Abstract
Circulating chromogranin A (CgA) level is a useful marker for diagnosis and treatment efficacy monitoring of neuroendocrine tumors (NETs). To evaluate the diagnostic value of serum CgA in well-differentiated non-functioning NETs and to investigate the correlation between changes in serum CgA levels and imaging responses in patients with locally advanced or metastatic disease, 60 healthy controls and 82 patients with NETs (28 with localized NETs and 54 with advanced NETs) treated between December 2010 and November 2014 were included. CgA levels were determined by ELISA. Receiver-operating characteristic (ROC) curve analysis was used to evaluate the diagnostic sensitivity and specificity of serum CgA. Correlation between CgA levels and tumor burden was analyzed. Serial CgA measurements and tumor responses (evaluated according to the RECIST 1.1 criteria) in 40 patients with locally advanced or metastatic disease were recorded. Using a cutoff value of 84 ng/mL, the sensitivity of serum CgA was 67 %, with a specificity of 78 %. Serum CgA levels of patients with different tumor burdens were significantly different. Progressions were observed in 38 out of 122 visits. Using a 28 % increase of serum CgA concentration as the best cutoff value, the sensitivity and specificity were 79 and 86 %, respectively, with positive and negative predictive values of 71 and 90 %, respectively, to determine disease progression. Serum CgA measurement had a modest sensitivity for the diagnosis of non-functioning NETs. However, increases of CgA levels combined with imaging might be helpful in detecting tumor progression in patients with NETs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumors (NETs) constitute a heterogeneous and probably underestimated group of neoplasms accounting for at least 2 % of human cancers [1–4]. They are derived from the diffuse neuroendocrine system (DNES), and are made up of hormone-producing cells with different hormonal profiles depending on their site of origin [1–4]. The most frequent sites of origin are the digestive and respiratory tracts [4, 5]. In clinical practice, the diagnosis of NETs can be based on the detection of tissue and/or circulating neuroendocrine markers [6].
Chromogranin A (CgA) is an acidic glycoprotein of 439 amino acids and a molecular mass of 48 kDa. It is widely expressed by neuroendocrine cells [7–9]. CgA is released by exocytosis from neuroendocrine-derived tumor cells and can be detected in the blood [10]. Circulating CgA has been proven to be a useful marker for NETs [11]. Measurement in NETs is established in the guidelines as an aid for diagnosis, treatment efficacy, and prognosis [12]. However, serum CgA is not a well-defined entity and is in reality a mixture of peptide fragments of varying lengths. Because the processing of tumor proteins varies considerably from tumor to tumor [13, 14], it is not surprising that varying sensitivities and specificities have been found according to tumor type and method used for CgA measurement [15]. Blood CgA levels seem to be closely related to tumor burden [11, 16–19]. CgA has also been suggested to be useful in the follow-up of patients with NETs. Indeed, previous studies have investigated the concordance between variations in serum CgA levels and changes in tumor size by imaging [7, 17, 20, 21]. Therefore, serial CgA monitoring might be useful for the assessment of tumor progression.
Hence, this study was designed to assess the value of serum CgA in non-functioning well-differentiated NETs in Chinese patients including its diagnostic and the monitoring values.
Methods
Controls
Healthy volunteers were enrolled at the Physical Examination Center of Peking Union Medical College Hospital from July 2013 to December 2013. Exclusion criteria were (1) any chronic or acute disease; (2) any cancer; (3) using proton pump inhibitors (PPI); or (4) serum creatinine ≥1.5 times the upper normal limit.
Patients
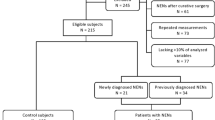
Eighty-two patients receiving treatment for NETs at the Peking Union Medical College Hospital from December 2010 to November 2014 were enrolled. Patients had to have a histologically proven well-differentiated non-functioning NET according to the WHO classification of tumors of the digestive system [5]. Exclusion criteria were (1) serum creatinine ≥1.5 times the upper normal limit; (2) alanine aminotransferase or aspartate aminotransferase ≥1.5 times the upper normal limit; or (3) neuroendocrine carcinoma.
Because treatment with somatostatin analogue or PPI can affect CgA levels, all baseline CgA levels were obtained from patients not using somatostatin analogue or PPI. However, since some patients might need these drugs, only follow-up visits when patients were under no or stable (>6 weeks) treatment with somatostatin analogue [22, 23] or PPI [24] were included for analysis of disease progression.
The patients were divided into two groups: localized disease group (group L) and advanced disease group (group A) according to the revised RECIST guidelines (version 1.1) [25]. Gastroenteropancreatic NETs (GEP-NETs) were diagnosed according to the World Health Organization (WHO) 2010 classification [5]. Atypical carcinoid and typical carcinoid were diagnosed according to the WHO 2004 classification [26].
The present study was approved by the ethical committee of the Peking Union Medical College Hospital. All participants provided a written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Treatment and follow-up
Group A patients were included for the analysis of the correlation between serum CgA changes and imaging disease progression. During follow-up, evaluation of tumor response and determination of serum CgA were performed at each visit. Tumor response was assessed by contrasted computed tomography (CT) or magnetic resonance imaging (MRI). Patients were considered assessable only if measurable disease was present. Response to treatment was evaluated using RECIST 1.1 [25]. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were defined according to the revised RECIST guidelines (version 1.1) [25].
Serum CgA determination
All samples were collected after an overnight fast. Measurement of serum CgA levels was performed with a commercial kit (Chromoa; Cis Bio International, France) using a sandwich-type ELISA assay. The intra-assay and inter-assay coefficients of variation are 5 and 7 %, respectively. At baseline, patients were divided into three groups according to tumor burden: (1) resected tumor; (2) ≤5 tumor sites; and (3) >5 tumor sites; baseline CgA levels were compared between these three groups.
ROC curve construction and analysis
Serum CgA levels of 60 healthy controls and initial CgA levels of 54 patients from group A measured before starting treatment were used to create a ROC curve evaluating the performance of CgA in the diagnosis of non-functioning NETs. Percentage changes in CgA levels on PD and non-progression (CR, PR, and SD) were used to create the ROC curve in order to determine the cutoff value predicting PD.
Statistics
ROC curves were constructed using SPSS 20.0 (IBM, Armonk, NY, USA). A stepwise step-down procedure for multiple comparisons and Cochran-Mantel-Haenszel (CMH) chi-square test were used to compare serum CgA levels in patients with different tumor burdens and different tumor origins, respectively. Spearman rank correlation analysis was performed to evaluate the correlation between CgA levels and tumor burden. McNemar’s test was used to compare serum CgA changes with imaging to determine disease progression. Statistical significance was established at P < 0.05.
Results
Characteristics of the patient
The group of patients included 46 males and 36 females, with a median age of 51 (range 15–84) years. The L group included 28 patients that have undergone resection, The A group included 54 patients: 6 had unresectable locally advanced disease and 48 had metastatic disease. Primary tumor site was the pancreas in 56 patients, lung or thymus in 10, rectum in 7, stomach in 3, small intestine in 3, and unknown primary in 3. The tumors in the 69 patients with GEP-NETs included 17 G1 tumors and 52 G2 tumors. The ten patients with thoracic primary tumor included nine cases of atypical carcinoid tumors and only one typical carcinoid tumor (Table 1).
The control group was composed of 60 healthy individuals including 33 males and 27 females. Median age was 43 (range 26–72) years.
Serum CgA levels
Serum CgA levels in group advanced and group control are shown in Fig. 1a. One patient with lung atypical carcinoid tumor and multiple bone and lung metastases was excluded because of outlying baseline serum CgA levels; hence, a total of 81 patients were divided into three groups according to tumor burden: (1) resected tumor; (2) ≤5 tumor sites; and (3) >5 tumor sites. At study entry, serum CgA levels were higher in group 3 compared to group 1 (P < 0.0001), while there was no difference between groups 1 and 2, and between groups 2 and 3 (Fig. 1b). CgA levels were significantly correlated to tumor burden (Spearman rank correlation, r = 0.4266, P < 0.0001). Serum CgA levels of patients with GEP-NETs and non-GEP-NETs were not significantly different (P = 0.9456) (Fig. 1c).
a Comparison of serum CgA levels between group A and controls. b Comparison of serum CgA levels according to different tumor burdens. CgA levels of patients with resected tumor and >5 tumor sites were significantly different. c Comparison of serum CgA levels according to different origins of NETs. CgA levels of patients with GEP-NETs and non-GEP-NETs were not significantly different
ROC analysis
ROC curve analysis was performed using baseline serum CgA levels of 60 healthy controls and 54 patients with locally advanced or metastatic well-differentiated non-functioning NETs. As shown in Fig. 2, the best cutoff value for NET diagnosis was 84 ng/mL, resulting in a sensitivity of 67 % and a specificity of 78 % for diagnosing well-differentiated non-functioning NETs with the Chromoa kit (Cis Bio International, France).
Receiver-operating characteristics (ROC) curve constructed with baseline serum CgA levels of 60 healthy controls and 54 patients with locally advanced or metastatic well-differentiated non-functioning NETs. The best cutoff value for NET diagnosis was 84 ng/mL, and the area under the curve was 0.69 (95 % CI 0.58–0.79)
Follow-up
Forty patients from group A (27 males and 13 females, median age of 55 years) with locally advanced disease (n = 4) or metastatic diseases (n = 36) were included for analysis between serum CgA changes and imaging disease progression. During follow-up, these 40 patients underwent 122 medical follow-up visits for treatments including 37 visits for targeted therapy, 29 visits for somatostatin analogue, 27 visits for chemotherapy, 16 visits for combined therapy (14 visits for somatostatin analogue with targeted therapy and 2 visits for somatostatin analogue with transarterial chemoembolization), 6 visits for transarterial chemoembolization, 6 visits for observation, and 1 visit for surgery.
All these patients had at least one follow-up visit after the initial study entry visit, and the mean number of visits per patient was 3 ± 2 (range 1–9). The median interval between visits was 3 (range 1.5–23) months. The median follow-up time was 10 (range 1.5–51) months.
Changes in CgA levels and disease progression
During the 122 visits of the 40 patients with locally advanced disease or metastatic disease, 24 PR events, 60 SD events, and 38 PD events were recorded. Based on the ROC curve, the best cutoff value for detecting disease progression was a 28 % increase over baseline CgA levels, with sensitivity and specificity of 79 and 86 %, respectively, to discriminate between disease progression and non-progression (Fig. 3).
Using imaging tumor response as reference, the calculated positive and negative predictive values of CgA changes for detecting disease progression were 71 and 90 %, respectively (Table 2). There was no significant difference between imaging and changes in CgA levels for the detection of disease progression (P = 0.5023).
Discussion
The aims of the present study were to evaluate the diagnostic value of serum CgA in well-differentiated non-functioning NETs and to investigate the correlation between changes in serum CgA levels and imaging responses in patients with locally advanced or metastatic disease. Results showed that using a cutoff value of 84 ng/mL, the sensitivity of serum CgA was 67 %, with a specificity of 78 %. Serum CgA levels of patients with different tumor burdens were significantly different. Using 28 % increase of serum CgA concentration as the best cutoff value, the sensitivity and specificity were 79 and 86 %, respectively. Therefore, these results suggest that serum CgA measurement had a modest sensitivity for the diagnosis of non-functioning NETs. However, increases of CgA levels combined with imaging might be helpful in detecting tumor progression in patients with NETs.
Different NETs release different molecular isoforms of CgA [27]. Due to differences between different CgA assays, results can be variable [28]. The CgA ELISA assay used in the present study is targeting the core of the molecule (amino acid residues 145–245) using two monoclonal antibodies to measure intact and fragmented CgA. Therefore, this assay should provide more stable and consistent results between different NETs.
Tumor functional status might also affect CgA levels. Indeed, a study by Nehar et al. has shown excellent specificity (98.4 %) using a CgA assay, but sensitivity was 73 % for secreting tumors and 45 % for non-secreting tumors in patients with GEP-NETs and multiple endocrine neoplasia type 1 (MEN-1) [17]. The results of an Italian study confirmed the finding that the median CgA levels were significantly higher in functioning GEP-NETs compared with non-functioning ones (295 vs. 43 U/L, P = 0.0001) [9]. Non-functioning NETs usually show intermediate CgA secretion potential compared to functioning NETs since the increases of CgA secretion are mainly associated with the increased hormone secretion potential of the tumor [8, 9]. On the other hand, a previous study showed that CgA levels were not affected by the functional status of the tumor [29].
The present study, however, focused on non-functioning NETs and showed that the sensitivity was 67 % using a CgA cutoff value of 84 ng/mL, which was comparable to previous studies [7, 15], while the specificity of 78 % was relatively lower. Considering the low sensitivity of CgA for the diagnosis of non-functioning NETs, increasing the cutoff value to 112 ng/mL would yield a specificity of 90 % with a lower sensitivity of 44 %, which was similar to a previous study [17].
Previous studies showed that CgA levels are increased in NETs from different organs including pheochromocytoma, carcinoid tumors, pancreatic neuroendocrine tumor, medullary carcinoma of the thyroid, and small-cell lung cancer [29]. Few studies examined the differences in CgA secretion between NETs from different organs. A previous study has shown that CgA levels are elevated in patients with NETs, irrespective of tumor location or functional status [29]. Another paper revealed that CgA levels cannot be used to differentiate different subtypes of NETs [30]. In this present study performed in non-functioning NETs, there was no difference in CgA secretion between GEP and non-GEP-NETs, as well as between pancreatic and non-pancreatic NETs, but the small number of tumors in each group might have prevented observing differences. Further study is necessary to refine these results.
Previous studies have shown that the levels of CgA were related to the extent of the metastatic spread [11, 18–20, 31]. In this study, serum CgA levels were correlated to tumor burden. Serum CgA levels were significantly different in patients without tumor and patients having >5 tumor sites. These results support the use of serum CgA measurement in monitoring disease progression in non-functioning NETs despite their relatively lower CgA secretion potential. This association is supported by previous studies [11, 18–20, 31].
Several published studies have also investigated the concordance between imaging tumor response and changes in CgA levels, showing that changes in CgA could indicate changes in tumor growth [5, 7, 17, 20]. Nevertheless, all studies were performed in heterogeneous groups of patients including both functioning and non-functioning NETs. Among these studies, the study by Jensen et al. focused on well-differentiated G1 and G2 ileo-cecal NETs [20], the study by Wang et al. enrolled both well-differentiated and poorly differentiated NETs [5], and other earlier studies did not use the 2010 WHO classification or RECIST 1.1 criteria to define their patient population or tumor responses [7, 17]. To the best of our knowledge, this study is the first to investigate the correlation between changes of serum CgA levels and imaging tumor response using the RECIST 1.1 criteria in non-functioning well-differentiated NETs.
Detecting disease progression is important in clinical practice to avoid continuing ineffective therapies and to prevent unnecessary side effects. Based on 38 events of disease progression, the present study suggests that using a cutoff value of 28 % CgA increase might be indicative of disease progression, which is comparable to the 25 % cutoff value proposed by Nehar et al. [17] and Jensen et al. [20]. Using imaging tumor response as the reference, the diagnostic sensitivity and specificity were 79 and 86 %, respectively, similar to the study by Jensen et al. [20]. Two other studies also examined the value of serial CgA measurements, but these studies were performed in heterogeneous populations of patients [7, 32]. A recent phase II study of NET treatment with temozolomide and bevacizumab showed that >50 % of patients with elevated CgA levels at baseline showed decreases during treatment, suggesting the usefulness of CgA measurements to monitor response to treatments and disease progression [33].
The use of CgA for monitoring disease progression might be performed at the same time as routine blood tests and might decrease the frequency of imaging examinations. Indeed, some imaging examinations are costly in time, money, and hospital resources, and expose the patients to radiation. CgA measurements could easily be performed, for example every 3 months, while computed tomography might be performed once a year or to confirm progression in case of the appearance of new symptoms or in case of CgA increase. Nevertheless, additional studies are necessary to determine the exact diagnosis and monitoring value of CgA for non-functioning NETs. Indeed, the usefulness of CgA is still impaired by differences in measurement methods, the heterogeneity of the study populations, and by CgA secretion by normal tissues [28].
The present study is not without limitations. This was a single-center study with a small sample size, and we cannot yet generalize its conclusions to the routine clinical practice. In addition, the small sample size prevented subgroup analyses. NETs from different organs might show different CgA levels. A study with a larger sample size is needed for refining and generalizing the conclusions of this study.
Conclusion
Although serum CgA determination was of modest sensitivity in diagnosing well-differentiated non-functioning NETs, serum CgA level might still reflect tumor burden. Serial CgA monitoring combined with imaging might be useful in the detection of tumor progression.
References
Donckier JE, Michel L. Phaeochromocytoma: state-of-the-art. Acta Chir Belg. 2010;110:140–8.
Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, et al. Adrenal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii131–138.
van der Lely AJ, de Herder WW. Carcinoid syndrome: diagnosis and medical management. Arq Bras Endocrinol Metabol. 2005;49:850–60.
Vaidakis D, Karoubalis J, Pappa T, Piaditis G, Zografos GN. Pancreatic insulinoma: current issues and trends. Hepatobiliary Pancreat Dis Int. 2010;9:234–41.
Bosman F, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010.
Berretta M, Cappellani A, Di Vita M, Berretta S, Nasti G, Bearz A, et al. Biomarkers in neuroendocrine tumors. Front Biosci (Schol Ed). 2010;2:332–42.
Bajetta E, Ferrari L, Martinetti A, Celio L, Procopio G, Artale S, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86:858–65.
d’Herbomez M, Do Cao C, Vezzosi D, Borzon-Chasot F, Baudin E, groupe des tumeurs endocrines (GTE France). Chromogranin A assay in clinical practice. Ann Endocrinol (Paris). 2010;71:274–80.
Massironi S, Rossi RE, Casazza G, Conte D, Ciafardini C, Galeazzi M, et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology. 2014;100:240–9.
O’Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986;314:1145–51.
Zatelli MC, Torta M, Leon A, Ambrosio MR, Gion M, Tomassetti P, et al. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian multicenter study. Endocr Relat Cancer. 2007;14:473–82.
Kocha W, Maroun J, Kennecke H, Law C, Metrakos P, Ouellet JF, et al. Consensus recommendations for the diagnosis and management of well-differentiated gastroenterohepatic neuroendocrine tumours: a revised statement from a Canadian national expert group. Curr Oncol. 2010;17:49–64.
Portela-Gomes GM, Stridsberg M. Selective processing of chromogranin A in the different islet cells in human pancreas. J Histochem Cytochem. 2001;49:483–90.
Laslop A, Doblinger A, Weiss U. Proteolytic processing of chromogranins. Adv Exp Med Biol. 2000;482:155–66.
Stridsberg M, Eriksson B, Oberg K, Janson ET. A comparison between three commercial kits for chromogranin A measurements. J Endocrinol. 2003;177:337–41.
Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose KJ, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol. 2008;6:820–7.
Nehar D, Lombard-Bohas C, Olivieri S, Claustrat B, Chayvialle JA, Penes MC, et al. Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf). 2004;60:644–52.
Janson ET, Holmberg L, Stridsberg M, Eriksson B, Theodorsson E, Wilander E, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–90.
Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622–8.
Jensen KH, Hilsted L, Jensen C, Mynster T, Rehfeld JF, Knigge U. Chromogranin A is a sensitive marker of progression or regression in ileo-cecal neuroendocrine tumors. Scand J Gastroenterol. 2013;48:70–7.
Wang YH, Yang QC, Lin Y, Xue L, Chen MH, Chen J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine (Baltimore). 2014;93:e247.
Tateishi K, Kitayama N, Matsuoka Y, Funakoshi A. Comparison of chromogranin A and pancreastatin levels in plasma of patients with pancreatic islet cell tumor. Life Sci. 1995;57:889–95.
Moattari AR, Deftos LJ, Vinik AI. Effects of sandostatin on plasma chromogranin-A levels in neuroendocrine tumors. J Clin Endocrinol Metab. 1989;69:902–5.
Igaz P, Mullner K, Hargitai B, Igaz I, Tombol Z, Racz K, et al. Marked chromogranin A elevation in a patient with bilateral adrenal incidentalomas, and its rapid normalization after discontinuation of proton pump inhibitor therapy. Clin Endocrinol (Oxf). 2007;67:805–6.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Tumours of the lung, pleura, thymus and heart. World Health Organization classification tumours. Pathology and genetics. Lyon: IARC Press; 2004.
Ferolla P, Faggiano A, Mansueto G, Avenia N, Cantelmi MG, Giovenali P, et al. The biological characterization of neuroendocrine tumors: the role of neuroendocrine markers. J Endocrinol Invest. 2008;31:277–86.
Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:111–34. viii.
Stivanello M, Berruti A, Torta M, Termine A, Tampellini M, Gorzegno G, et al. Circulating chromogranin A in the assessment of patients with neuroendocrine tumours. A single institution experience. Ann Oncol. 2001;12 Suppl 2:S73–77.
de Herder WW. Biochemistry of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:33–41.
Borglum T, Rehfeld JF, Drivsholm LB, Hilsted L. Processing-independent quantitation of chromogranin A in plasma from patients with neuroendocrine tumors and small-cell lung carcinomas. Clin Chem. 2007;53:438–46.
Abou-Saif A, Gibril F, Ojeaburu JV, Bashir S, Entsuah LK, Asgharian B, et al. Prospective study of the ability of serial measurements of serum chromogranin A and gastrin to detect changes in tumor burden in patients with gastrinomas. Cancer. 2003;98:249–61.
Chan JA, Stuart K, Earle CC, Clark JW, Bhargava P, Miksad R, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30:2963–8.
Acknowledgments
We are highly thankful to the patients for their invaluable participation, as well as to the radiology and laboratory staff.
Compliance with ethical standards
ᅟ
Conflicts of interest
None
Ethics approval
The study involved human participants and was approved by the ethical committee of the Peking Union Medical College Hospital. All participants provided a written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuejuan Cheng and Zhao Sun contributed equally to this study.
Rights and permissions
About this article
Cite this article
Cheng, Y., Sun, Z., Bai, C. et al. Serum chromogranin A levels for the diagnosis and follow-up of well-differentiated non-functioning neuroendocrine tumors. Tumor Biol. 37, 2863–2869 (2016). https://doi.org/10.1007/s13277-015-4114-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4114-7