Abstract
Sex-determining region Y-related high-mobility group box 4 (SOX4) has been proven to serve as a critical role in cancer progression. However, the pathological role of SOX4 in colorectal cancer (CRC) remains unknown. The aim of this study was to investigate the role of SOX4 in CRC. In this study, we investigated the expression of SOX4 in CRC tissues by immunohistochemistry, quantitative reverse transcription polymerase chain reaction (qRT-PCR), and Western blot. We also evaluated the effect of SOX4 on cell proliferation and invasion by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and transwell assay. The SOX4 messenger RNA (mRNA) and protein expression were markedly higher in CRC tissues compared with adjacent normal mucosa tissues. Inhibition of SOX4 could suppress CRC cell proliferation, and invasion in vitro. Our findings indicate that targeting SOX4 might provide a new therapeutic modality for the treatment of CRC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is one of the most common malignant neoplasms and the third leading cause of cancer patient’s death worldwide, accounting for approximately 10 % of all cancer incidence and mortality [1, 2]. In China, the incidence of this disease has been increased in the last decades [2, 3]. Due to the high local tumor recurrence, further progression, and distant metastases, poor clinical outcome has been shown for CRC patients [4, 5]. Although surgical techniques and adjuvant therapies have been established, the overall survival rate remains unfavorable [6, 7]. Therefore, it is urgent to identify useful biomarkers and explore novel therapeutic targets for early diagnosis and for the development of more efficient treatment.
The sex-determining region Y (SRY) related high-mobility group (HMG) box family includes 20 highly conserved transcription factors that could tumor progression [8]. Sex-determining region Y-related high-mobility group box 4 (SOX4), a 47 kDa protein, is essential to endocardial development and lymphocyte differentiation [9–11]. SOX4 is overexpressed in various types of human cancers, often correlating with tumor angiogenesis and resistance to radiation and chemotherapy [12, 13]. However, the precise role of the SOX4 in human CRC progression is still unknown. In this study, we demonstrated that SOX4 expression is upregulated in CRC, and SOX4 promotes CRC cell proliferation, migration, and invasion in vitro. Furthermore, we demonstrate that SOX4 promotes cell migration and invasion via epithelial-mesenchymal transition (EMT) pathway. Taken together, these data provide substantial new evidence that SOX4 is involved in CRC progression.
Materials and methods
Patients and tissue samples
Surgically resected primary CRC tissues and adjacent normal mucosa tissues from 119 CRC patients were obtained from the archives of Xiangya Hospital, Central South University with the approval of the medical ethics committee and with informed consent. Paraffin-embedded blocks of these tissues were included in the present study. None of our study patients had received preoperative chemotherapy and/or radiotherapy. For qRT-PCR and Western blot analyses, 30 cases of freshly resected CRC tissues and adjacent normal mucosa tissues were immediately frozen in liquid nitrogen and stored at −80 °C until use.
Immunohistochemistry
Tissue microarrays were constructed. Tissue paraffin blocks were stained with hematoxylin-eosin to confirm the diagnoses and marked at fixed points with most typical histological characteristics under a microscope. Two 2-mm cores per donor block were transferred into a recipient block tissue microarrayer, and each dot array contained fewer than 160 dots. Three-micron-thick sections were cut from the recipient block and transferred to glass slides with an adhesive tape transfer system for ultraviolet cross-linkage. Immunoreactivity of SOX4 was evaluated according to both of the proportion of stained cells and their intensity. The extent of SOX4 staining was semi-quantitatively scored as follows: 0 = negative, 1 = 1–25 % of cells, 2 = 26–50 % of cells, 3 = 51–75 % of cells, and 4 = 76–100 % of cells were stained. Staining intensity was scored as 0 = negative, 1 = weak, 2 = medium, and 3 = strong. The sum of the intensity and extent score was used as the final staining score. Low expression was defined as a total score ≤4 and high expression with a total score ≥4. Immunohistochemical staining results were scored by two independent pathologists without prior knowledge of patients’ detail medical history, and mean percentage values of two scores were taken.
Cell culture
The human CRC cell lines HCT116, SW480, SW620, and LoVo were grown in RPMI-1640 medium supplemented with 10 % fetal bovine serum (FBS). The human colon epithelial cell line NCM460 was cultured in MEM medium supplemented with 10 % FBS. Cells were maintained in a humidified atmosphere at 37 °C and 5 % CO2. After cultures became 80 % confluent (usually after 3 days), cells were trypsinized, centrifuged, and suspended in fresh medium. All cells used for experiments displayed >95 % viability and were seeded in 96-well plates, 6-well culture plates, 60-mm culture plates, or ultra low-attachment plates (Corning Inc, Lowell, MA, USA). All experiments were carried out in duplicate and repeated at least three times.
RNA extraction and semi-quantitative RT-PCR
Total cellular RNA was extracted from cells using the RNeasy Plus Mini Kit from Qiagen. The quality and yield of the RNA samples were determined by ultraviolet spectrophotometer. Total RNAs (1 μg) were reverse transcribed to complementary DNA (cDNA) (20 μL) using PrimeScriptTM RT Kit (TaKaRa) according to the manufacturer’s instructions. PCR reaction was conducted with 2 μL cDNA sample, 0.4 μL forward primer (10 μmol/L), 0.4 μL reverse primer (10 μmol/L), 11.2 μL RNase-free water, and 6 μL 2× EsayTaq PCR SuperMix (TransGen BIotech, Beijing, China). PCR reaction was performed using the following cycle parameters: 95 °C for 5 min, (94 °C for 30 s, 56 °C for 30 s, 72 °C for 45 s) for 30 cycles, 72 °C for 7 min. RT-PCR products were separated on 2 % agarose gels. After stained with ethidium bromide, gel images were photographed with ChemiImagerTM 4400. RT-PCR was performed at least three times for each sample. The sequences of human SOX4 primers were 5′-GGTCTCTAGTTCTTGCACGCTC-3′ (forward) and 5′-CGGAATCGGCACTAAGGAG-3′ (reverse). The primers for human GAPDH were 5′-GCAGTGGCAAAGTGGAGATT-3′ (forward) and 5′-TGAAGTCGCAGGAGACAACC-3′ (reverse).
Western blot analysis
The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked and then incubated overnight at 4 °C with primary antibodies: anti-SOX4, anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-β-actin and anti-GAPDH (Abcam, Cambridge, MA, USA). After washing, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized by enhanced chemiluminescence kit (Pierce, Rockford, IL).
Knockdown of SOX4 by siRNA transfection
Sequence-specific small interfering RNA (siRNA) against SOX4 was purchased from Thermo Scientific Dharmacon (Lafayette, CO, USA). Cells were seeded into 6-well plates at a density of 3 × 104 to 5 × 104 cells per well and transfected with siRNA using Lipofectamine 2000 reagent (Invitrogen). After 48 h, SOX4 expression levels were detected by RT-PCR and/or Western blot.
MTT assay
Cells were plated in 96-well plates in medium containing 10 % FBS at about 3000 cells per well 24 h after transfection. Then, 20 μL of 5 mg/mL 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; thiazolyl blue) solution was added to each well and incubated for 4 h at 37 °C, the media was removed from each well, and the resultant MTT formazan was solubilized in 150 μL of DMSO. The results were quantitated spectrophotometrically using a test wavelength of 570 nm.
Colony formation assay
At 48 h after transfection, 300 cells were planted into 6-well plates per well. After incubation for 14 days, plates were stained with crystal violet, and colonies containing more than 50 cells were scored.
Transwell cell invasion assay
In vitro invasion assay was determined using Matrigel invasion chambers (BD Bioscience). CRC cells were seeded into inserts at 2 × 104 per insert in serum-free medium and then transferred to wells filled with the culture medium containing 10 % FBS (Sijiqing Biotech, Hangzhou, China) as a chemoattractant. After 24 h of incubation, non-invading cells on the top of the membrane were removed by scraping. Invaded cells on the bottom of the membrane were fixed, followed by staining with 0.05 % crystal violet. The number of invaded cells on the membrane was then counted under a light microscope (CA-4500, Chinan Electronic Technology Co., Shanghai, China).
Statistics
Experimental results are presented as mean ± SEM. The difference between SOX4 mRNA or protein expression in tumor tissue and that in adjacent normal mucosa tissues was evaluated using Student’s t test, and differences were considered to be statistically significant when P value is less than 0.05.
Results
Expression of SOX4 in CRC cells and tissues
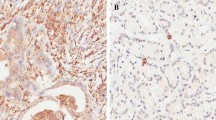
We first examined the expression levels of SOX4 in primary CRC and adjacent normal mucosa tissues by qRT-PCR and Western blot. The results showed that the mRNA levels of SOX4 were approximately threefold higher in CRC compared to adjacent normal mucosa tissues (Fig. 1a). Moreover, SOX4 protein expression levels were also upregulated in CRC tissues (Fig. 1b). Next, we analyzed SOX4 protein expression in a series of 119 paraffin-embedded CRC tissues and corresponding non-tumor tissues using immunohistochemistry. We found that SOX4 expression levels were considerably overexpressed in CRC when compared to corresponding non-tumor tissues (Fig. 2a, b). We observed that SOX4 was positively expressed in 93 of the 119 (78.15 %) patients, and SOX4-positive staining was mainly in the cytoplasm of the cancer cells.
To determine the effect of SOX4 expression on CRC progression, we next analyzed the levels of SOX4 mRNA and protein in CRC cell lines using qRT-PCR and Western blot. As shown in Fig. 3a, b, SOX4 was overexpressed in CRC cell lines when compared to human colon epithelial cell line NCM460. According to the expression of SOX4 in CRC cells, both LoVo and SW480 cells which have a relatively high level of SOX4 were selected to estimate the effects of SOX4 inhibition (Fig. 3a, b).
SOX4 knockdown impairs CRC cell proliferation in vitro
We examined the effects of SOX4 knockdown on cell proliferation, migration, and invasion of CRC cells by siRNA. MTT assay showed that SOX4 depletion significantly suppressed the ability of proliferation of LoVo and SW480 cells compared to the controls (Fig. 4a, b). Moreover, colony formation assays revealed that colony number and colony size were smaller in SOX4-siRNA LoVo and SOX4-siRNA SW480 cells than control cells (Fig. 4c, d). These results reveal that SOX4 increases the proliferation and colony formation of CRC cells.
Inhibition of SOX4 reversed EMT and suppress the invasion of CRC cells
Next, the transwell assay demonstrated that SOX4 depletion resulted in a significant reduction of cell migration and invasion (Fig. 5). To study whether SOX4 could regulate EMT markers, we performed Western blotting to test the effects of SOX4 on the expression of N-cadherin, vimentin, and E-cadherin. Our results indicated that knockdown of SOX4 decreased N-cadherin and vimentin mRNA levels and enhanced E-cadherin protein level (Fig. 6a, b). All these results show that overexpression of SOX4 can induce EMT and increase migration and invasion capabilities of CRC cells.
a Western blots showing that knockdown of SOX4 reverses the EMT process through downregulation of N-cadherin and vimentin and upregulation of E-cadherin in LoVo cells. b Western blots showing that knockdown of SOX4 reverses the EMT process through downregulation of N-cadherin and vimentin and upregulation of E-cadherin in SW480 cells
Discussion
So far, treatment options for CRC patients have been very limited mostly due to the high rate of distant metastasis, leading to poor overall survival; there is an urgent demand for the development of novel molecule targets and therapeutic approaches. SOX4 is a 47 kDa protein member of SOX family encoded by a single exon gene, and it preferentially binds the A/T A/TCAAAG sequence motif through HMB domain and regulates transcription of target genes [14, 15]. The abnormal expression of SOX4 has been reported in various types of cancer, and considerable attention is focused on understanding of the physiological and pathophysiological mechanisms of SOX4 in cancer development. These observations suggest an important role of SOX4 in CRC development.
Aberrant expression of SOX4 has been identified in several human cancers, and it is often associated with the progression of cancer [16–18]. Our findings verified that expression of SOX4 was significantly increased in the resected tissues of CRC patients compared to non-cancerous colon specimens and was highly expressed in a panel of CRC cell lines compared with normal colon epithelial cells. This study provided some insights into the mechanism underlying SOX4 tumorigenic property.
Previous studies reported that SOX4 promotes esophageal tumor cell proliferation and invasion by silencing miR-31 via activation of EZH2 and HDAC3. To further investigate the role of SOX4 in the progression of CRC, we knock downed SOX4 in LoVo and SW480 cells. According to our Western blotting data, we confirmed that our SOX4 siRNA really did work and had a high interference efficiency. We found that the downregulated SOX4 inhibited the growth and proliferation of LoVo and SW480 cells. In addition, ablation of SOX4 inhibited the colony formation of CRC cells. Furthermore, many studies have proved that even tumor metastasis is correlated with SOX4 overexpression. In HCC and breast cancer, SOX4 expression has been demonstrated to be particularly elevated in metastases, which was associated with disease progression, recurrence, and poor metastasis-free survival [19, 20]. In our study, we found that depletion of SOX4 significantly inhibits colon cancer cell invasion and migration in LoVo and SW480 cells, suggesting that SOX4 contributes to malignant progression of CRC.
EMT is characterized by the loss of intercellular adhesion (E-cadherin), the upregulation of mesenchymal markers (vimentin), and the acquisition of a fibroblast-like motile and invasive phenotype [21, 22]. Recently, SOX4 has been demonstrated to induce EMT in human mammary epithelial cells [23]. Ectopic expression of SOX4 in these cells induced EMT and conferred metastatic properties which were associated with increased migration and invasion in vitro [24]. RNA interference-mediated knockdown of SOX4 reverses the EMT process through downregulation of E-cadherin transcription repressors including N-cadherin and vimentin and also through upregulation of E-cadherin in LoVo and SW480 cells, indicating that SOX4 depletion could reverse the EMT process.
In conclusion, we reported for the first time that inhibition of SOX4 expression by RNAi can suppress proliferation, metastasis, and invasion of CRC cells in vitro and reverse EMT through upregulation of mesenchymal markers vimentin and downregulation of epithelial markers E-cadherin in CRC cell line. SOX4 was shown to play a crucial role in the development and progression of CRC. And, further efforts to investigate the mechanisms of CRC development and progression induced by SOX4 would be valuable.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57.
Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR, et al. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann Surg. 2014;259(4):735–43.
Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks ArchSurg. 2009;394:973–83.
Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3:167–70.
Perencevich M, Stoffel EM. A multidisciplinary approach to the diagnosis and management of multiple colorectal polyps. Gastroenterol Hepatol. 2011;7:420.
Wilson M, Koopman P. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. CurrOpin Genet Dev. 2002;12:441–6.
Penzo-Méndez AI. Critical roles for SoxC transcription factors in development and cancer. Int J Biochem Cell Biol. 2010;42:425–8.
Jafarnejad SM, Ardekani GS, Ghaffari M, Li G. Pleiotropic function of SRY-related HMG box transcription factor 4 in regulation of tumorigenesis. Cell Mol Life Sci. 2013;70:2677–96.
Schilham MW, Oosterwegel MA, Moerer P, Ya J, de Boer PA, et al. Defects in cardiac outflow tract formation and pro-Blymphocyte expansion in mice lacking Sox-4. Nature. 1996;380:711–4.
Castillo SD, Matheu A, Mariani N, Carretero J, Lopez-Rios F, Lovell-Badge R, et al. Novel transcriptional targets of the SRY-HMG box transcription factor SOX4 link its expression to the development of small cell lung cancer. Cancer Res. 2012;72:176–86.
Wang L, Zhang J, Yang X, Chang YW, Qi M, Zhou Z, et al. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013;16:301–7.
Vervoort SJ, van Boxtel R, Coffer PJ. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: friend or foe? Oncogene. 2013;32:3397–409.
Andersen CL, Christensen LL, Thorsen K, Schepeler T, Sørensen FB, et al. Dysregulation of the transcription factors SOX4, CBFB and SMARCC1 correlates with outcome of colorectal cancer. Br J Cancer. 2009;100:511–23.
Madan A, Yoon JG, Fang X, Yan X, Kim TK, et al. Massively parallel signature sequencing and bioinformatics analysis identifies up-regulation of TGFBI and SOX4 in human glioblastoma. PLoS One. 2010;5:e10210.
Lee AK, Ahn SG, Yoon JH, Kim SA. Sox4 stimulates ss-catenin activity through induction of CK2. Oncol Rep. 2011;25:559–65.
Saegusa M, Hashimura M, Kuwata T. Sox4 functions as a positive regulator of beta-catenin signaling through upregulation of TCF4 during morular differentiation of endometrial carcinomas. Lab Invest. 2012;92:511–21.
Iqbal MS, Otsuyama K, Shamsasenjan K, Asaoku H, Kawano MM. CD56 expression in human myeloma cells derived from the neurogenic gene expression: possible role of the SRY-HMG box gene SOX4. Int J Hematol. 2010;91:267–75.
Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–52.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54.
Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–9.
Liao YL, Sun YM, Chau GY, Chau YP, Lai TC, Wang JL, et al. Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008;27:5578–89.
Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao X, et al. SOX4 induces epithelial–mesenchymal transition and contributes to breast cancer progression. Cancer Res. 2012;72:4597–608.
Acknowledgments
This study was supported by the National Natural Science Foundation of Hainan (no. 814309)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Wang, B., Li, Y., Tan, F. et al. Increased expression of SOX4 is associated with colorectal cancer progression. Tumor Biol. 37, 9131–9137 (2016). https://doi.org/10.1007/s13277-015-4756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4756-5