PDAC) cell lines. AsPC-1 cells were maintained in RPMI-1640 (Invitrogen), and Miapaca-2 cells were maintained in DMEM medium (Invitrogen). Gemcitabine was dissolved in water. Cells for RNA interference were transfected with integrinβ1 siRNA duplexes using Lipofectamine RNAi-MAX and Opti-MEM mediums (Invitrogen) according to the manufacturers’ protocols. Transfection efficiency was examined using RT-PCR and Western blot assays in triplicate.
Colony formation and cell death assays
Single-cell suspensions were plated in 6-well plates with 500 cells per plate. After 1 day, cells were incubated for 24 h with gemcitabine. Media was replaced every 48 h for 10–12 days, and cells were fixed in methanol for 10 min, stained overnight with 5 % Giemsa (Sigma-Aldrich, MO, USA), washed in phosphate-buffered saline and dried. Pictures were obtained using a digital camera, and colonies were counted. Cells were seeded at 70 % confluency and treated with different doses of gemcitabine for 72 h for cell death analyses. Cells were washed in phosphate-buffered saline and prepared for caspase-3 immunofluorescence using an anti-cleaved caspase-3 antibody (1:500; Sigma-Aldrich). Five random fields were chosen for each gemcitabine dose, and at least 200 cells per field were counted. Positive cells were counted using fluorescence microscopy. A Guava Nexin kit and Guava PCA system (Guava Technologies, Hayward, CA, USA) were used to assess apoptosis according to the manufacturer’s protocol. AnnexinV-PE was used to detect all stages of apoptotic cells. 7-Amino actinomycin-D (7-AAD) was the cell-impermeable dye that was used to identify late-stage apoptotic and dead cells. AnnexinV-PE fluorescence was analysed using Cytosoft software (Guava Technologies, Hayward. CA, USA). A minimum of 2000 events was counted. All tests were performed in triplicate.
PCR analyses
RNA was extracted using TRIzol (Invitrogen) and converted to cDNA using an RNA PCR kit (Applied Biosystems, CA, USA) according to the manufacturer’s recommendations. RT-PCR and quantitative real-time PCR analysis were performed using an RT-PCR kit and StepOne Real-Time PCR System (Applied Biosystems) according to the manufacturers’ protocols. Expression levels were normalised to GAPDH gene levels. Table 1 lists that primer sequences used in this analysis. Tests were performed in triplicate.
Protein extracts and Western blot analysis
Cells were resuspended in a RIPA buffer, which included 50 mM of Tris (pH 7.4), 150 mM of sodium chloride, 0.5 % sodium deoxycholate, 1.0 % NP-40, 0.1 % SDS, 0.5 mM of NaVO4, 1 mM of dithiothreitol and a protease inhibitor cocktail (Sigma-Aldrich). Extracts were centrifuged at 12,000g for 10 min after 10 min on ice, and supernatants were collected and used for Western blotting assays. Primary antibody incubation (1:1000) was performed using the following antibodies: MRP-3, MRP-5, integrinα1, α4, α5, αv, β1, β3, Ki-67, AKT, P-AKT(T308), Cdc42, GTP-Cdc42 and GAPDH (Sigma-Aldrich). Images of Western blots were acquired as TIFF files. Tests were performed in triplicate.
Histology and immunohistochemistry
Human PDAC tissue samples were fixed in a 10 % phosphate-buffered formalin and embedded in paraffin. Sections were cut 4 μm thick, mounted on glass slides and dried for 30 min at 60 °C. Sections were stained with haematoxylin and eosin (H&E) (Dako, CA, USA) according to standard histopathological procedures. Sections for IHC were incubated with anti-human antibodies of integrinβ1 (Cell Signaling Technology, MA, USA). Immunodetection was performed using Envision-Flex (Dako, Glostrup, Denmark). Two pathologists blindly and independently scored the staining of integrinβ1 in neoplastic cells based on distribution and intensity in five sections per case. Distribution was scored as 0 (0 %), 1 (1–50 %) and 2 (51–100 %). Intensity was scored as 0 (no signal), 1 (mild), 2 (intermediate) and 3 (strong). Values were summed in a total score from 0 to 5. Samples were classified as ‘low integrinβ1’ expression (score ≤3) and ‘high integrinβ1’ expression (score >3). Five random high-powered fields per slide were observed.
Statistical analysis
Clinical and histopathological data, time of tumour recurrence and survival for each patient were recorded. Statistical analyses were performed using SPSS v.17.0 (SPSS Inc., Chicago, USA). Differences for continuous variables and categorical variables were evaluated using a Student’s t test and Pearson’s chi-square test, respectively. Survival probabilities were estimated using Kaplan-Meier curves. Relapse-free survival (RFS) differences were analysed using the log-rank test, and overall survival (OS) differences were compared using the Breslow test. Univariate and multivariate analyses for risk factors affecting survival were performed using Cox’s proportional hazards regression model. A P value <0.05 was considered statistically significant.
Results
Characteristics and clinical outcome of study patients
All 63 patients who were diagnosed with primary PDAC without metastases received adjuvant gemcitabine chemotherapy after pancreatectomy, and three patients also received neoadjuvant treatments with gemcitabine. ATP-TCA identified 36, 11 and 9 patients as ‘sensitive’, ‘medium’ and ‘resistant’ cases, respectively. Seven cases were excluded because of contamination, or the cancer cells were missed. Table 2 briefly shows the characteristics and clinical outcome of patients.
Selection of GR-pPDAC cells and culture of primary cell lines
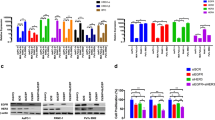
We isolated DR cancer cells from resistant tumour tissues and cultured these cells in DMEM to establish GR-pPDAC cell lines (Fig. 1a). GR-pPDAC cells can be subcultured stably and exhibit strong colony formation ability or even sphere formation ability (Fig. 1b, left panels). GR-pPDAC cell lines were less sensitive to gemcitabine than Ctrl-pPDAC cells. Low drug doses caused more cell death, and high doses caused massive cell death in Ctrl-pPDAC cells (Fig. 1b, middle and right panels). Drug-resistant markers, such as ABCC1, ABCC3, ABCC5 and ABCB1, were higher in PDAC cells than normal pancreatic cells, and ABCC3 expression was significantly enhanced. ABCC3 and ABCC5 mRNA were higher in GR-pPDAC cells compared to Ctrl-pPDAC cells, which suggests that PDAC displayed intrinsic multidrug resistance that was stronger in GR-pPDAC cells (Fig. 1c, d).
Selection of GR-pPDAC cells by ATP-TCA. a Schematic routine of the procedures used to identify GR-pPDAC from clinical tumour samples. b Representative images of the cell clusters in GR- (upper panel) and Ctrl-pPDAC cells (bottom panel) cultured with different drug doses in medium for 72 h (×40 magnification). c RT-PCR analysis in GR- and Ctrl-pPDAC cells (C) and adjacent normal pancreatic cells (N) of DR markers. d Bar graphs show their relative value of ABCC1, ABCC3, ABCC5 or ABCB1 mRNA levels from three experiments (n = 3, mean ± s.d.) as measured by qRT-PCR. e Western blot analysis in GR- and Ctrl-pPDAC (C) cells and adjacent normal pancreatic (N) cells of MRP-3 and MRP-5. f Bar graphs show their relative value of MRP-3 (left panel) or MRP-5 (right panel) protein levels from three experiments (n = 3, mean ± s.d.) as measured by Western blot. Data were analysed by paired or independent Student’s t test. **P ≤ 0.01; ns not significant
Isolation of GR-PDAC cells and propagation of cell lines
The following two cell lines were exposed to chronic gemcitabine (0.1 μM) treatment to isolate GR-PDAC cell subpopulations: AsPC-1, which was more resistant to the drug, and MiaPaCa-2, which displayed less resistance [27]. Viable clones were pooled, amplified, cultured and maintained in media (Fig. 2a, b). We evaluated cell survival using colony formation assays to confirm that GR-PDAC cell lines were more resistant to chemotherapy than PCL. The number of colonies in PCL was reduced in a drug dose-dependent manner, and GR-PDAC cells were more resistant to low doses of gemcitabine and less sensitive to high doses (Fig. 2c, d). These results indicate that the selected subpopulations acquired gemcitabine resistance.
Isolation of GR-PDAC cells by chronic exposure to gemcitabine. a Schematic routine of the procedures used to induce GR-PDAC cells from PCL. b Representative phase-contrast images of PCL- and GR-AsPC-1 (left panels) or Miapaca-2 (right panels) cells (×40 magnification). c, d Representative images of the colony assay performed in PCL- and GR-AsPC-1 (c, upper panels) or Miapaca-2 (d, upper panels), and bar graphs (c, d, bottom panels) show the percentage of survival with respect to treatment with different drug doses from three experiments (n = 3, mean ± s.d.). Data were analysed by paired or independent Student’s t test. **P ≤ 0.01
Integrinβ1 is upregulated in DR-PDAC cells
Recent evidence suggests that integrins play a key role in the acquisition of oncogenic features and drug resistance by human cancer cells [14–17]. Therefore, we investigated whether GR-pPDAC and Ctrl-pPDAC cells displayed changes in the expression of a subset of cancer-relevant integrins. We selected a group of integrins (α1, α4, α5, αv, β1 and β3) that are involved in drug resistance in cancer cells [28–30]. Western blot analyses demonstrated that α1, α5, αv and β3 expression was not different between GR-pPDAC and Ctrl-pPDAC cells. In contrast, α4 and β1 expression was upregulated in GR-pPDAC cells, especially integrinβ1 (Fig. 3a), which suggests that α4 and β1 correlated to intrinsic drug resistance in PDAC cells. Therefore, we focused on integrinβ1 expression, and changes in protein levels were confirmed using Western blot analyses. GR-pPDAC and GR-PDAC cells expressed higher levels of integrinβ1, and integrinβ1 was lower compared to Ctrl-pPDAC and PCL cells (Fig. 3b, c), which suggests that integrinβ1 correlated with intrinsic and acquired drug resistance in PDAC cells. We also found that integrinβ1 expression in PDAC cells was higher than adjacent normal cells (Fig. 3b). AsPC-1 cells, which were more resistant to gemcitabine, also expressed higher levels of integrinβ1 than MiaPaCa-2 cells (Fig. 3c). These observations indicate that the DR phenotype of PDAC cells correlates with increased integrinβ1 expression.
Integrinβ1 protein level is upregulated in DR-PDAC cells. a Western blot analyses of integrins related to malignant features in GR- and Ctrl-pPDAC cells (left panel). Bar graphs (right panel) show the relative value of integrin protein levels from three experiments (n = 3, mean ± s.d.) as measured by Western blot. b, c Western blot analyses of integrinβ1 in GR- and Ctrl-pPDAC (C) cells and adjacent normal (N) cells (b, upper panels), or PCL- and GR-AsPC-1 or Miapaca-2 cells (c, upper panels). Bar graphs (b, c, bottom panels) show the relative value of integrinβ1 protein levels from three experiments (n = 3, mean ± s.d.) as measured by Western blot. Data were analysed by paired or independent Student’s t test. *P ≤ 0.05, **P ≤ 0.01; ns not significant
Integrinβ1 expression correlates with drug response and PDAC patient survival
We investigated integrinβ1 protein levels using IHC in samples resected from 63 patients to assess the relevance of integrinβ1 with gemcitabine resistance in vivo. Our hypothesis was that patients expressing high levels of integrinβ1 would be more resistant to gemcitabine and display a worse clinical outcome. The neoplastic lesions of all 63 samples (100 %) exhibited positive integrinβ1 staining in the cytoplasm and cytomembrane. A linear score of staining (range 0–5) was assigned to each sample, and patients were subdivided into two groups (Table 3): the ‘high integrinβ1’ group was comprised of 29 samples (Fig. 4a, upper panels) and the ‘low integrinβ1’ group was comprised of 34 samples (Fig. 4a, lower panels).
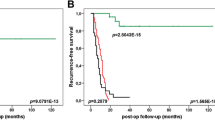
Integrinβ1 protein level correlates with the outcome of PDAC patients. a Representative images of integrinβ1 immunohistochemistry in PDAC tissues (×100 magnification). Upper panels show neoplastic lesions with strong staining (high integrinβ1; group score >3); bottom panels show neoplastic glands with weak staining (low integrinβ1; group score ≤3). b, c Kaplan-Meier patient survival curves analysis of RFS (b, log rank P = 0.005) or OS (c, Breslow P = 0.014). Low integrinβ1 group comprised 34 patients (green line), whereas high integrinβ1 group comprised 29 patients (blue line)
No differences in age, sex or pathological features (tumour site, mean tumour size, grade, TNM stage and resection margins) were found between the two groups (Table 3). However, the response to gemcitabine was significantly different. Six of the 29 high integrinβ1 patients displayed chemoresistance in ATP-TCA compared to 3 of the 34 low integrinβ1 patients (P = 0.044, Pearson’s chi-square test; Table 3). Therefore, integrinβ1 expression was likely related to the gemcitabine resistance of PDAC cells, and integrinβ1 may be a chemoresistance marker in PDAC.
Nine of the 34 patients whose tumours exhibited low integrinβ1 staining developed recurrence within 12 months after surgery compared to 16 of the 29 patients with high integrinβ1 staining (P = 0.020, Pearson’s chi-square test; Table 3). RFS was defined as the time from surgery to disease recurrence, and it was significantly shorter in the former group than the latter group when tested with a Kaplan-Meier analysis (log-rank P = 0.005, Fig. 4b). Integrinβ1 was an independent risk factor that was significantly associated with shorter RFS in a Cox regression analysis (OR 2.488; 95 % confidence interval 1.197–5.170, P = 0.015; Table 4). Seventeen of the 34 patients with low integrinβ1 died, and 22 of the 29 patients with high integrinβ1 died (P = 0.035, Pearson’s chi-square test; Table 3). The Kaplan-Meier curve also revealed significantly longer OS in low integrinβ1 patients than high integrinβ1 cases (Breslow P = 0.014, Fig. 4c). A Cox regression analysis demonstrated that integrinβ1 expression was an independent predictor of OS (OR 2.375; 95 % confidence interval 1.186–5.194, P = 0.030; Table 5). These data strongly suggest that tumours with higher integrinβ1 basal expression display more aggressive behaviour and worse responses to chemotherapy.
Knockdown of integrinβ1 sensitises DR-PDAC cells to gemcitabine and impairs cdc42 and AKT activities in cells
We analysed proliferation and apoptosis in integrinβ1-silenced DR cells (Fig. 5a) treated with gemcitabine to investigate whether integrinβ1 expression is necessary for the resistance of DR-PDAC cells to chemotherapy agents. Notably, we found a depletion of integrinβ1 in the GR-pPDAC cell line induced enhanced apoptosis even in the absence of chemotherapeutic treatments, but the two GR-PDAC cell lines did not exhibit apparent changes (Fig. 5b). The downregulation of integrinβ1 significantly rescued the sensitivity of GR-PDAC cells to gemcitabine treatment, and immunofluorescence analyses of cleaved caspase-3 and annexin V-PE were not different from PCL cells (Fig. 5b, c, left and middle panels). Western blot analysis of Ki-67 revealed a significant inhibition of cell proliferation compared to control-silenced cells (Fig. 5d). An obvious increased sensitivity to chemotherapy drugs was also observed in GR-pPDAC cells, which exhibited enhanced apoptosis and reduced proliferation in integrinβ1-silenced cells compared to control-silenced cells (Fig. 5b, c, right panel; Fig. 5d). Therefore, knockdown of integrinβ1 impaired resistance to gemcitabine, which suggests that integrinβ1 expression is involved in PDAC cell survival and escape from genotoxic stress.
Integrinβ1 downregulation impairs gemcitabine resistance and activation of Cdc42 and AKT in DR-PDAC cells. a RT-PCR and Western blot analyses (left panels) to assess integrinβ1 silencing efficiency in DR-PDAC cells transfected with either a control (si ctrl) or integrinβ1 (siβ1) small interfering RNAs (siRNAs). Bar graphs show the relative value of integrinβ1 mRNA (middle panel) and protein (right panel) levels from three experiments (n = 3, mean ± s.d.) as measured by qRT-PCR and Western blot. b, c Bar graphs show the percentage of apoptotic cells from three experiments (n = 3, mean ± s.d.) as assessed by immunofluorescence analysis of the cleaved caspase-3 (b) and Annexin V-PE (c) in PCL-, DR-, si ctrl or β1 DR-PDAC cells without or with gemcitabine. d Western blot analyses to detect expression level of Ki-67 for proliferation analysis in DR-PDAC cells (left panels) transfected with control (si ctrl) or integrinβ1 (si β1) siRNAs. Bar graphs (right panels) show relative values of Ki-67 from three experiments (n = 3, mean ± s.d.) with gemcitabine treatment. e Western blot analyses to evaluate expression levels of Cdc42, GTP-Cdc42, AKT and P-AKT in DR-PDAC cells (left panels) transfected with control (si ctrl) or integrinβ1 (si β1) siRNAs. Bar graphs show activities of Cdc42 (middle panel) or AKT (right panel) from three experiments (n = 3, mean ± s.d.) as assessed by values of GTP-Cdc42/Cdc42 or P-AKT/AKT. Statistical analyses were performed by paired Student’s t test. *P ≤ 0.05, **P ≤ 0.01; ns not significant
We knocked down integrinβ1 in GR-PDAC and GR-pPDAC cells to examine the signalling pathway that contributed to integrinβ1-related chemoresistance. Alterations in cdc42 and AKT activities, which promote oncogenic features, such as proliferation, survival, migration, invasion and chemoresistance in cancer cells, were also investigated. Knockdown of integrinβ1 decreased GTP-cdc42 and P-AKP protein levels in DR-PDAC cells and reduced cdc42 and AKT activity (Fig. 5e). These results suggest that cdc42 and AKT activation is involved in integrinβ1-related chemoresistance in DR-PDAC cells.
Collectively, these results demonstrated that high integrinβ1 expression levels were required for the maintenance of the drug-resistant phenotype of PDAC cells, and the signalling pathway that conferred chemoresistance to PDAC cells included cdc42 and AKT activation.
Discussion
PDAC is one of the most lethal cancers because of its very poor prognosis. Chemotherapies are largely ineffective, and treatment with the standard agent gemcitabine rarely improves survival in patients in advanced stages [1]. We followed 63 patients with invasive PDAC who received adjuvant gemcitabine chemotherapy after surgery who also experienced poor 1-year survival rates that correlated with differentiation, lymph nodal status, TNM stage and drug response of tumours. Therefore, elucidation of the mechanism of chemoresistance in PDAC patients may contribute to the development of novel therapeutic strategies for advanced PDAC. This study demonstrated that chronic gemcitabine exposure led to the isolation of DR cells that displayed higher resistance to gemcitabine, a prototype genotoxic drug that is a first-line treatment in human PDAC therapy [31]. These findings suggest that PDAC cells had strong capabilities to adapt to hostile factors, which led to the selection of DR subpopulations. We also identified GR-pPDAC cells from clinical samples using ATP-TCA and established a primary GR-pPDAC cell line. GR-pPDAC cell exhibited a lower response to gemcitabine and a multidrug-resistant phenotype that was characterised by a higher expression of DR markers, such as ABCC3, compared to Ctrl-pPDAC.
We focused on integrinβ1 to elucidate the molecular mechanisms involved in the intrinsic and acquired DR phenotype of PDAC cells because this molecule is a key determinant of survival, proliferation, angiogenesis, invasion and migration in human cancers [15–19]. Genotoxic stresses imposed by chemotherapies strongly stimulate some adaptive alterations that improve survival and protect cancer cells [32, 33]. Our study identified integrinβ1 as a novel contributor to cell survival as a result of intrinsic and acquired drug resistance. We found that integrinβ1 correlated most closely with chemoresistance in human PDAC in the subset of cancer-relevant integrins analysed. Notably, integrinβ1 was definitely relevant to the resistance to chemotherapy drugs. Integrinβ1 protein levels were enhanced in GR-pPDAC and GR-PDAC cells compared to Ctrl-pPDAC and PCL cells, respectively. Integrinβ1 expression in AsPC-1 was higher than the MiaPaCa-2 cell line. The relationship between integrinβ1 and DR phenotype was further supported because integrinβ1 expression was significantly related to drug response of human PDAC patients based on follow-up data analysis. We also found that high integrinβ1 expression was an independent risk factor that was significantly associated with shorter RFS and OS in patients. These results suggest that increased integrinβ1 expression is responsible for the lower response of residual cancer cells to chemotherapy. These results support integrinβ1 as a novel potential prognostic marker and therapeutic target to overcome DR. However, studies with a larger cohort of patients are required to further assess the use of integrinβ1 as a marker for the prediction of disease severity and response to chemotherapy.
The role of integrinβ1 in cancer is not fully elucidated. We found that this molecule is expressed at higher levels in neoplastic lesions, including tumour cells and the intercellular matrix, versus their normal counterparts, and the downregulation of integrinβ1 in GR-pPDAC increased cell apoptosis. These results support a correlation of integrinβ1 with malignant features. Our findings document that the upregulation of integrinβ1 in DR-PDAC cell lines was required for survival in the presence of gemcitabine because integrinβ1 knockdown restored the sensitivity of DR-PDAC cells to this agent. The influence of integrinβ1 on cell apoptosis was variable, which may relate to intrinsic or acquired high integrinβ1. PDAC cell lines are somewhat less dependent on this molecule for viability without gemcitabine. However, integrinβ1 was necessary for survival during genotoxic stress in DR-PDAC cells. Integrinβ1 expression promotes resistance to multiple therapeutic modalities, including cytotoxic drugs, radiotherapy and targeted treatments [20–22]. Our work adds to these scenarios and suggests that the suppression of integrinβ1 is a potential therapeutic tool to induce apoptosis of cancer cells with high endogenous integrinβ1 and increase the efficacy of standard chemotherapies in advanced PDAC. This strategy may be a promising approach. A humanised neutralising β1 integrin monoclonal antibody, OS2966, is currently under development for clinical trial to cure other cancers, and improvements in its design and administration may ensure its use as a cancer therapy in the near future [19].
The activation of Rho GTPases and AKT correlates to cancer onset, progression and response to therapies [34–37]. Our findings indicated that changes in Cdc42 activity, which is a Rho GTPase, and AKT were related to the modulation of integrinβ1 expression in DR-PDAC cells. A role for integrinβ1 in Rho GTPases and AKT activity was demonstrated in ovarian and lung cancer, respectively [38, 39]. Therefore, our result combined with the current record [38–40] and supports integrinβ1 as the upstream factor in the regulation of Cdc42 and AKT activities during the acquisition of a DR phenotype in PDAC cells. We demonstrated that high integrinβ1 expression accounted for the inherent and acquired resistance to genotoxic drugs because GR-PDAC cells with integrinβ1 suppression became sensitive to gemcitabine similar to PCL cells, and resistance to gemcitabine was impaired as integrinβ1 levels decreased in GR-pPDAC cells. Therefore, our results suggest that upregulated integrinβ1 plays a key role in the acquisition of chemoresistance in PDAC cells, and this resistance results from changes in Cdc42 and AKT activity.
Conclusions
We demonstrated that integrinβ1 was upregulated in PDAC cell lines and clinical samples. Our data demonstrated that integrinβ1 regulated Cdc42 and AKT activity, and the suppression of integrinβ1 significantly impaired PDAC cells resistance to gemcitabine partially via reductions in Cdc42 and AKT activity. Our identification of Cdc42 and AKT activity as targets of integrinβ1 provides new insights into the pathways of resistance to chemotherapies in PDAC and supports integrinβ1 as a novel therapeutic target for PDAC.
PDAC, pancreatic ductal adenocarcinoma cancer; DR, drug-resistant; ATP-TCA, ATP-based tumour chemosensitivity assay; PCL, parent cell line; qRT-PCR, quantitative real-time PCR; IHC, immunohistochemistry; RNAi, RNA interference; siRNAs, small interfering RNAs; CSC, cancer stem cell; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; TDC, test drug concentration; IC50, inhibitory concentration of 50 %; RFS, relapse-free survival; OS, overall survival.
References
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–72.
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–81.
Oettle H, Neuhaus P. Adjuvant therapy in pancreatic cancer: a critical appraisal. Drugs. 2007;67(16):2293–310.
Neoptolemos JP, Cunningham D, Friess H, Bassi C, Stocken DD, Tait DM, et al. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol. 2003;14(5):675–92.
Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62(2):317–26.
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–17.
Tang SC, Chen YC. Novel therapeutic targets for pancreatic cancer. World J Gastroenterol. 2014;20(31):10825–44.
Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol. 2010;75(3):173–234.
Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17(17):5530–7.
Zhang L, Wu Z, Zhou Q. Epithelial-mesenchymal transition and tumor chemoresistance. Chin J Lung Canc. 2013;16(1):54–7.
Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–76.
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61.
Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66(4):2146–52.
Weaver VM, Lelièvre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2(3):205–16.
Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–26.
Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31.
Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005;24(11):1942–53.
Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362–74.
Jahangiri A, Aghi MK, Carbonell WS. β1 integrin: critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014;74(1):3–7.
Huang C, Park CC, Hilsenbeck SG, Ward R, Rimawi MF, Wang YC, et al. Beta1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res. 2011;13(4):R84.
Nam JM, Chung Y, Hsu HC, Park CC. Beta1 integrin targeting to enhance radiation therapy. Int J Radiat Biol. 2009;85(11):923–8.
Mocanu MM, Fazekas Z, Petras M, Nagy P, Sebestyen Z, Isola J, et al. Associations of ErbB2, beta1-integrin, and lipid rafts on Herceptin (Trastuzumab) resistant and sensitive tumor cell lines. Cancer Lett. 2005;227(2):201–12.
Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Löhr JM, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121(4):699–705.
Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9(8):454–67.
Ugurel S, Schadendorf D, Pföhler C, Neuber K, Thoelke A, Ulrich J, et al. In vitro drug sensitivity predicts response and survival after individualized sensitivity-directed chemotherapy in metastatic melanoma: a multicenter phase II trial of the Dermatologic Cooperative Oncology Group. Clin Cancer Res. 2006;12(18):5454–63.
Michalski CW, Erkan M, Sauliunaite D, Giese T, Stratmann R, Sartori C, et al. Ex vivo chemosensitivity testing and gene expression profiling predict response towards adjuvant gemcitabine treatment in pancreatic cancer. Br J Cancer. 2008;99(5):760–7.
Hamed SS, Straubinger RM, Jusko WJ. Pharmacodynamic modeling of cell cycle and apoptotic effects of gemcitabine on pancreatic adenocarcinoma cells. Cancer Chemother Pharmacol. 2013;72(3):553–63.
Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000;6(8):324–9.
Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33(7):405–12.
Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–99.
Costello BA, Borad MJ, Qi Y, Kim GP, Northfelt DW, Erlichman C, et al. Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Invest New Drugs. 2014;32(4):710–6.
Dutertre M, Sanchez G, Barbier J, Corcos L, Auboeuf D. The emerging role of pre-messenger RNA splicing in stress responses: sending alternative messages and silent messengers. RNA Biol. 2011;8(5):740–7.
Busà R, Geremia R, Sette C. Genotoxic stress causes the accumulation of the splicing regulator Sam68 in nuclear foci of transcriptionally active chromatin. Nucleic Acids Res. 2010;38(9):3005–18.
Kutys ML, Yamada KM. An extracellular-matrix-specific GEF-GAP interaction regulates Rho GTPase crosstalk for 3D collagen migration. Nat Cell Biol. 2014;16(9):909–17.
Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23(9):1415–23.
Reymond N, Im JH, Garg R, Vega FM, Borda d’Agua B, Riou P, et al. Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J Cell Biol. 2012;199(4):653–68.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Miao H, Li S, Hu YL, Yuan S, Zhao Y, Chen BP, et al. Differential regulation of Rho GTPases by β1 and β3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci. 2002;115(Pt10):2199–206.
Kanda R, Kawahara A, Watari K, Murakami Y, Sonoda K, Maeda M, et al. Erlotinib resistance in lung cancer cells mediated by integrin β1/Src/Akt-driven bypass signaling. Cancer Res. 2013;73(20):6243–53.
Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, et al. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of β1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol. 2012;29(4):2512–8.
Acknowledgments
The study was supported by Department of Cell Biology, Basic Research Institute, Second Military Medical University (Shanghai, China).
Authors’ contributions
Qingping Cai conceived and designed the experiments. Dejun Yang and Ronglin Ran revised the manuscript and carried out the further experiments. Dejun Yang, Jian Shi, Hongbing Fu, Ziran Wei, Jiapeng Xu and Yu Zhang performed the experiments. Hongbing Fu and Yu Zhang collected the samples and analysed the data. Dejun Yang wrote the paper. All authors are in agreement with the content of the manuscript and this submission. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Informed consent was obtained from patients, and the study was approved by the Second Military Medical University Research Ethics Committee (Institutional Review Board), Shanghai, China.
Conflicts of interests
None
Additional information
Dejun Yang, Jian Shi, and Hongbing Fu are first authors.
Dejun Yang, Jian Shi and Hongbing Fu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, D., Shi, J., Fu, H. et al. Integrinβ1 modulates tumour resistance to gemcitabine and serves as an independent prognostic factor in pancreatic adenocarcinomas. Tumor Biol. 37, 12315–12327 (2016). https://doi.org/10.1007/s13277-016-5061-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5061-7