Abstract
Accurate evaluation of human epidermal growth factor receptor 2 (HER2) status is quite crucial for invasive breast tumor patients in order to select anti-HER2 therapy for effective clinical outcomes. Immunohistochemistry (IHC) assay is routinely used to evaluate the HER2 oncoprotein overexpression but is unable to explain the chromosomal and genetic alterations and has been considered as a hot issue in IHC-equivocal cases. We investigated these molecular aberrations in correlation with prognostic factors. A cohort of 154 IHC-equivocal (+2) cases was selected and retrospectively analyzed by dual-probe fluorescence in situ hybridization (FISH) assay by using locus-specific HER2 and centromere enumeration probes (CEP17) for the identification of HER2 proto-oncogene amplification and chromosomal copy number per cell, respectively. The data were analyzed by SPSS 16.0 version using chi-square test (p < 0.05). We identified 36 out of 154 cases (23.4 %) showing HER2 gene amplification (average HER2 gene copies per cell >4 or <4 with HER2/CEP17 ratio >2) in concordance with HER2 oncoprotein overexpression, and significant correlation was observed with prognostic parameters including histological type, tumor grade II to III, histology and pathological type, lymphatic invasion, ductal carcinoma in situ (DCIS), and estrogen-positive and progesterone-negative receptors. Of the 154 cases, 18 cases (11.7 %) showed polysomy 17 with CEP17 probe signals per cell ≥3 and 22 cases (14.3 %) presented monosomy 17 (CEP17 probe signals per cell ≤1). Our data indicate that the use of anti-HER2 therapy should not be suggested unless true evaluation of HER2 protein expression is made regarding gene amplification essentially in IHC-ambiguous invasive breast tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Pakistan, among the top ten commonest malignancies, breast cancer is the leading one and ranks second in position worldwide [1]. Invasive breast cancer constitutes 75–80 % of breast tumors [2] and 15–30 % is being caused by HER2 gene amplification/overexpression [3, 4]. Human epidermal growth factor receptor 2 (HER2) gene is located at chromosome 17 q12–21.32 and acts as a proto-oncogene involved in modulating proliferation, invasion, and apoptosis [5]. It encodes a p185 HER2 oncoprotein, also known as HER-2/neuorerbB-2, an important member of the HER family which is a cell membrane receptor having tyrosine kinase activity that can stimulate cell proliferation through the RAS–MAPK downstream signaling cascades and inhibit cell death through the phosphatidylinositol 3′-kinase–AKT–mammalian target of rapamycin (mTOR) pathway [6].
The overexpression of HER2 protein may result by increased gene copy numbers (amplification) under different aberrative genetic mechanisms such as increased replicating amplicons (repeated HER2 gene segments) on chromosome 17 and chromosomal aneusomy which could associate with various types of cancers remarkably breast, ovarian, gastric, pancreatic, prostate, colorectal, and lung cancer and tumors of the female genital tract [3, 7].
Invasive breast cancer patients with HER2 protein overexpression are being subjected to trastuzumab therapy (Herceptin, Genentech), a recombinant monoclonal antibody to target the HER2 extracellular domain to suppress the downstream signaling pathways [8]. Trastuzumab is a Food and Drug Administration (FDA)-approved drug and has been recommended as a monotherapy or in combination with ado-trastuzumabemtansine [9] for first-line treatment of HER2-positive invasive breast tumors [10, 11].
American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) has recommended new guidelines for diagnostic approach of HER2 protein overexpression by immunohistochemistry (IHC) categorized with IHC score as 0/1+ (IHC-negative), 2+ (IHC-equivocal), and 3+ (IHC-positive). The IHC-equivocal or borderline 2+ cases have ambiguous tumor nature with weak HER2 oncoprotein expression in this subgroup and highly crucial for medical practitioners to mark the patient’s eligible for Herceptin therapy. In situ hybridization techniques are standard alternative cytogenetic methodology to identify increased number of gene loci with reference CEP17 DNA sequence for accurate HER2 assessment [12].
The aim of the present study was the clinical assessment of HER2 proto-oncogene amplification and chromosome 17 copy numbers per cell by dual-probe fluorescence in situ hybridization (FISH) assay for demonstration of HER2 protein overexpression in correlation with clinicopathologic parameters in the subgroup of IHC-equivocal (borderline 2+) patients for better selection of anti-HER2 therapy.
Materials and methods
Patients/specimen selection
A cohort of 154 IHC-equivocal (2+) cases of female invasive breast carcinoma patients were selected between 2012 and February 2014 from histopathology tissue bank of Shifa International Hospital (SIH), Pakistan (including both in-hospitalized and referral cases). The paraffin-embedded tissue specimens immunohistochemically diagnosed as weak HER2 oncoprotein overexpression were included in the study and retrospectively analyzed by dual-probe FISH assay for the clinical assessment of HER2 oncogene and aneusomy 17 in the Division of Histopathology and Cytogenetic Pathology Lab at SIH in collaboration with Cancer Genetics Lab of Quaid-I-Azam University, Islamabad, Pakistan. The study protocol was approved by the Institutional Review Board and Ethical Committee (IRB-EC) of SIH and Shifa College of Medicine (SCM) with a reference number (IRB-EC #2069-118-2013).
Clinicohistopathological and immunohistochemical parameters
Patient’s age, sex, and histopathological parameters including microscopic tumor size, histological type and grades, pathological stage, lymph-vascular invasion, and ductal carcinoma in situ (DCIS) were noted for each case. All the selected cases were immunohistochemically proven for HER2 oncoprotien (2+) at borderline stage and esterogen and progesterone hormone receptor statuses (ER and PR) were also noted for each case.
Dual-probe fluorescence in situ hybridization assay
FISH assay was performed on tissue specimens using the FDA-approved PathVysion HER2 DNA Probe kit (Abbott Molecular Inc., Des Plaines, IL, USA), composed of two probes: LSI HER2 (locus-specific identifier HER2) Spectrum Red fluorophore-labeled DNA probe for the HER2 gene locus (17q12–21.32) to determine HER2 gene amplification status and CEP17 (centromere enumeration probe 17) Spectrum Green fluorophore-labeled alpha satellite DNA sequence probe for chromosome 17 to enumerate copy number of chromosome 17 per cell. The FISH procedure was carried out according to kit manufacturer instructions. The tissue sections of 4–6-μm thickness were cut from formalin-fixed paraffin-embedded tissue blocks using rotary microtome and floated in a protein-free water bath at 40 °C to mount the sections on organosilane-coated slides. After air-dried, the slides were baked overnight at 56 °C. The slides were deparafinized in Hemo-De for 10 min at room temperature, dehydrated in 100 % ethanol for 5 min at room temperature, and air-dried. Subsequently, the slides were pretreated with protease K solution at 37 °C for 10 min. Denaturation of specimen DNA was done in humidified hybridization chamber at 72 °C for 30 min and dehydrated the slides through ethanol gradients 70, 85, and 100 %. The denatured DNA specimen was hybridized with pre-warmed probes mixture on a 45–50 °C slide-warmer for 2–5 min, covered with cover slip, and further incubated at 37 °C overnight, followed by post-hybridization treatment with wash buffer (2× SSC/0.3 % NP-40), and finally, slides were counterstained with DAPI. The slides were visualized under fluorescence microscope (Olympus, USA) and fluorescent probe signals were recorded in a minimum of 20 selected tumor nuclei for each case using CCD camera (Olympus) to enumerate HER2 gene copies (red signals) and chromosome 17 copies (green signals). In case of variable result, the assay was repeated. Subjective interpretations of FISH results were done by cooperation of pathologists and the average copy numbers and ratio of HER2 (red signals) and CEP17 (green signals) were calculated in the fields containing invasive tumor component with non-overlapping tumor nuclei for the assessment of HER2 gene amplification and chromosome 17. ASCO/(CAP) guidelines were followed for interpretation of HER2 gene status [12].
HER2 gene interpretation criteria
Average HER2 copy number (signals/cell) | HER2/CEP17 ratio | HER2 gene status |
≥4 or <4 | ≥2 | Amplified |
≥6 | <2 | Amplified |
≥4 to <6 | <2 | Equivocal (FISH borderline)* |
<4 | <2 | Non-amplified |
*Must order a reflex test (same specimen using IHC), test with alternative ISH chromosome 17 probe, or order a new test (new specimen if available, ISH or IHC)
Chromosome 17 aneusomy was determined by calculating an average CEP17 fluorescent green signals per cell nucleus and defined as CEP17 copies ≥3 (polysomy 17), CEP17 copies 1.5–2.5 (disomy), and CEP17 copies ≤1 (monosomy 17) [13].
Statistical evaluation
SPSS statistical program software (version 16.0, IBM Chicago, IL, USA) was used to analyze the data. The results of HER2 gene amplification by FISH method were correlated with aforementioned clinical and histological parameters using chi-square test with 95 % confidence interval; p < 0.05 was considered significant.
Ethical aspects
The study protocol, consent, and quaternary forms were approved by the Institutional Review Board and Ethical Committee (IRB-EC) of SIH and SCM, Islamabad, Pakistan. Reference of approval was IRB #2069-118-2013. All collected information including patient names and medical record numbers were handled anonymously.
Results
Biostatistics features
We studied 154 invasive breast carcinoma cases; all had HER2 protein overexpression with 2+ score by IHC assay. In a cohort of 154 cases, the patient’s age ranged between 33 and 93 years (mean, 53.04 years and mode 58, Std. Dev. ± 11.43) with a majority of occurrence above 50 years. The majority of the cases were invasive ductal carcinomas (IDC; 132 cases, 85.71 %) and the rest were invasive lobular carcinoma (ILC; 22 cases, 14.28 %).
Dual-probe fluorescence in situ hybridization evaluation
Dual-probe FISH assay was performed for HER2 gene amplification (red signals) and chromosome 17 copy number (CEP17 green signals) per tumor cell. In a total of 154 cases, 36 cases (23.4 %) had HER2 gene amplification (average HER2 copies/cell ≥4 with HER2/CEP17 ratio >2), 114 (74.0 %) cases non-amplified (average HER2 gene copies <4 with HER2/CEP17 ratio <2), and 4 cases (2.6 %) equivocal (average HER2 copies 4–6 with HER2/CEP17 ratio <2) as shown in Table 1 and Fig. 1.
Of the 154 cases, a total of 18 cases (11.7 %) showed polysomy 17 (average CEP17/cell copies >3) in non-amplified, while more 13 cases (8.4 %) and 9 cases (5.8 %) presented monosomy 17 (average CEP17/cell) in amplified and non-amplified cases, respectively. One hundred fourteen cases (74 %) had disomy with HER2 gene status distributed as 23 cases (14.9 %), 4 cases (4.6 %), and 87 cases (56.5 %) in FISH-amplified, equivocal, and non-amplified, respectively, and significant correlation between aneusomy 17 and HER2 gene amplification was observed as p < 0.001 (Table 1 and Fig. 1).
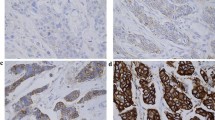
In Fig. 1a, the FISH results show HER2 (red) and CEP17 (green) probe signals for detection of an average HER2 gene and chromosome 17 copy numbers per cell in invasive breast carcinoma by dual-probe FISH. In panel a, average HER2 copies 4> and HER2/CEP17 >2.86 indicated HER2 gene amplification (×1,000). In panel b, HER2 copies 2.05 and HER2/CEP17 >1.9 FISH-equivocal. In panel c, HER2 copies 3 with ratio 1.5 presenting non-amplification. In panel d, CEP17 green signals counted with an average CEP17 copy number/cell nucleus found 3.4 greater than >3 indicated presence of polysomy 17; in panel e, green signals/cell nucleus found 0.8 l lesser than <1 indicated monosomy; and in panel f, green signals/ell were 1.53 that is in the range of 1.5–2.5 demonstrated as disomy (normal).
Frequency of HER2 gene amplification in correlation with estrogen and progesterone receptors
Of the 154 IHC2+ cases, 128 (83.1 %) cases were estrogen receptor-positive (ER+) and 83 (53.9 %) cases were progesterone receptor-positive (PR+); among these, 20 cases (13.0 %) showed relatively high and 6 cases (3.9 %) showed lower HER2 gene amplification, respectively. On the other hand, 26 (16.9 %) ER-negative and 71 (46.1 %) PR-negative cases showed the frequency of gene amplification as 16 (10.4 %) and 30 (19.5 %), respectively, which demonstrated that negative cases of either ER or PR had more gene amplification frequency as compared to ER- or PR-positive cases. This difference is highly correlated and statistically significant (p < 0.001, Table 2).
Association of HER2 gene amplification with clinicohistopathologic variables
An excellent correlation was found between gene amplification and prognostic factors in borderline (IHC 2+)/HER2 oncoprotein overexpressed cases. It was clear that HER2 gene amplification was more frequent in older patients, ductal carcinoma, high-grade tumors, and hormone receptor-negative tumors, but no significant difference was observed in other factors. It was found that incidence of HER2 gene amplification (n = 20 (13.0 %)) is more in older >50 years patients than <50 years (n = 16 (10.4 %)) but correlation was nonsignificant (p = 0.072). IDC (n = 132 (85.7 %)) patients had a gene amplification frequency in 36 cases (23.45 %) versus ILC patients (n = 22 (14.3 %)) presented no case of gene amplification; thus, a significant correlation was found between histological type and gene amplification as p = 0.011 (Table 3).
Microscopic tumor size recorded in 154 cases had been characterized between less than ≤2 cm (n = 33 (21.4 %)) and greater than >2 (n = 121 (78.6 %)), of these, 12 (7.8 %) while 24 (15.6 %) showed gene amplification, respectively; the correlation of microscopic tumor size and gene amplification was nonsignificant (p = 0.094, Table 3).
Seventy-seven cases (50 %) were high grade (grade III), 65 (42.2 %) were grade II, and 12 (7.8 %) were of low grade (grade I). The frequency of gene amplification was then determined for clinical stages I–III, for which the rates were observed as 0 %, 13 % (8.4 %), and 23 % (14.9 %) HER gene amplification, respectively. Thus, it appeared from the data that the rate of gene amplification is more in clinical stage III while decreased rate found in grade II with 2.6 % gene amplification at a cutoff point (equivocal) and non-amplified cases were n = 54 (35.1 %). Our results show that the rates of gene amplification tumors in IHC 2+ cases of patients were significant (p = 0.024; Table 3).
The majority of pathological staged pT2 cases (n = 114 (74.0 %)) showed that a gene amplification 17 (11 %) relatively had a higher rate than the rest of the stages such as pT0, pT1, pT3, and pT4 (p = 0.024, Table 3).
Among prognotic markers Lymphatic-positive tumors n = 82 (53.2 %) had gene amplification n = 23 (14.9 %) while DCIS-positive tumors n = 55 (35.7 %) showed gene amplification n = 23 (14.9 %). Prognostic markers presented significant correlations for gene amplification with p values of 0.043 and <0.001, respectively, compared to non-significant vascular involvement with p = 0.152 (Table 3).
Discussion
Overexpressed HER2 protein is a potent predictive and prognostic biological marker in invasive breast carcinomas, but its accurate diagnostic evaluation by IHC method has been considered uncertain particularly in subgroup showing IHC 2+ score (IHC-equivocal) [14]. In the present study, we investigated HER2 gene amplification and chromosomal 17 aneusomy for true molecular assessment of HER2 status cytogenetically using dual-probe FISH assay in a cohort of IHC-equivocal cases of invasive breast cancer patients.
HER2 gene amplification has been reported in numerous studies in the range of 18–20 to 25–30 % of invasive breast tumors as a whole [15] but concordance between HER2 protein overexpression and gene amplification drastically varies that is very low in IHC-negative (0/+1) to high in IHC-positive (3+) cases; however, IHC-equivocal (2+) cases have heterogeneous observations. According to the new HER2 guidelines from American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) [12] for the interpretation of FISH results, we observed 23.4 % HER2 gene amplification identification (average gene copies >4 with HER2/CEP17 ratio >2) in paraffin-embedded tissue specimens showing that IHC2+/FISH+ indicated breast tumor is true HER2-positive and patients with these findings should be subjected for anti-HER2 treatment. In previous studies, 6–25 % incidence of IHC 2+/FISH amplified cases had been reported [16, 17].
In a study, 28 out of 90 had IHC-equivocal (2+) cases and showed 17.8 % FISH-equivocal cases [18] but standard ASCO/CAP guidelines recommend that FISH-equivocal results should be less than 3 % in all specimens to ensure better FISH procedure [17]. In our study, four cases (2.6 %) were FISH-equivocal (HER2 gene copies range between ≥4 and >6) indicating more reliability in FISH procedure and interpretation and support the new ASCO/CAP recommendations for the accurate evaluation of true gene amplification on chromosome 17. Among this subset of patients, further studies for the validation and new criteria are needed for better interpretations of such cases in order to get potential benefits from anti-HER2 therapy and to avoid any potential risk of chemotherapeutic agents. Thus, all breast cancer patients with HER2 IHC score of 2+ should be recommended for HER2 gene amplification assay for molecular genetic testing using dual-probe FISH analysis.
Interestingly, FISH non-amplified cases presented a secondary alternative mechanism of polysomy 17 for increase HER2 gene copies resulting by extra gain of chromosome17 (as defined ≥3 CEP17 copies/cell). In the present study, aneusomy 17 was observed and CEP17 probe (centromere enumeration probe) plays the key role to identify chromosome 17 aneusomy and is helpful to resolve such chromosomal genetic discripancies in this subgroup of breast carcinoma with HER2 protein weak overexpression in non-amplified cases. Breast carcinomas harbor the frequency range from 10 to 50 % of polysomy in all IHC cases (negative 0, 1+, 2+, 3+) [17, 19]. Numerous studies have also postulated that it is more likely that the weak HER2 protein overexpression in non-amplified cases might be resulting from polysomy 17, an unconventional mechanism to increase the additional copies of HER2 gene per cell nucleus [17, 20]. In the ASCO/CAP guidelines, approximately 8 % equivocal cases had the evidence of polysomy 17 [17], while in our study, 11.7 % IHC-equivocal (2+) breast tumors (18 out of 154) presented polysomy 17 cases (CEP17 copies ≥3 per cell) in 74 % unamplified invasive breast cancers associated with adverse histopathological biomarkers such as histological grade III, advanced DCIS, and negative ER and PR receptors.
However, polysomy 17 has been found both in amplified and non-amplified tumors; more frequently observed are the non-amplified cases and our findings support these observations [13]. The occurrence of chromosome 17 polysomy is not rare in breast tumors and it has been suggested to explain the HER2 protein overexpression without true gene amplification that is falsely interpreted as positive HER2 protein overexpression in IHC analysis [18] but molecular analysis by dual-probe FISH assay resolves this discrepancy that the weak HER2 protein expression may be caused by extra gain of chromosome 17. Yet, insufficient data has been published to recommend potential benefit from anti-HER2 therapy in this genetically heterogeneous subclass. In this study, polyploidy phenomenon was explained by using CEP17 probe that reflects the number of chromosome copies per cell; this is particularly important where FISH-HER2/CEP17 ratios are <2 but the HER2 gene copy numbers >4 per cell.
Single-gene overexpression phenomenon had been observed in tumor cell harboring single chromosome 17 copy per cell (monosomy 17, less than two copies of CEP17 signals per cell) than normal state (disomy, two copies per cell) [19]. Various studies have been reported to address these variabilities and about 10 % of these alterations have been proposed to be caused by gene overexpression at the mRNA transcriptional level or at posttranslational levels [21]. In our study, we found that in nine cases (5.8 %) HER2 protein overexpression might be caused by single-gene copy overexpression reflected by decreased CEP17 probe signals per cell (CEP17 ≤ 1), thus exploring and elaborating the phenomenon of HER2 gene overexpression and not gene amplification in this subset of breast carcinoma patients.
Thirteen percent ER+ and 19 % PR-negative receptors showed a significant correlation with HER2 gene amplification in the present study, relating to near similar observations in previous studies like 16 % cases showed both expression of ER and HER2 amplification [15, 22, 23]. The relation between hormone receptor and HER2 amplification is proposed to be complex interactive signaling pathway in breast cancer cells [24].
In the present study, there is no statistically significant correlation of HER2 gene amplification with microscopic tumor size and age; however, patients aged greater than 50 years present more occurrences. Fifty percent high-grade (III) breast tumors cases had 14.9 % gene amplification relatively high than low grades (I and II) which is concordant with the previous studies [18, 25]. Previous studies correlated with ductal carcinomas in situ (DCIS) had a high proportion of gene amplification, while our study data presented gene amplification in almost all cases of invasive breast cancer (IDC) [18]. Pathological factors including pathological type (pT2) and positive lymphatic invasion show significant correlation with HER2 gene amplification in the present study.
In case of 23.4 % HER2 gene amplification cases of invasive breast carcinoma patients, distinguished with protein overexpression evaluation by dual-probe FISH assay, targeting of HER2 receptor with adjuvant herceptin therapy can be used as a better approach. Our findings support to interpret and distinguish the weak HER2 oncoprotein overexpression in ambiguous heterogeneous nature of invasive breast tumors because they could either be resulting from HER2 oncogene amplification (multiple HER2 gene amplicons on same chromosome 17), polysomy 17 (increased HER2 gene copies by extra gain of chromosome per cell) mostly in FISH non-amplified cases, or by monosomy 17 (single HER2 gene overexpression). The data provides information that combined HER2 assessment at protein and gene level is quite vital for the recruitment of anti-HER2 treatment eligibility and to rule out false positive and negative IHC results to avoid the potential chemotherapy risks in this subgroup.
References
Annual-Cancer-Registry-Report. Lahore: Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan, (CRCDM) CRaCDM;2012.
Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046–52.
Montemurro F, Cosimo SD, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol. 2013;24:2715–24.
Han X, Shi Y, Ma L, Lyu Z, Yang H, Yao J, et al. Comparison of immunohistochemistry with fluorescence in situ hybridization in determining the human epidermal growth factor receptor 2 status of breast cancer specimens: a multicenter study of 3,149 Chinese patients. Chin Med J Engl. 2014;127(2):246–53.
Dang HZ, Yu Y, Jiao SC. Prognosis of HER2 over-expressing gastric cancer patients with liver metastasis. World J Gastroenterol. 2012;18(19):2402–7.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34(6):791–808.
Takashima T, Nakayama T, Yoshidome K, Kawajiri H, Kamigaki S, Tsurutani J, et al. Phase II Study of S-1 in combination with trastuzumab for HER2-positive metastatic breast cancer. Anticancer Res. 2014;34:3583–8.
Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–63.
Jackisch C. HER-2-positive metastatic breast cancer: optimizing trastuzumab-based therapy. Oncologist. 2006;11 Suppl 1:34–41.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013.
Orsaria M, Khelifa S, Buza N, Kamath A, Hui P. Chromosome 17 polysomy: correlation with histological parameters and HER2NEU gene amplification. J Clin Pathol. 2013;66(12):1070–5.
Couturier J, Vincent-Salomon A, Nicolas A, Beuzeboc P, Mouret E, Zafrani B, et al. Strong correlation between results of fluorescent in situ hybridization and immunohistochemistry for the assessment of the ERBB2 (HER-2/neu) gene status in breast carcinoma. Mod Pathol. 2000;13(11):1238–43.
Panjwani P, Epari S, Karpate A, Shirsat H, Rajsekharan P, Basak R, et al. Assessment of HER-2/neu status in breast cancer using fluorescence in situ hybridization and immunohistochemistry: experience of a tertiary cancer referral centre in India. Indian J Med Res. 2010;132:287–94.
Perez EA, Suman VJ, Davidson NE, Martino S, Kaufman PA, Lingle WL, et al. HER2 testing by local, central, and reference laboratories in specimens from the north central cancer treatment group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–8.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45.
Goud KI, Dayakar S, Vijayalaxmi K, Babu SJ, Reddy PV. Evaluation of HER-2/neu status in breast cancer specimens using immunohistochemistry (IHC) & fluorescence in-situ hybridization (FISH) assay. Indian J Med Res. 2012;135:312–7.
Wang S, Hossein Saboorian M, Frenkel EP, Haley BB, Siddiqui MT, Gokaslan S, et al. Aneusomy 17 in breast cancer: its role in HER-2/neu protein expression and implication for clinical assessment of HER-2/neu status. Mod Pathol. 2002;15(2):137–45.
Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol. 2001;19(10):2714–21.
Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18(21):3651–64.
Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, et al. Hormone receptors do not predict the HER2/neu status in all age groups of women with an operable breast cancer. Ann Oncol. 2005;16(11):1755–61.
Prati R, Apple SK, He J, Gornbein JA, Chang HR. Histopathologic characteristics predicting HER-2/neu amplification in breast cancer. Breast J. 2005;11(6):433–9.
Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13 Suppl 1:S15–24.
Crowe JP, Patrick RJ, Rybicki LA, Escobar PF, Weng D, Thomas Budd G, et al. A data model to predict HER2 status in breast cancer based on the clinical and pathologic profiles of a large patient population at a single institution. Breast. 2006;15(6):728–35.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-016-5419-x.
Rights and permissions
About this article
Cite this article
Afzal, M., Amir, M., Hassan, M.J. et al. Clinical role of HER2 gene amplification and chromosome 17: a study on 154 IHC-equivocal cases of invasive breast carcinoma patients. Tumor Biol. 37, 8665–8672 (2016). https://doi.org/10.1007/s13277-015-4657-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4657-7