Abstract
Ephrin-A2, a member of the Eph/ephrin family, is associated with tumorigenesis and tumor progression. This study aimed to assess the diagnostic and prognostic value of both serum and tissue levels of Ephrin-A2 in prostate cancer (PCa) management. One hundred and forty-five frozen prostate tissues, 55 paraffin-embedded prostate tissues, 88 serum samples, and seven prostate cell lines (RWPE-1, LNCaP, LNCaP-LN3, PC-3, PC-3M, PC-3M-LN4, and DU145) were examined via quantitative reverse transcription-PCR (qRT-PCR), immunohistochemistry, enzyme-linked immunosorbent assay, and western blotting. Induced Ephrin-A2 messenger RNA (mRNA) or protein expression was detected in 8.6 % (5/58) benign prostatic hyperplasia (BPH), 59.8 % (52/87) PCa, and five prostate cancer cell lines. Ephrin-A2 immunostaining was present in 6.7 % (1/15) patients with BPHs and 62.5 % (25/40) clinically localized PCa. Accordingly, serum Ephrin-A2 was significantly higher in PCa patients compared to those in the BPH patients and controls (P < 0.001). The expression of Ephrin-A2 was higher in tumor patients with an elevated Gleason score or T3–T4 staging. Ephrin-A2 expression was correlated with Ki-67 expression in PCa patients, both at the gene scale and protein level. Our data indicate that Ephrin-A2 is a potential diagnostic and prognostic biomarker and a promising molecular therapeutic target to attenuate prostate cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignancy in male and the second leading cause of death from cancer for men in the USA and Western countries [1]. With the rapidly aging population and changing food consumption in developing countries all around the world, more and more PCa cases have been reported in recent years [2]. So far, the prostate-specific antigen (PSA) testing still remains the most widely used method for diagnosis and prognosis of PCa [3]. Recently, however, PSA screening has fallen under controversy, as other nonmalignant prostate diseases, such as benign prostate hyperplasia (BPH), also display elevated serum PSA levels, which can lead to over-diagnosis [4]. In addition, PSA is a poor indicator of aggressiveness, leading to potential over-treatment of many PCa patients [5]. Thus, there is an urgent need for more sensitive and specific biomarkers for PCa diagnosis and prognosis.
The Eph receptors, the largest family of receptor tyrosine kinases (RTKs), include 14 human type 1 transmembrane protein members in human [6]. They are divided into two subgroups designated EphA and EphB based on both sequence similarity and affinity to their ligands, the ephrins. In general, EphA receptors bind to glycosylphosphatidylinositol (GPI)-anchored ephrin ligands (Ephrin-A), while EphB receptors bind to Ephrin-B, which are transmembrane proteins [6, 7].
Growing evidence has indicated the import role of Ephs and ephrins in angiogenesis, axon guidance, tissue border formation, cell migration, repulsion, and adhesion [6]. Aberrant expression of Ephs and ephrins in human cancer is implicated in malignant transformation, tumor metastasis, tumor differentiation, and outcome [7]. For example, upregulation of EphA2 has been observed in many malignant tumors and is associated with accelerated cell proliferation, enhanced neovascularization, and promoted cell migration and invasion [8–12]. Several studies [13–15] on EphB4 have revealed that its overexpression can promote tumor development by stimulating angiogenesis, increasing cancer cell survival, and facilitating invasion and migration. We recently completed a series of studies on Eph receptor in PCa tumorigenesis and progression, which not only illustrates that EphA6 expression is increased consistently and significantly in PCa tumor tissues compared with their adjacent nontumor tissues and is associated with PCa progression [16], but also confirms that normal prostate tissue expresses a high level of EphA5, which is progressively lost during PCa development [17]. However, research of Ephrin members in PCa is still rare.
Ephrin-A2, located on chromosome 19p13.3, is a cognate ligand to several Eph receptors, including EphA3, EphA5, EphA6, EphA7, and EphA8 [18]. It is widely expressed in normal tissue including lung, small intestinal, kidney, bladder, thymus, and colon tissue [19, 20]. Overexpression of Ephrin-A2 has been observed in hepatocellular carcinoma, renal cell carcinoma, and breast carcinoma cell lines and PCa cell lines [20–23]. Moreover, Ephrin-A2 could be cleaved by the A disintegrin and metalloproteinase 10 (ADAM10) from the membrane and naturally generated soluble Ephrin-A2 into the supernatant of Neuro2a neuroblastoma cell medium [24]. However, knowledge on the role of Ephrin-A2 in the carcinogenesis of prostate carcinoma remains limited. In the present study, we firstly evaluated the expression of Ephrin-A2 messenger RNA (mRNA) and protein of prostate cell lines and tissues. Secondly, we analyzed serum concentrations of Ephrin-A2 in men both with and without PCa. Finally, we explored the association between Ephrin-A2 and clinicopathologic parameters such as patients’ age, prostate volume, PSA levels, tumor-node-metastasis (TNM) staging, and Gleason score.
Materials and methods
Cell culture
The human prostate cell lines RWPE-1, LNCaP, PC-3, and DU145 were purchased from American Type Culture Collection (ATCC). The LNCaP-LN3(a more metastatic subclone of LNCaP from Lymph node metastasis), PC-3M (a more invasive subclone of PC-3), and PC-3M-LN4 cells (a more metastatic subclone of PC-3M from lymph node metastasis) were obtained from Dr. Zhang (Biomedical Research Institute, Shenzhen PKU-HKUST Medical Center, Shenzhen, China). All cells were cultured in a 5 % CO2 and 95 % atmosphere at 37 °C. RWPE-1 was grown in Defined Keratinocyte-SFM (Gibco, Carlsbad, USA) containing 50 μg/mL bovine pituitary extract (Gibco), 5 ng/mL epidermal growth factor (Gibco), 100 U/mL penicillin, and 100 U/mL streptomycin. LNCaP, LNCaP-LN3, PC-3, PC-3M, PC-3M-LN4, and DU145 were cultured in DMEM/F-12 medium (Gibco) containing 10 % fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, and 100 U/mL streptomycin.
Clinical samples
All specimens were obtained between March 2013 and November 2014 at the Urology Department of Huashan Hospital (Shanghai, China) under an institutional review board-approved protocol. Overall, 288 samples of 205 men [107 with PCa, 78 with benign prostate hyperplasia (BPH), and 20 healthy controls] divided into three partly overlapping cohorts were included in this study: (1) 145 frozen tissue samples (87 PCa and 58 BPH) analyzed by quantitative reverse transcription (RT) real-time PCR, (2) 55 paraffin-embedded tissues (40 PCa and 15 BPH) analyzed by immunohistochemistry (IHC), and (3) 88 serum samples (48 PCa, 20 BPH, and 20 controls) assessed by ELISA. The clinical characteristics of the subjects are listed in Supplementary Table 1.
All tissue specimens were obtained from patients undergoing prostate needle biopsies (PNB) or radical prostatectomy (RPE). All the tumors were confirmed to contain more than 80 % tumor cells by histological examination of sequential sections. Staging was assessed after pathological examination of formalin-fixed specimens according to the 1997 TNM classification. Control serum samples were collected from age-matched men who participated in PCa screening and were deemed of low risk of PCa based on digital rectal examination and low PSA level. Written informed consent was obtained from each patient before sample acquisition, and the study was carried out in accordance with the ethical standards of the Helsinki Declaration II and approved by the Institution Review Board of Huashan Hospital.
Quantitative reverse transcription-PCR
RNA was isolated from cells and tissues using the AllPrep DNA/RNA/Protein (Qiagen, Hilden, Germany) and reverse transcribed with the PrimeScript™ RT Reagent Kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol. The mRNAs were quantified by real-time reverse transcriptase polymerase chain reaction (RT-PCR), based on TaqMan Gene Expression Assay (Applied Biosystems, CA, USA): Hs01023290_ml for Ephrin-A2, Hs01032434_ml for Ki-67, and Hs99999905_ml for GAPDH, respectively (www.appliedbiosystems.com). The comparative cycle threshold (Ct) method was used to determine the relative expression for Ephrin-A2 and Ki-67 mRNA to GAPDH control gene (ΔCt = CT analyzed gene-CT control gene GAPDH). The relative expression levels of analyzed genes were calculated as 2-ΔCt according to Applied Biosystems instruction.
Western blotting
Total protein was extracted from prostate cell lines using radioimmunoprecipitation assay buffer, and protein concentrations were determined using the BCA Protein Reagent Kit (Beyotime, Nantong, China) as described in the manufacturer’s instructions. Proteins (30 μg) were separated on 12 % SDS-PAGE, transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, USA), and incubated with primary antibodies against Ephrin-A2 (1:1000, Abcam ab123877, Cambridge, USA) and GAPDH (1:5000, Proteintech 10494-1-AP, Wuhan, China) overnight at 4 °C. After washing, the membranes were incubated with secondary antibodies (1:5000, Abcam) for 2 h and visualized with enhanced chemiluminescent substrate (Millipore). Then, immunoreactive bands were quantified using the LAS-3000 system (Fuji Film, Tokyo, Japan).
Immunohistochemistry
Immunohistochemical staining of Ephrin-A2 and Ki-67 was performed according to a standard method. Briefly, tissues were fixed in 10 % formalin, embedded in paraffin, and sectioned at a thickness of 5 μm. The sections were dewaxed, rehydrated, and microwaved for 30 min in sodium citrate buffer (pH 9.0) to retrieve antigen epitopes. After endogenous peroxidase had been quenched with aqueous 3 % H2O2 for 10 min, the sections were permeabilized by 0.1 % TritionX-100 in PBS for 15 min, washed in PBS, incubated overnight with a mouse anti-Ephrin-A2 monoclonal antibody (1:100, Abcam ab123877, Cambridge, USA) or a mouse anti-Ki-67 monoclonal antibody (1:50, BD Biosciences 563462, NY, USA), and then washed with PBS. Next, the sections were incubated with secondary antibody (Dako, Glostrup, Denmark) for 30 min at room temperature. Color development was performed with 3,3′-diaminobenzidine (DAB). Nuclei were lightly counterstained with hematoxylin. Negative control sections with the omission of the primary antibody were included in each run. Two pathologists independently assessed the immunostained slides. For subsequent statistical analysis, immunohistochemical staining of cancer cells was semiquantitatively assessed according to the staining intensity and percentage of positive cells. Ephrin-A2 expression was assessed for intensity (0 = no staining, 1 = weak, 2 = moderate, 3 = strong) and the percentage of positive cells (0 = 0 %, 1 ≤ 10 %, 2 = 10 % to 50 %, 3 = 51 % to 80 %, 4 ≥ 80 % positive cells) as defined previously. The scores for intensity and percentage were multiplied and a cutoff of 6 was used. The percentage of Ki-67 positivity was recorded in all cases at the time of diagnosis. The Ki-67 labeling index (LI) was grouped as ≥4 % or <4 %.
Measurement of serum Ephrin-A2 levels
Blood serum Ephrin-A2 levels were quantified using a sandwich ELISA kit (TSZ, NJ, USA) according to the manufacturer’s instructions. Briefly, an anti-human Ephrin-A2 coating antibody was adsorbed onto microwells and bound to human Ephrin-A2 present in the samples or the standard. Serum samples were diluted (1:4) with sample diluent and placed in designated microwells. In addition, calibrators (the different concentrations of standard: 30, 60, 120, 240, 480, and 960 ng/L) and negative and positive controls were added to the designated microwells to construct a standard curve ranging from 0.1 to 960 ng/L. Then, the plates were incubated for 30 min at 37 °C and washed with wash buffer three times. After the incubation of horseradish peroxidase (HRP)-conjugated anti-human Ephrin-A2 antibody and washing, each well was added 100 μL tetramethylbenzidine (TMB) substrate and incubated for 30 min at 37 °C, protected from intense light. Following washing, 100 μL of stop solution was added to each well, using the same order as for the substrate, and incubated for 5 min. The absorbance of the resulting product was read at 450 nm within 15 min using a microplate reader (BioTek Instruments Inc, Winooski, USA). All samples were examined in duplicate, and the mean values were used for statistical analysis.
Statistical analysis
Associations of the expression of Ephrin-A2 with clinicopathological parameters and the correlation between Ephrin-A2 and Ki-67 protein expression in PCa tissues were evaluated by a chi-square test. Pearson correlation coefficients (r) were calculated to evaluate the relationship between Ephrin-A2 transcript and Ki-67 mRNA expression in PCa tissues. Association between Ephrin-A2 serum concentrations and clinicopathological parameters was assessed using Mann–Whitney U tests. For paired group comparisons, the nonparametric, two-sided Wilcoxon rank-sum test was applied. Receiver operating characteristic (ROC) analysis was done to verify whether Ephrin-A2 serum levels could be used to distinguish patients with PCa from patients with BPH. All statistical analyses were performed by using SPSS16.0 software program (SPSS, Chicago, USA). A two-sided P value of less than 0.05 was considered statistically significant in all of the statistical tests.
Results
Ephrin-A2 was overexpressed in PCa cell lines
To explore the potential role of Ephrin-A2 in PCa, we first analyzed the mRNA expression of Ephrin-A2 by real-time quantitative reverse transcription-PCR (qRT-PCR) in a panel of human nonmalignant (RWPE-1) and PCa (LNCaP, LNCaP-LN3, PC-3, PC-3M, PC-3M-LN4, and DU145) cell lines. Figure 1a shows that Ephrin-A2 was highly expressed in all cancer cell lines except PC-3 cell when compared with RWPE-1cells. In addition, we also observed that the level of Ephrin-A2 transcript is increased consistently and significantly in both of lymph node derivative cell lines compared with their parental PCa cells LNCaP and PC-3. In order to confirm the overexpression of Ephrin-A2 in PCa cell, we next analyzed Ephrin-A2 expression at the protein level by western blotting, in which Ephrin-A2 protein could be detected in LNCaP, LNCaP-LN3, and DU145 cell lines (Ephrin-A2 mRNA high-expressing cells). These data are consistent with the pattern of Ephrin-A2 mRNA expression, further demonstrating that Ephrin-A2 was overexpressed in most PCa cell lines compared to the nonmalignant cells (Fig. 1b).
Ephrin-A2 expression in prostate cell lines. a Ephrin-A2 mRNA expression was analyzed by qRT-PCR in seven prostate cell lines. GAPDH was amplified as an internal control. b Western blotting analysis of Ephrin-A2 protein expression in seven prostate cell lines. GAPDH was used as a control for equal loading of cell lysates. The relative expression of Ephrin-A2 in prostate cell lines was also normalized to that in LNCaP cells. Representative results of triplicate experiments are shown as mean ± SD (n = 3). *P < 0.05 vs. the RWPE-1 cell line; **P < 0.05 vs. the LNCaP cell line, ***P < 0.05 vs. the PC-3 cell line
Overexpression of Ephrin-A2 transcript in prostatic cancer tissues
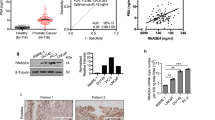
To determine whether overexpression of the Ephrin-A2 gene also occurs in primary prostate tumors, Ephrin-A2 expression was analyzed by qRT-PCR in 58 BPH tissues, 87 primary prostate tumor tissues, and 23 matched adjacent tissues. Compared to the matched adjacent tissues, Ephrin-A2 was upregulated in 52 of 87 (59.8 %) PCa samples and in 5 of 58 (8.6 %) BPH samples compared to matched adjacent tissues (Fig. 2a).
Ephrin-A2 expression in prostate tissues. a Expression of Ephrin-A2 mRNA in 87 primary PCa tissues, 23 matched adjacent prostate tissues, and 58 BPH tissues was measured by qRT-PCR and normalized to the endogenous control GAPDH. Ephrin-A2 mRNA expression was determined in BPH and PCa tissue relative to the mean of 23 matched adjacent normal tissues. b Representative Ephrin-A2 IHC staining of paraffin-embedded normal and malignant prostate tissues. I Negative staining of Ephrin-A2 in normal prostate tissues. II BPH tissues show no Ephrin-A2 expression. III Weak Ephrin-A2 staining in prostate cancer. IV Strong Ephrin-A2 staining in prostate cancer (scale bar = 50 μm)
We next analyzed the association between Ephrin-A2 transcript and various clinicopathologic parameters. As shown in Table 1, the high Gleason score group (Gleason score 8–10) had significantly high expression of Ephrin-A2 mRNA than low Gleason score group (Gleason score 6–7) (73.8 vs. 46.7 %; P = 0.010). Moreover, we also observed a positive correlation between the overexpression of Ephrin-A2 transcript and TNM staging (P = 0.008). However, no significant association was observed between Ephrin-A2 transcript level and patient age, PSA levels, or prostate volume.
Immunohistochemical expression of Ephrin-A2
To further characterize Ephrin-A2 expression, we examined Ephrin-A2 protein expression via immunohistochemistry in 15 BPH tissues, 22 primary prostate tumor tissues, and 18 paired prostate carcinomas and noncancerous tissues. All 18 prostate noncarcinoma specimens and 93.3 % (14/15) BPH samples exhibited undetectable or weak immunostaining. Strong immunostaining of the Ephrin-A2 protein was observed in the cell membrane and cell cytoplasm of the prostate cells in one BPH tissue and 25 tumor tissues. Fifteen cancerous tissues exhibited weak immunostaining (Fig. 2b). Ephrin-A2 immunopositivity was correlated with Gleason score (P = 0.014) (Table 1). However, there was no correlation between Ephrin-A2 immunostaining and patients’ age, prostate volume, PSA value, or TNM staging (Table 1). Interestingly, the correlations between Ephrin-A2 protein and TNM staging are inconsistent with the pattern of Ephrin-A2 transcript in PCa tissues. One possible explanation for this finding is that mRNA expression of Ephrin-A2 is earlier than the expression of Ephrin-A2 protein in PCa tissues.
Ephrin-A2 serum levels and clinicopathological parameters
The averages of serum circulating Ephrin-A2 concentrations were respectively 1.4-fold and 2-fold higher in PCa patients (521.7 ± 35.8 ng/L) compared to BPH patients (394.0 ± 37.9 ng/L) and controls (256.8 ± 46.3 ng/L). Moreover, serum circulating Ephrin-A2 concentrations were significantly higher in PCa patients with high Gleason sore (8–10) compared to those with low Gleason sore (6–7) (P = 0.032). However, no correlation was observed between Ephrin-A2 serum concentrations and patients’ age, prostate volume, PSA levels, or TNM staging (Table 1).
Based on the differential serum level of Ephrin-A2 in patients with PCa and BPH, ROC curve analyses of the serum Ephrin-A2 levels were performed, in comparison to analysis of the pre-surgical serum PSA levels, to distinguish PCa from BPH patients (Fig. 3). The serum Ephrin-A2 levels differentiated PCa from BPH patients (area under curve (AUC) = 0.86; confidence interval (CI) = 0.72–0.95; P < 0.001), with a more favored ROC than serum PSA levels (AUC = 0.59; CI = 0.45–0.73; P = 0.258) in this group of samples analyzed. For the discrimination between PCa and BPH, the optimal cutoff point of Ephrin-A2 concentration was 394.6 ng/L, with a sensitivity of 83.3 % and a specificity of 85.5 %, whereas the serum PSA value cutoff was 5.35, with a sensitivity of 79.2 % and a specificity of 37.5 %. The cutoff values were estimated in order to obtain the best discriminatory power between PCa and BPH patients. These results suggest that serum Ephrin-A2 levels can distinguish PCa from BPH patients and that serum Ephrin-A2 concentration is a better parameter than pre-surgical serum PSA level measurement for the differential diagnosis of these prostatic diseases. The low serum Ephrin-A2 levels are associated with the presence of BPH, while the high Ephrin-A2 levels are associated with the presence of PCa.
ROC for Ephrin-A2 and PSA serum levels. a The ROC curve discrimination of PCa and BPH patients according to serum circulating Ephrin-A2 concentrations (AUC = 0. 86; CI = 0.72–0.95; P < 0.001) with a cutoff value of 394.6 ng/L (specificity 83.3 %; sensitivity 85.5 %). b The ROC curve discrimination of PCa and BPH patients according to serum PSA level (AUC = 0.59; CI = 0.45–0.73; P = 0.258) with a cutoff value of 5.35 ng/mL (specificity 79.2 %; sensitivity 37.5 %)
Ki-67 expression correlates with increased Ephrin-A2 expression in PCa
qRT-PCR has shown that there was a significant correlation between Ki-67 and Ephrin-A2 in mRNA expression level (Pearson coefficient = 0.582) (Fig. 4). In positive cases, Ki-67 IHC shows a nuclear staining of the prostatic adenocarcinoma cells (Fig. 5). Furthermore, this positive correlation between Ephrin-A2 and Ki-67 was also observed in the protein level (Table 2), suggesting that Ephrin-A2 may play an important role in PCa development.
Discussion
The present study was the first to systematically evaluate the expression profile of Ephrin-A2 in both prostate tissues and serum samples of PCa patients, BPH patients, and healthy controls. We observed significantly elevated Ephrin-A2 levels in both tissue and serum samples of PCa patients comparing to those of BPH patients and healthy controls. Moreover, high Ephrin-A2 serum concentrations and tissue expressions were associated with Gleason score. This gradually increasing expression pattern paralleled with deterioration of this disease, suggesting a potential role of Ephrin-A2 in the progression of PCa.
There is emerging evidence demonstrating that Ephrin-A2 is involved not only in the developmental processes [25, 26], but also in carcinogenesis and tumor progression of many tumor types [20–23]. For example, Feng et al. [21] carried out a study of hepatocellular carcinoma (HCC) and found that Ephrin-A2 is significantly upregulated in both cancerous cell lines and clinical tissue samples of HCC compared with the normal ones. Several studies [20, 22] on the Eph/ephrin expression profiles also identified that Ephrin-A2 transcript was overexpressed in breast cancer and renal cell carcinoma, implying that Ephrin-A2 plays an important role in cancer progression. In addition, Fox et al. [23] reported that the expression of Ephrin A2 mRNA is significantly higher in CPTX cell (human local prostate tumor) compared to NPTX cell (normal human prostate epithelium), suggesting that Ephrin-A2 may promote the transformation of the normal prostate epithelial cell into one with a malignant phenotype. The transcript for Ephrin-A2 is also highly present in DU145 and LNCaP and shows a relative increase in the more aggressive PC-3ML as compared to its parent cell line PC-3, implying that Ephrin-A2 may also play an important role in promoting PCa invasion. Consistent with the previous studies [20–23], upregulation of Ephrin-A2 transcript expression was also observed in 52 of 87 (59.8 %) PCa tissues with various histological stages and in five PCa cell lines, whereas only 8.6 % (5/58) of BPH tissues exhibited upregulation of the Ephrin-A2 gene expression. Moreover, Ephrin-A2 IHC positivity rate was significantly higher in PCa tissues (62.5 %) than in BPH tissues (6.7 %). These findings suggest that Ephrin-A2 could be a potential marker to distinguish malignant prostate tissues from the nonmalignant.
Diagnosing cancers by serum profiling is a particularly attractive concept. As a biomarker, serum prostate-specific antigen (PSA) remains the gold standard for detecting PCa in males [3, 4]. In a normal healthy male, serum PSA concentrations are usually well below 4 ng/mL while men with PCa generally present elevated concentrations of PSA [4]. However, the serum PSA level may fluctuate in a patient depending on his diet and exercise conditions [27]. Furthermore, the serum PSA concentration of patients with other prostatic diseases such as benign prostate hyperplasia (BPH) can also be increased [28], which could lead to unnecessary intervention such as prostate biopsies. Therefore, better biomarkers are desirable for distinguishing PCa from BPH.
Ephrin-A ligands belong to a membrane-bound protein with a glycosylphosphatidylinositol (GPI) lipid anchor and include five members (A1–A5) in human [6, 7]. Research and clinical trials have confirmed proteolytic shedding of membrane-bound Ephrin-As, which released soluble fragments at the cellular level [24, 29, 30]. For example, a recent report has identified that ADAM12 cleaved Ephrin-A1 at the primary tumor, and naturally generated soluble Ephrin-A1 was released into blood [30]. Studies on tumor markers have demonstrated that secreted Ephrin-A1 was detected in the medium of several tumor cell lines and in the serum from patients with HCC [31, 32]. In addition, membrane-bound Ephrin-A2 and Ephrin-A5 have been identified as the substrate of ADAM10 and released soluble Ephrin-A2 fragments and Ephrin-A5 fragments into the cell medium [24, 29]. Moreover, Kälin M et al. [33] carried out a study in 57 patients with castration-resistant PCa and found a significant prognostic value of elevated serum Ephrin-A5 levels. The above data suggested that secreted Ephrin-As may be useful serum markers for the diagnosis and prognosis of many tumors including PCa [34]. In the present study, the Ephrin-A2 serum level was found to be significantly higher in patients with PCa than those with BPH or health controls, suggesting that the high serum Ephrin-A2 concentration might help identify patients with PCa. Since ADAM-10 (protein and/or mRNA) is overexpressed in prostatic cancer tissues [35, 36], the potential reason of elevated Ephrin-A2 serum concentration is that ADAM10 cleaved Ephrin-A2 at the prostate tumor tissue and released soluble Ephrin-A2 fragments into the blood of patient with PCa. What is more, the use of serum Ephrin-A2 levels to distinguish PCa and BPH groups was efficient in our cohort of samples, with a desired sensitivity (83.3 %) and specificity (85.5 %). Conversely, in the same patient cohort, the use of serum PSA levels had lower sensitivity (79.2 %) and specificity (37.5 %) to distinguish PCa from BPH samples. These data imply that the serum Ephrin-A2 concentration could be a valuable tool for PCa patient identification.
Ki-67, a proliferation marker, is strongly associated with the prognosis of many tumors, including breast cancer, nonsmall cell lung cancer, and rectal/recto sigmoid cancer, especially PCa [37–40]. Previous studies [41–44] have demonstrated the independent prognostic value of Ki-67 staining both in clinically localized disease treated by RPE or radiation and in patients with advanced disease. Recently, Gunia et al. [44] reported that Ki-67 SI >5 % is an independent predictor of biochemical recurrence following RPE. Moreover, Feng et al. [21] reported that overexpression of Ephrin-A2 promotes tumor growth and knock down of Ephrin-A2 inhibits HCC cell growth in vivo, which imply that Ephrin-A2 promotes HCC cell proliferation in vivo. In our study, the positive relation was observed between expression of Ki-67 and Ephrin-A2 in both transcript level and protein level in prostate tumor tissues. Based on the above data, we hypothesized that Ephrin-A2 might be a valuable prognostic predictor of PCa. Since Gleason score is one of the most important parameters for PCa prognosis evaluation, the significant associations of Ephrin-A2 with higher Gleason score also suggest that Ephrin-A2 has a favored prognosis value.
Taken together, we have identified that increased Ephrin-A2 expression may promote PCa cell proliferation and can be used both in disease diagnosis and in prognosis, although much more follow-ups should be performed to validate this hypothesis. Yet, the potential application of Ephrin-A2 as a prognostic marker for PCa would be addressed in future work.
In sum, we found upregulated Ephrin-A2 levels in tissue and serum samples of PCa patients compared to those without PCa. Moreover, increased Ephrin-A2 expression was found to correlate with Ki-67 expression in PCa patients. Those results indicate that Ephrin-A2 is a potential diagnostic and prognostic biomarker and a useful molecular therapeutic target to attenuate PCa progression.
References
Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51.
Gu F. Changing constituents of genitourinary cancer in recent 50 years in Beijing. Chin Med J (Engl). 2003;116:1391–3.
Dimakakos A, Armakolas A, Koutsilieris M. Novel tools for prostate cancer prognosis, diagnosis, and follow-up. Biomed Res Int. 2014;2014:890697.
Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–78.
Diamandis EP. Prostate-specific antigen: its usefulness in clinical medicine. Trends Endocrinol Metab. 1998;9:310–6.
Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013;5:9.
Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–80.
Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6.
Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–52.
Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–50.
Taddei ML, Parri M, Angelucci A. EphA2 induces metastatic growth regulating amoeboid motility and clonogenic potential in prostate carcinoma cells. Mol Cancer Res. 2011;9:149–60.
Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20.
Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–32.
Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–80.
Rutkowski R, Mertens-Walker I, Lisle JE, Herington AC, Stephenson SA. Evidence for a dual function of EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand. Int J Cancer. 2012;131:E614–24.
Li S, Ma Y, Xie C, Wu Z, Kang Z, Fang Z, et al. EphA6 promotes angiogenesis and prostate cancer metastasis and is associated with human prostate cancer progression. Oncotarget. 2015.
Li S, Zhu Y, Ma C, Qiu Z, Zhang X, Kang Z, et al. Downregulation of EphA5 by promoter methylation in human prostate cancer. BMC Cancer. 2015;15:18.
Roberta N, Rubio Dela Torre E, Pasquale EB. Profiling Eph receptor expression in cells and tissues: a targeted mass spectrometry approach. Cell Adh Migr. 2012;6:102–12.
Aasheim HC, Pedeutour F, Grosgeorge J, Logtenberg T. Cloning, chromosal mapping, and tissue expression of the gene encoding the human Eph-family kinase ligand ephrin-A2. Biochem Biophys Res Commun. 1998;252:378–82.
Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–9.
Feng YX, Zhao JS, Li JJ, Wang T, Cheng SQ, Yuan Y, et al. Liver cancer: EphrinA2 promotes tumorigenicity through Rac1/Akt/NF-kappaB signaling pathway. Hepatology. 2010;51:535–44.
Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882–92.
Fox BP, Tabone CJ, Kandpal RP. Potential clinical relevance of Eph receptors and ephrin ligands expressed in prostate carcinoma cell lines. Biochem Biophys Res Commun. 2006;342:1263–72.
Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–5.
Yates NJ, Martin-Iverson MT, Rodger J. The role of ephrin-A2 and ephrin-A5 in sensorimotor control and gating. Behav Brain Res. 2014;275:225–33.
Diercke K, Sen S, Kohl A, Lux CJ, Erber R. Compression-dependent up-regulation of ephrin-A2 in PDL fibroblasts attenuates osteogenesis. J Dent Res. 2011;90:1108–15.
Ornish D. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174:1065–9.
Barry MJ. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58:25–32.
Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304.
Ieguchi K, Tomita T, Omori T, Komatsu A, Deguchi A, Masuda J, et al. ADAM12-cleaved ephrin-A1 contributes to lung metastasis. Oncogene. 2014;33:2179–90.
Wykosky J, Palma E, Gibo DM, Ringler S, Turner CP, Debinski W. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–73.
Cui XD, Lee MJ, Yu GR, Kim IH, Yu HC, Song EY, et al. EFNA1 ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma. Int J Cancer. 2010;126:940–9.
Kälin M, Cima I, Schiess R, Fankhauser N, Powles T, Wild P, et al. Novel prognostic markers in the serum of patients with castration-resistant prostate cancer derived from quantitative analysis of the pten conditional knockout mouse proteome. Eur Urol. 2011;60:1235–43.
Lisle JE, Mertens-Walker I, Rutkowski R, Herington AC, Stephenson SA. Eph receptors and their ligands: promising molecular biomarkers and therapeutic targets in prostate cancer. Biochim Biophys Acta. 1835;2013:243–57.
McCulloch DR, Akl P, Samaratunga H, Herington AC, Odorico DM. Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res. 2004;10:314–23.
Arima T, Enokida H, Kubo H, Kagara I, Matsuda R, Toki K, et al. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Sci. 2007;98:1720–6.
Li H, Han X, Liu Y, Liu G, Dong G. Ki67 as a predictor of poor prognosis in patients with triple-negative breast cancer. Oncol Lett. 2015;9:149–52.
Berghoff AS, Ilhan-Mutlu A, Wöhrer A, Hackl M, Widhalm G, Hainfellner JA, et al. Prognostic significance of Ki67 proliferation index, HIF1 alpha index and microvascular density in patients with non-small cell lung cancer brain metastases. Strahlenther Onkol. 2014;190:676–85.
Salminen E, Palmu S, Vahlberg T, Roberts PJ, Söderström KO. Increased proliferation activity measured by immunoreactive Ki67 is associated with survival improvement in rectal/recto sigmoid cancer. World J Gastroenterol. 2005;11:3245–9.
Zellweger T, Günther S, Zlobec I, Savic S, Sauter G, Moch H, et al. Tumour growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int J Cancer. 2009;124:2116–23.
Rubio J, Ramos D, López-Guerrero JA, Iborra I, Collado A, Solsona E, et al. Immunohistochemical expression of Ki-67 antigen, cox-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. Eur Urol. 2005;48:745–51.
Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92–02. J Clin Oncol. 2004;22:2133–40.
Tomaszewski JJ, Cummings JL, Parwani AV, Dhir R, Mason JB, Nelson JB, et al. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011;71:1287–93.
Gunia S, Albrecht K, Koch S, Herrmann T, Ecke T, Loy V, et al. Ki67 staining index and neuroendocrine differentiation aggravate adverse prognostic parameters in prostate cancer and are characterized by negligible inter-observer variability. World J Urol. 2008;26:243–50.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (No. 81272386), the Shanghai Municipal Health Bureau Hundred Talents Program (XBR2011044), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120071110073). We gratefully acknowledge the Nature Publishing Group Language Editing for linguistic assistance in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Shibao Li and Zhiyuan Wu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 55 kb)
Rights and permissions
About this article
Cite this article
Li, S., Wu, Z., Chen, Y. et al. Diagnostic and prognostic value of tissue and circulating levels of Ephrin-A2 in prostate cancer. Tumor Biol. 37, 5365–5374 (2016). https://doi.org/10.1007/s13277-015-4398-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4398-7