Abstract
This study investigated the expression of nucleolin in tissue samples in patients with non-small cell lung cancer (NSCLC). Nucleolin was studied to determine whether it has a prognostic value and if its levels correlate with various clinicopathologic parameters. The relationship between nucleolin and expression of DNA-PKcs was also evaluated. Immunohistochemistry was used for detecting the expression levels of nucleolin and DNA-PKcs in tissues from 225 stage IA to IIIB NSCLC patients who underwent lung surgery. Nucleolin was observed predominantly in the cytoplasm, and some levels were observed in the nucleus. Nucleolin expression was higher in NSCLC tissues than adjacent normal lung tissues. Among 225 NSCLC patients, 117 (52.0 %) had high expression of nucleolin. The expression of nucleolin was significantly associated with pathologic stage (P = 0.013) and T status (P = 0.043). Multivariate analysis revealed that nucleolin, cytoplasmic nucleolin, and nuclear nucleolin expression were independent prognostic factors for both overall survival (OS) (P < 0.001) and disease-free survival (DFS) (P < 0.001). A high level of nuclear nucleolin served as an independent prognostic factor for better survival, while a high level of cytoplasmic nucleolin was closely associated with worse prognosis in NSCLC patients. The expression of nucleolin and cytoplasmic nucleolin positively correlated with DNA-PKcs (P < 0.001). These data suggest that nucleolin could be an effective treatment target and prognostic factor for patients with NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common malignant tumors and leading causes of cancer deaths, with an increasing incidence and a poor prognosis [1]. Therefore, new treatment modalities that improve survival rates are urgently needed. A clearer understanding of the biology of NSCLC at the molecular level will aid in the development of new treatment strategies, including methods to accurately predict the prognosis of patients with certain clinical-pathological characteristics and identification of new targets for radiation therapy.
Resistance to cell death is a hallmark of cancer that contributes to progression to high-grade malignancy and therapeutic resistance. Although the cellular conditions that trigger apoptosis remain to be fully enumerated, it has been reported that abnormalities in DNA damage sensory play a key role in tumor development [2].
Nucleolin (C23) is a 100–110-kDa protein that is ubiquitously expressed in exponentially growing eukaryotic cells, accounting for approximately 10 % of the total protein content within the nucleolus [3]. It is able to bind DNA, RNA, and protein and has been demonstrated to play an essential role in tumorigenesis by promoting cell proliferation and inhibiting cell apoptosis [4]. A study by Willimott et al. reported that upregulating the expression of C23 stabilized Bcl-2 messenger RNA (mRNA), which increased Bcl-2 expression level and inhibited apoptosis [5]. Similarly, upregulating C23 in endothelial cells was shown to inhibit hydrogen peroxide-induced apoptosis [6]. Other studies found that downregulating Hsp70 in laryngeal cancer cells increased the fracture and degradation of C23, which subsequently led to increased radiosensitivity [7].
As a multifunctional protein, C23 is involved in DNA damage repair [8]. C23 binding to Rad51 may regulate the repair of chromosomal DNA by homologous recombination (HR) [9]. C23 is able to interact with Rad50 and is recruited to DNA double-strand breaks (DSBs) in an MRE11-NBS1-RAD50 complex-dependent manner [10]. Notably, C23 knockdown decreased ATM-dependent phosphorylation following exposure to γ-radiation or activation of cell cycle checkpoints. Furthermore, C23 knockdown may inactivate the catalytic subunit of autophosphorylation of DNA-dependent protein kinase (DNA-PKcs) [11].
There are currently few reports which study the effects of nucleolin on DNA damage response in patients with NSCLC. We hypothesized that nucleolin may serve as an important marker for DNA damage response. In this study, the relationship between nucleolin expression and the prognosis of NSCLC patients was investigated to determine whether nucleolin could be a new prognostic marker for the diagnosis and treatment of NSCLC.
Materials and methods
Patients
This is a retrospective study approved by the Medical Ethical Committee of the Third Affiliated Hospital of Harbin Medical University, Harbin, China. The informed consent letters were provided by all participants. This study comprised primary NSCLC specimens and 42 adjacent normal lung tissue specimens which corresponded to primary tumors from the 225 patients. Patients included stage IA to IIIB NSCLC who underwent surgery at the Third Affiliated Hospital of Harbin Medical University between January 2005 and December 2007. No patients received preoperative chemotherapy and radiotherapy. The age of patients ranged from 28 to 82 years, with a median age of 58 years. All patients were followed up for survival analysis until March 31, 2014. Among the patients, 62 (27.6 %) had stage I disease, 87 (38.7 %) had stage II disease, and 76 (33.8 %) had stage III disease. Histological classification showed that patient tumor samples consisted of 121 squamous cell carcinomas, 103 adenocarcinomas, and 1 adenosquamous carcinoma. In total, 187 patients underwent lobectomy, 33 patients underwent pneumonectomy, and 5 patients underwent segmentectomy. One hundred and sixty-six patients received 3 to 4 cycles of adjuvant chemotherapy, and 62 patients received postoperative radiotherapy (total dose range, 50–66 Gy), with 2 Gy per fraction given 5 days per week. The patients’ clinicopathological characteristics are presented in Table 1.
Immunohistochemistry
Paraffin-embedded NSCLC specimens were cut into 4-μm sections. The sections were placed on Gold Seal microscope frosted slides (VWR Scientific, West Chester, PA, USA) and incubated at 65 °C for 30 min. The slides were deparaffinized in the first bottle containing xylene for 10 min, followed by treatment for 10 min in dimethyl benzene. The slides were rehydrated with graded alcohol concentrations using anhydrous ethanol for 5 min, 90 % ethanol for 5 min, 70 % ethanol for 5 min, and deionized water for 5 min and washed with phosphate-buffered saline (PBS, 0.1 M, pH 7.4) three times each for 5 min. Endogenous peroxidase activity was blocked by incubation in 3 % hydrogen peroxide (H2O2) for 10 min at room temperature. The slides were then submerged in citrate buffer (0.01 M, pH 6.0) and heated in a pressure cooker for 5 min for antigen retrieval. The slides were washed with PBS three times for 5 min each. After treatment with 5 % BSA, the slides were incubated with a mouse monoclonal antibody against nucleolin (dilution 1:200; Santa Cruz Biotechnology) and a rabbit polyclonal antibody against DNA-PKcs (1:500; Thermo Scientific) overnight at 4 °C. After washing with PBS, the slides were incubated with the biotinylated secondary antibody followed by peroxidase-conjugated streptavidin for 30 min. For visualizing the reaction, we added 3 μl of 3 % H2O2 to 1 ml of 3-3′-diaminobenzidine tetrahydrochloride (Dako, Denmark) and blend it fully. Each section was then added with a drop of 50 μl of the mixture and chromogenic for 20 to 30 min. The slides were rinsed with distilled water, counterstained with hematoxylin, dehydrated, and mounted for further analysis. Mouse IgG and rabbit IgG (Cell Signaling Technology, Beverly, MA, USA) were used as a negative control at the same dilution as the primary antibody.
Immunohistochemical analysis
Evaluation of the staining for nucleolin and DNA-PKcs expression was conducted by bright-field light microscopy with two independent and experienced pathologists without prior knowledge of the clinicopathology of the tumors. Levels of nucleolin and DNA-PKcs in tumor samples were classified semiquantitatively by the total combined scores, which was based on the proportion and intensity of positively stained cells. The percentage of positively stained cells was scored as follows: no positive cells (0), ≤10 % positive cells (1), 11–50 % positive cells (2), 51–75 % positive cells (3), and >75 % positive cells (4). The staining intensity was scored as follows: colorless (0), slightly yellow (1), yellow brown (2), and dark brown (3). The expression levels of nucleolin and DNA-PKcs were determined by multiplying the staining intensity score with the percentage of positive stained cells. Levels of cytoplasmic nucleolin and nuclear nucleolin were also determined. The groups’ scores acquired from the two pathologists were entered for statistical analyses. Subsequently, the mean scores to calculate the median value were obtained, which was used as a cutoff to categorize the patients into two groups: low expression and high expression.
Statistical analysis
The association between the immunohistochemistry (IHC) parameters and IHC/clinicopathological parameters was analyzed using the chi-square test, Fisher’s exact test, and Pearson rank correlation. Overall survival (OS) and disease-free survival (DFS) were calculated using the Kaplan-Meier method. A univariate analysis for each prognostic variable was performed by using the log-rank test. Multivariate analysis was conducted using the Cox proportional hazards regression model to identify independent prognostic factors which influenced the OS or DFS. P < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Expression of nucleolin and DNA-PKcs in NSCLC patients
Immunohistochemistry was used to assess levels of nucleolin and DNA-PKcs in 225 primary NSCLC and 42 adjacent normal lung tissue specimens. Nucleolin expression was detected in 142 (62.8 %) NSCLC tumor samples and in 15 (35.7 %) adjacent normal lung tissues. Nucleolin was observed predominantly in the cytoplasm and in the nucleus. Based on the staining and the positive staining score, 108 (48.0 %) tumor specimens showed low expression and 117 (52.0 %) tumor specimens showed high expression of nucleolin. Overall, nucleolin was highly detectable in tumor cells compared to neighboring normal lung tissues (Fig. 1).
DNA-PKcs expression was detected in 162 (72.4 %) tumor samples and in 20 (47.6 %) adjacent normal lung tissues. DNA-PKcs was mostly observed in the nucleus. The adjacent normal lung epithelial cells had weak staining intensity for DNA-PKcs compared to tumor tissues (Fig. 2). Based on the staining scores, 105 (46.7 %) tumor specimens showed low expression and 120 (53.3 %) tumor specimens showed high expression of DNA-PKcs.
Correlation with clinicopathological parameters
We next assessed whether nucleolin or DNA-PKcs expression correlated with clinicopathological parameters in NSCLC. Table 1 demonstrated that nucleolin expression was significantly associated with the pathologic stage and T status. Patients diagnosed with higher pathologic stage (P = 0.013) and T status (P = 0.043) had higher expression of nucleolin. There was no statistically significant difference between nucleolin expression with age, gender, histological type, differentiation grade, and lymph node status (P > 0.05).
The expression of DNA-PKcs was significantly associated with pathological stage and lymph node status. Patients diagnosed with higher pathologic stage had higher expression of DNA-PKcs (P = 0.029). DNA-PKcs expression in patients with lymph node metastasis was significantly higher than patients without lymph node metastasis (P = 0.027). There was no statistically significant difference between DNA-PKcs expression with age, gender, histological type, differentiation grade, and T stage (P > 0.05, Table 1).
Correlation of nucleolin or DNA-PKcs expression with prognosis
Based on the survival analysis of 225 NSCLC samples, the median OS time was 51.8 months and the median DFS time was 27.8 months.
We observed that nucleolin expression was significantly correlated with OS and DFS. The low- and high-nucleolin expression groups had a median OS of 85.5 and 37.1 months, respectively. The median DFS of the low- and high-nucleolin expression groups was 71.9 and 29.0 months, respectively. Additionally, there was a significant negative correlation between nucleolin expression levels with OS (P < 0.001, Fig. 3a) and DFS (P < 0.001, Fig. 3b).
Kaplan-Meier survival curves for patients with NSCLC categorized by nucleolin and DNA-PKcs expression. a Overall survival of patients with high nucleolin expression compared to patients with low nucleolin expression, P < 0.001. b Disease-free survival of patients with high nucleolin expression compared to patients with low nucleolin expression, P < 0.001. c Overall survival of patients with high cytoplasmic nucleolin expression compared to patients with low cytoplasmic nucleolin expression, P < 0.001. d Disease-free survival of patients with high cytoplasmic nucleolin expression compared to patients with low cytoplasmic nucleolin expression, P < 0.001. e Overall survival of patients with high nuclear nucleolin expression compared to patients with low nuclear nucleolin expression, P = 0.020. f Disease-free survival of patients with high nuclear nucleolin expression compared to patients with low nuclear nucleolin expression, P = 0.003. g Overall survival of patients with high DNA-PKcs expression compared to patients with low DNA-PKcs expression, P < 0.001. h Disease-free survival of patients with high DNA-PKcs expression compared to patients with low DNA-PKcs expression, P < 0.001
The expression of cytoplasmic nucleolin was significantly correlated with OS and DFS. The low- and high-cytoplasmic nucleolin expression groups had a median OS of 84.6 and 36.1 months, respectively. The median DFS of the low- and high-cytoplasmic nucleolin expression groups was 70.0 and 29.0 months, respectively. There was also a significant negative association between cytoplasmic nucleolin expression levels with OS (P < 0.001, Fig. 3c) and DFS (P < 0.001, Fig. 3d).
Nuclear nucleolin expression level significantly correlated with OS and DFS. In this group, the median OS in the low- and high-nuclear nucleolin expression groups was 54.6 and 70.6 months, respectively. The median DFS of the low- and high-nuclear nucleolin expression groups was 44.2 and 61.6 months, respectively. Expression of nuclear nucleolin significantly correlated with OS (P = 0.020, Fig. 3e) and DFS (P = 0.003, Fig. 3f).
The expression of DNA-PKcs also significantly correlated with OS and DFS. Groups with low DNA-PKcs had a lower median OS than those expressing high levels of DNA-PKcs (68.7 vs 25.7 months). The median DFS of the low- and high-DNA-PKcs expression groups was 58.2 and 19.6 months, respectively. There was a significant negative correlation between expression levels of DNA-PKcs with OS (P < 0.001, Fig. 3g) and DFS (P < 0.001, Fig. 3h).
Multivariate analysis revealed that tumor histological type, pathologic stage, nucleolin, cytoplasmic nucleolin, nuclear nucleolin, and DNA-PKcs expression were independent prognostic factors for both OS and DFS (Table 2).
We analyzed the histological type which correlated with prognosis. The OS (67.5 vs 50.3 months) and DFS (57.5 vs 38.1 months) were much improved in patients with squamous cell carcinoma than in those with adenocarcinoma, respectively (Fig. 4a, b). We further investigated the relationship between the histological type and localization of nucleolin (Fig. 5). We observed that high expression of cytoplasmic nucleolin was associated with poorer OS and DFS in patients with squamous cell carcinoma or adenocarcinoma (Fig. 4c–f). Additionally, high expression of nuclear nucleolin was also associated with improved DFS in patients with squamous cell carcinoma (P = 0.011, Fig. 4h).
Kaplan-Meier survival curves for patients with NSCLC categorized by squamous cell carcinoma and adenocarcinoma. a Overall survival of patients with squamous cell carcinoma compared to adenocarcinoma, P < 0.001. b Disease-free survival of patients with squamous cell carcinoma compared to adenocarcinoma, P < 0.001. c Overall survival of patients with high cytoplasmic nucleolin expression compared to patients with low cytoplasmic nucleolin expression in squamous cell carcinoma, P < 0.001. d Disease-free survival of patients with high cytoplasmic nucleolin expression compared to patients with low cytoplasmic nucleolin expression in squamous cell carcinoma, P < 0.001. e Overall survival of patients with high cytoplasmic nucleolin expression compared to patients with low cytoplasmic nucleolin expression in adenocarcinoma, P < 0.001. f Disease-free survival of patients with high cytoplasmic nucleolin expression compared to patients with low cytoplasmic nucleolin expression in adenocarcinoma, P < 0.001. g Overall survival of patients with high nuclear nucleolin expression compared to patients with low nuclear nucleolin expression in squamous cell carcinoma, P = 0.149. h Disease-free survival of patients with high nuclear nucleolin expression compared to patients with low nuclear nucleolin expression in squamous cell carcinoma, P = 0.011. i Overall survival of patients with high nuclear nucleolin expression compared to patients with low nuclear nucleolin expression in adenocarcinoma, P = 0.098. j Disease-free survival of patients with high nuclear nucleolin expression compared to patients with low nuclear nucleolin expression in adenocarcinoma, P = 0.208
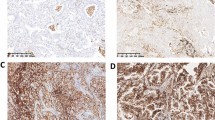
Immunohistochemical staining of cytoplasmic and nuclear nucleolin in NSCLC specimens. A, B Nuclear nucleolin expression in adenocarcinoma tissues. C, D Cytoplasmic nucleolin expression in adenocarcinoma tissues. E, F nuclear nucleolin expression in squamous cell carcinoma tissues. G, H Cytoplasmic nucleolin expression in squamous cell carcinoma tissues
Correlation between nucleolin and DNA-PKcs gene
We further investigated the association between nucleolin and DNA-PKcs expression (Table 3). The analysis demonstrated that nucleolin and cytoplasmic nucleolin positively correlate with DNA-PKcs expression, while no association was observed between nuclear nucleolin and DNA-PKcs expression.
In total, 81.7 % (98/120) of patients with high DNA-PKcs had high nucleolin expression. This is in contrast to 18.3 % (21/120) of patients with high DNA-PKcs and low nucleolin expression (P < 0.001). Similar to nucleolin expression, 78.3 % (94/120) of patients with high DNA-PKcs had concurrent high cytoplasmic nucleolin expression. This is in contrast to 21.7 % (26/120) of patients with high DNA-PKcs and low cytoplasmic nucleolin expression (P < 0.001). In nucleolin-negative cases, DNA-PKcs expression significantly influenced OS (53.8 vs 91.5 months; P = 0.001) and DFS (45.6 vs 77.0 months; P = 0.014). In patients that did not express cytoplasmic nucleolin, DNA-PKcs expression significantly influenced OS (55.6 vs 89.8 months; P = 0.010) and DFS (47.8 vs 74.5 months; P = 0.030).
Discussion
In this study, levels of nucleolin were reported to be highly expressed in NSCLC tissues. As determined by IHC, nucleolin expression was detected in 62.8 % (142/225) of patients with stage IA to IIIB NSCLC who underwent lung surgery and in 35.7 % (15/42) of patients with adjacent normal lung tissues. This indicated that nucleolin expression was significantly high in tumor tissues compared to adjacent normal lung tissues.
However, only a few studies have assessed the role of nucleolin in NSCLC. Nucleolin was detected in 34.2 % (50/146) of patients with NSCLC [12]. Nucleolin levels were detected at higher levels in NSCLC samples compared to a previous study [12]. This could be attributed to sample size and the cutoff point used to define levels of nucleolin. A total of 267 specimens (225 tumor specimens and 42 adjacent normal lung tissues) were used for the IHC analyses, which is higher than other previous published studies. Moreover, the cutoff point used was based on the median score of nucleolin expression that allow categorization of patients into low- or high-nucleolin expression groups. The selected median score used in this study as the cutoff point may reduce the maximal deviation.
Nucleolin expression was an independent prognostic factor for poor survival [12]. In this study, nucleolin expression level was demonstrated to be independent prognostic factors for both OS and DFS. The median OS of the low- and high-nucleolin expression groups was 85.5 and 37.1 months, respectively. The median DFS of the low- and high-nucleolin expression groups was 71.9 and 29.0 months, respectively.
In our study, the cellular localization of nucleolin was assessed as a prognostic factor. Based on the staining scores and localization that were obtained, 109 (48.4 %) tumor specimens showed low expression and 116 (51.6 %) tumor specimens showed high expression of cytoplasmic nucleolin as well as 154 (68.4 %) tumor had low expression and 71 (31.6 %) tumor had high expression of nuclear nucleolin. Another study demonstrated that among the 124 samples from patients with gastric cancer, 85 (68.5 %) had a high expression level of nucleolin. A high level of cytoplasmic nucleolin was associated with poorer survival, while high nucleolar staining was associated with better prognosis [13], which was consistent with the results from our study. We observed that high levels of nuclear nucleolin were an independent prognostic factor for better survival, while high levels of cytoplasmic nucleolin were closely associated with poorer prognosis in NSCLC patients.
Nucleolin is found to be most abundant in the nucleolus, which comprised approximately 10 % of the total nucleoli protein. As a multifunctional protein, nucleolin is involved in DNA damage repair. It is reported to function as a stress-responsive mRNA-binding protein, binding to untranslated regions (UTRs) of mRNA in a sequence-nonspecific manner to stabilize the mRNAs [14] or regulate their translation. Nucleolin repressed p53 translation by binding to both the 5′- and 3′-UTRs of p53 mRNA and binding to the same 5′- and 3′-UTR interaction region that is critical for the recruitment of RPL26 to p53 mRNA during DNA damage [15]. In our previous study, knockdown of nucleolin inhibited DNA-PKcs phosphorylation activity at the S2056 and T2609 sites, thus reducing radiation-induced damage repair and increasing the radiosensitivity of NSCLC cells [16]. Therefore, we hypothesized that nucleolin may serve as an important biomarker for DNA damage response and investigated the relationship between nucleolin and DNA-PKcs.
DNA-PKcs, a key protein in the NHEJ pathway, consists of a 465-kDa catalytic subunit (DNA-PKcs) and a heterodimeric regulatory complex (Ku) that comprises a 70-kDa subunit (Ku70) and an 86-kDa subunit (Ku80) [17]. The expression of DNA-PKcs was significantly higher in tumor tissues than in normal tissues. Patients with high DNA-PKcs expression were associated with a significantly worse OS and DFS than those with lower expression [18]. Our results demonstrated that DNA-PKcs expression is highly over-expressed in NSCLC tissues and correlates with a worsen OS and DFS.
We next analyzed the relationship between nucleolin with DNA-PKcs expression in tissue samples obtained from patients with NSCLC. The analysis showed that there was a positive correlation between nucleolin, cytoplasmic nucleolin, and DNA-PKcs. In samples that were negative for DNA-PKcs, high expression of nucleolin and cytoplasmic nucleolin was associated with a poorer OS and DFS. In patient samples with high expression of DNA-PKcs and nucleolin, the OS (29.6 vs 89.8 months) and DFS (19.6 vs 74.5 months) of patients were significantly reduced compared to samples that had low expression. In samples with high expression of DNA-PKcs and cytoplasmic nucleolin, the OS (22.6 vs 91.5 months) and DFS (17.6 vs 77.0 months) of patients were significantly reduced compared to those of lower expression. These results demonstrated that high expression of nucleolin and cytoplasmic nucleolin correlated with DNA-PKcs levels and was associated with worse survival. In our previous study, nucleolin knockdown inhibited DNA-PKcs phosphorylation activity in NSCLC cells, suggesting that nucleolin may affect the tumor treatment through DNA damage repair pathways.
We also observed that nucleolin levels are associated with DNA damage repair (e.g., DNA-PKcs) and could serve as a potential biomarker of treatment outcome. Nucleolin may be an effective treatment target and prognostic factor in patients with NSCLC. There is currently a phase II study to assess AS1411-targeted nucleolin for the treatment of metastatic renal cell carcinoma [19]. Further studies will include targeting nucleolin to potentially improve survival benefits in patients with NSCLC.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Bicknell K, Brooks G, Kaiser P, Chen H, Dove BK, Hiscox JA. Nucleolin is regulated both at the level of transcription and translation. Biochem Biophys Res Commun. 2005;332:817–22.
Derenzini M, Sirri V, Trere D, Ochs RL. The quantity of nucleolar proteins nucleolin and protein B23 is related to cell doubling time in human cancer cells. Lab Investig. 1995;73:497–502.
Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans. 2010;38:1571–5.
Zhang B, Wang H, Jiang B, Liang P, Liu M, Deng G, et al. Nucleolin/C23 is a negative regulator of hydrogen peroxide-induced apoptosis in HUVECs. Cell Stress Chaperones. 2010;15:249–57.
Xu J, Wang K, Zhang X, Qiu Y, Huang D, Li W, et al. HSP70: a promising target for laryngeal carcinoma radiotherapy by inhibiting cleavage and degradation of nucleolin. J Exp Clin Cancer Res. 2010;26:106.
Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112(Pt 6):761–72.
De A, Donahue SL, Tabah A, et al. A novel interaction [corrected] of nucleolin with Rad51. Biochem Biophys Res Commun. 2006;344:206–13.
Goldstein M, Derheimer FA, Tait-Mulder J, Castro NE, Mraz N, Cruise JL, et al. Nucleolin mediates nucleosome disruption critical for DNA double-strand break repair. Proc Natl Acad Sci U S A. 2013;110:16874–9.
Kobayashi J, Fujimoto H, Sato J, Hayashi I, Burma S, Matsuura S, et al. Nucleolin participates in DNA double-strand break-induced damage response through MDC1-dependent pathway. PLoS One. 2012;7:e49245.
Zhao H, Huang Y, Xue C, Chen Y, Hou X, Guo Y, et al. Prognostic significance of the combined score of endothelial expression of nucleolin and CD31 in surgically resected non-small cell lung cancer. PLoS One. 2013;8:e54674.
Qiu W, Zhou F, Zhang Q, Sun X, Shi X, Liang Y, et al. Overexpression of nucleolin and different expression sites both related to the prognosis of gastric cancer. APMIS. 2013;121:919–25.
Zhang J, Tsaprailis G, Bowden GT. Nucleolin stabilizes Bcl-X L messenger RNA in response to UVA irradiation. Cancer Res. 2008;68:1046–54.
Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J Biol Chem. 2010;287:16467–76.
Xu JY, Lu S, Xu XY, Hu SL, Li B, Qi RX, et al. Knocking down nucleolin expression enhances the radiosensitivity of non-small cell lung cancer by influencing DNA-PKcs activity. Asian Pac J Cancer Prev. 2015;16:3301–6.
Beskow C, Skikuniene J, Holgersson A, Nilsson B, Lewensohn R, Kanter L, et al. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer. 2009;101:816–21.
Hu S, Qu Y, Xu X, Xu Q, Geng J, Xu J. Nuclear survivin and its relationship to DNA damage repair genes in non-small cell lung cancer investigated using tissue array. PLoS One. 2013;8:e74161.
Rosenberg JE, Bambury RM, Van Allen EM, Drabkin HA, Lara Jr PN, Harzstark AL, et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig New Drugs. 2014;32:178–87.
Acknowledgments
We are grateful to Dr. Geng Jing-shu at the Third Affiliated Hospital of Harbin Medical University. Xu Jian-yu was supported by a hospital research startup fund at the Third Affiliated Hospital of Harbin Medical University (JJZ2011-19). Xiang-ying Xu was supported by the Heilongjiang Provincial Science and Technology projects (WB12C101) and Special Fund for Innovative Talent in Science and Technology Research of Harbin (2012RFXXS063).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Xu, Jy., Lu, S., Xu, Xy. et al. Prognostic significance of nuclear or cytoplasmic nucleolin expression in human non-small cell lung cancer and its relationship with DNA-PKcs. Tumor Biol. 37, 10349–10356 (2016). https://doi.org/10.1007/s13277-016-4920-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4920-6