Abstract
Background
Chromogranin A (CgA) is a valuable biomarker for detection and follow-up of patients with neuroendocrine neoplasms (NENs). However, various comorbidities may influence serum CgA, which decreases its diagnostic accuracy. We aimed to investigate which laboratory parameters are independently associated with increased CgA in real-life setting and to develop a scoring system, which could improve the diagnostic accuracy of CgA in detecting patients with NENs.
Methods
This retrospective study included 55 treatment naïve patients with NENs and160 patients with various comorbidities but without NEN (nonNENs). Scoring system (CgA-score) was developed based on z-scores obtained from receiver operating curve analysis for each parameter that was associated with elevated serum CgA in nonNENs.
Results
CgA correlated positively with serum BUN, creatinine, α2-globulin, red-cell distribution width, erythrocyte sedimentation rate, plasma glucose and correlated inversely with hemoglobin, thrombocytes and serum albumin. Serum CgA was also associated with the presence of chronic renal failure, arterial hypertension and diabetes and the use of PPI. In the entire study population, CgA showed an area under the curve of 0.656. Aforementioned parameters were used to develop a CgA-score. In a cohort of patients with CgA-score <12.0 (N = 87), serum CgA >156.5 ng/ml had 77.8% sensitivity and 91.5% specificity for detecting NENs (AUC 0.841, 95% CI 0.713–0.969, P < 0.001). Serum CgA had no diagnostic value in detecting NENs in patients with CgA-score >12.0 (AUC 0.554, 95% CI 0.405–0.702, P = 0.430).
Conclusions
CgA-score encompasses a wide range of comorbidities and represents a promising tool that could improve diagnostic performance of CgA in everyday clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromogranin A (CgA) is an acidic glycoprotein and a member of the granin family, which can be found in secretory granules of all endocrine and neuroendocrine cells [1]. It has an important regulatory role in the process of formation and exocytosis of secretory granules that contain various peptide hormones [2]. CgA is secreted along with peptide hormones and metabolized in various tissues into biologically active peptides, such as vasostatin, pancreastatin, catestatin, and serpinins [2]. Some studies suggest that these peptides might be involved in various biological processes; however, their exact role has not been completely elucidated. For instance, vasostatin inhibits a series of effects induced by vascular endothelial growth factor on endothelial cells, which may be very important in terms of tumorgenesis [3]. Moreover, evidence exists that CgA counteracts the effects of tumor necrosis factor-α on the endothelial cells, which is an important role in the setting of systemic inflammation [4]. Serpinin peptides act as myocardial β-adrenergic-like agonists that increase the cardiac inotropy and lusitropy. They may also affect the cell survival and exert neuroprotective effects [5]. Pancreastatin and catestatin display beneficial effects on glucose metabolism, dyslipidemia and arterial hypertension in patients with metabolic syndrome [6, 7]. It is notable that CgA not only controls exocytosis of secretory granules in systemic neuroendocrine response to various disease conditions, but is also a molecular precursor of all these peptides. Thus, it is common to find increased CgA serum levels in patients with sepsis [8], acute exacerbation of rheumatoid arthritis [9], inflammatory bowel disease [10, 11], various metastatic malignancies [12], heart and renal failure [13, 14], complicated myocardial infarction [15], arterial hypertension [16], and chronic atrophic gastritis [17]. Moreover, the use of proton pump inhibitors (PPIs) may also increase CgA serum levels [18]. Thus, CgA can be found in every human and its abundance is not per se an indicator of neuroendocrine neoplastic disease, but the increase above a normal distribution may indicate the presence of neuroendocrine neoplasm (NEN).
CgA is routinely used only in the diagnosis and follow-up of patients with NENs [19]. NEN tumor cells secrete CgA, which has a diagnostic, predictive and prognostic role in this population of patients. CgA has a relatively good predictive accuracy, so the rise of CgA in patients with resected NEN is the first sign of recurrence in 85% of patients [20]. Moreover, the short-term decrease in serum CgA treated with everolimus, predicts long-term treatment response [21].
However, due to its non-specific nature, the exact diagnostic accuracy of CgA as a screening method in detecting patients with NENs remains controversial. Sensitivity of CgA ranges from 53 to 85%, while specificity ranges from 84 to 96% in detecting patients with NENs in the general population [22]. This greatly differs between studies, depending mainly on the control group. Studies focusing on the diagnostic accuracy of CgA may be distinguished to those which exclude or include subjects with interfering factors [22]. Studies that used healthy blood donors had highest diagnostic accuracy of CgA in detecting NENs, while the diagnostic accuracy substantially decreased when control group consisted of patients using PPI or patients with other malignant diseases [22]. Moreover, in a study by Marotta V et al. that included 42 subjects affected with NEN, 120 subjects affected with non-endocrine neoplasias and 100 non-neoplastic subjects affected with benign nodular goiter, serum CgA had no diagnostic value in detecting patients with NENs [23]. In addition, the diagnostic accuracy of CgA also depends on the stage of the disease and tumor volume [24, 25], primary tumor site [25] and the method of measurement [26, 27].
The concept of this study was to find which anthropometric and laboratory parameters are associated with increased serum CgA in patients without NEN. In addition, we aimed to deduce a scoring system consisting of the relevant of those parameters and hypothesized that this scoring system could stratify subjects into cohorts that have different diagnostic accuracy of CgA. Thus, the scoring system could potentially select subjects in whom CgA does not have any diagnostic accuracy due to their substantial comorbidities and could help to avoid unnecessary costly diagnostic work-up. On the other hand, it could also help to select patients in whom increased CgA suggests the presence of NEN and thus warrants further investigation.
Patients and methods
Patients
This single-center retrospective study was performed in a tertiary referral center for patients with NEN at University hospital center Sisters of charity, Zagreb, Croatia and included all patients in whom serum CgA was measured between 2012 and 2016. Medical charts from patients were retrieved and analyzed by physicians with clinical expertise in diagnosis and treatment of patients with NEN. Initially, we included all patients for whom more than 90% of analyzed variables were available at the time when CgA was measured. In patients who had several CgA measurements, only clinical and laboratory parameters during the first CgA measurement were included in the final analysis. Repeated measurements of CgA and other laboratory parameters were not analyzed.
The indication for CgA measurement was set by physicians of different specialties in case of suspicion of NEN. All subjects with increased CgA were referred to a multidisciplinary NEN team and all of them had undergone further diagnostic work up. It included chest X-ray and abdominal ultrasound, 24-h urinary 5-hydroxyindoleacetic acid collections and serum gastrin (in patients with suspected pancreatic NEN) in all subjects. Based on the ongoing clinical suspicion of a NEN after this initial diagnostic work-up, some patients continued a more specific diagnostic process with computed tomography (CT) and/or magnetic resonance imaging (MRI) and 99mTc-Tektrotyd scintigraphy and/or 18F-fludeoxyglucose positron emission tomography (18F-FDG PET). CT protocols included early arterial phase sequences and MRI protocols included both contrast-enhanced and diffusion-weighted sequences. All the patients with previously established diagnosis of NEN have undergone all the aforementioned diagnostic tests. If even after this workup, the presence of a NEN could not be demonstrated, these subjects were defined as controls (nonNENs). Patients with NENs were included only if they were treatment naïve and had active disease at the time of CgA measurement. Patients who underwent curative surgery and did not have radiological evidence of recurrence or metastases were excluded from the study. Patients with NEN were classified based on ENETS guidelines of 2012 [28]. Finally, of 460 CgA measurements, 160 subjects could be included into the control group and 55 subjects into the NEN group; a flowchart of the study subjects is presented in Fig. 1.
The following anthropometric and the following clinical parameters were recorded in all subjects: age, gender, body mass, body height, body mass index, the history of arterial hypertension, diabetes, heart failure, chronic obstructive pulmonary disease, liver cirrhosis, renal disease, other malignant disease, gastritis, atrophic gastritis, inflammatory bowel disease, autoimmune diseases (other than atrophic gastritis), current infectious disease and current malignant disease (besides NEN), recent weight loss (within 3 months prior to CgA measurement), smoking and the use of PPIs. Active malignant disease was defined as newly diagnosed malignant disease (other than NEN), systemic treatment for metastatic disease or terminal phase of malignant disease.
The study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the University Hospital Center Sisters of charity.
Laboratory analyses
In addition to the anthropometric an clinical parameters the following laboratory values were analyzed: complete blood cell count, erythrocyte sedimentation rate (ESR), red cell distribution width (RDW), blood urea nitrogen (BUN), serum creatinine, serum cholesterol, high-density lipoprotein, low-density lipoprotein (LDL), serum triglycerides, serum bilirubin, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, lactate dehydrogenase, fibrinogen, C-reactive protein, protein electrophoresis, prothrombin time (PT), thrombin time, serum amylase, alkaline phosphatase, creatine kinase, serum sodium, potassium, chloride, phosphate, calcium, iron, total iron-binding capacity, unsaturated iron-binding capacity and serum glucose. Parameters analyzed from urine were: urine specific gravity, amylase, calcium, creatinine, pH, phosphorus, presence of epithelial cells, erythrocyte and leukocyte count. All analyses were made with the analytic system on the automatic analyzer AU 2700 (Beckman 40) with original chemicals (Beckman Coulter International S. A.).
CgA level was measured via ELISA using a commercially available kit (Demeditec Diagnostics GmbH, Germany) [29]. Blood samples were collected by venipuncture into serum-separator tubes without anticoagulant. Serum was separated by centrifugation and immediately stored at −20 °C until analysis, which was performed as described in the manufacturer’s instructions. Normal range was considered to be 12.5–100 μg/L, as provided by the manufacturer. Elevated serum CgA was considered >100 μg/L.
Statistical analyses
Patient characteristics were analyzed with descriptive statistics and presented as a median and interquartile range. Since the majority of parameters did not follow normal distribution we used nonparametric tests as follows: independent continuous variables were compared with Mann–Whitney U test and categorical variables were compared using Fisher’s exact test. Receiver operating characteristic (ROC) analysis was used to analyze the diagnostic accuracy of CgA in the entire study population. Afterwards, Spearman correlation was performed in non-NEN patients in order to detect the association between CgA and anthropometric and laboratory parameters. Eta statistic was used for correlation between continuous and categorical variables. ROC analyses were performed for parameters that showed significant correlation coefficient in order to establish a cut-off value for each variable. Variables available in more than 90% of controls, which showed significant association with CgA on ROC analysis, were included into the CgA scoring system (CgA-score). CgA-score was calculated by multiplying z-scores obtained in ROC analysis. Afterwards, the entire study population was divided into five subgroups based on CgA-score values and reassessed the diagnostic accuracy of CgA in each subgroup. The results of (ROC) analysis were presented with sensitivity, specificity and positive likelihood ratio for each CgA cut-off. Two-tailed P-values < 0.05 were considered significant. “We did not perform p-value adjustment for multiple testing, due to hypothesis and primary aim of the study. The aim of the correlation analysis was to detect which parameters have the greatest impact within the CgA-score, and not to claim the actual strength of these correlations with other parameters.” Statistical analyses were performed by using SPSS Version 20.0.
Results
General characteristics
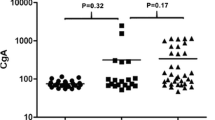
The median age of our study population was 56.0 years (41.0–66.0), 97 (45.1%) of them were males and median CgA was 88.0 μg/L (38.0–280.0). A total of 102 (47.4%) of subjects had serum CgA above the upper reference range value. A median CgA level was lower in non-NEN group (Table 1). The presence of atrophic gastritis was higher in NEN group, while the presence of infectious diseases was higher in non-NEN group. Five patients had gastric NENs. Three patients had G1 and two patients had G2 NENs. All of them were categorized as type 1 gastric carcinoids and all had localized disease. Patients with gastric NENs had higher serum CgA levels when compared with ten patients with localized panNENs [197 (130–335) vs. 68 (29–110) ng/ml], but the difference was not statistically significant (P = 0.221). There were no significant differences in laboratory parameters between non-NEN and NEN group (Supplemental Table 1). The presence of all other comorbidities was similar in both groups. Ki67 index was available in 49 (89.1%) patients. The group of patients with NEN were further subdivided based on Ki67 index into three groups, patients with G1 NENs had significantly lower CgA level (161.0 [68.0–466.0] μg/L), compared to G2 (308.0 [29.0–863.0] μg/L) and G3 NENs (357.0 [237.0–1100.0] μg/L) (P = 0.001). The difference between CgA serum levels between G2 and G3 was not statistically significant (P = 0.573). Metastatic disease was more prevalent in patients with G3 NENs (Table 1). In the entire study population, CgA had the area under the curve (AUC) of 0.656 (95% CI 0.564–0.749) (Fig. 2f). The cut-off value of CgA of 189 μg/L had a sensitivity of 56.4% (95% CI 42.3–69.7), specificity of 76.9% (95% CI 69.6–83.2) and positive predictive value of 2.44 (95% CI 1.7–3.5). In a subgroup analysis that included only patients with localized NENs and all non-NEN subjects, CgA had no diagnostic value in detecting patients with NENs (AUC 0.522, 95% CI 0.392–0.652, P = 0.710).
We have also performed a subgroup analysis of patients with non-NEN malignant disease in order to evaluate the diagnostic performance of CgA in patients with evident malignant disease. There were 21 patients with non-NEN metastatic disease and 27 metastatic NENs. Among 21 patients with non-NEN metastatic disease, 16 had adenocarcinoma, 2 melanoma, 1 plasmacytoma and 2 undefined high-grade carcinomas. CgA was increased above the ULN in 12 (57.1%) patients in the non-NEN group and 22 (81.5%) in the NEN group (P = 0.095). However, patients with NENs had significantly higher CgA levels [117 (35–211) vs. 296 (134–920), P < 0.001]. Consequently, the diagnostic accuracy of CgA was substantially better in patients with metastatic disease (AUC 0.820, 95% CI 0.698–0.941, P < 0.001). In this subgroup of patients with non-NEN and NEN metastatic disease, CgA of 211.5 μg/L had both a sensitivity and specificity of 81% each in detecting patients with NENs.
Correlation between clinical and biochemical parameters and CgA in non-NEN group
A total of 66 (41.2%) of non-NEN patients had increased serum CgA. Subjects with increased CgA had also lower erythrocyte and thrombocyte count, hemoglobin, MCHC, PT, LDL cholesterol, albumins and urinary phosphate levels, but higher alpha2-globulins, BUN and creatinine levels when compared with subjects with normal CgA (Supplemental Table 2). CgA correlated positively with urinary leukocyte and erythrocyte count, serum BUN and creatinine concentration, α2-globulin fraction, RDW, ESR, plasma glucose, previously established diagnosis of chronic renal failure, arterial hypertension, diabetes, and the use of PPI. CgA was inversely associated urinary phosphate, hemoglobin, thrombocyte count, serum albumins and body mass. Correlation coefficients for each parameter are presented in Table 2 and categorized based on the correlation strength. ROC analysis was performed for each of the previously mentioned parameter, cut-off values, AUC and z-statistic values are presented in Table 2. Only parameters that showed statistically significant association with CgA in both Spearman correlation and ROC analysis, and parameters available in ≥140 control subjects were included into a scoring system (CgA-score). The correlation plots and box-plots were made for these variables and presented in Fig. 3.
Correlation between serum chromogranin A and hemoglobin (a), blood urea nitrogen (b), serum albumins (c), alpha2 globulins (d), red distribution width (e), thrombocytes (f), serum creatinine (g), fasting plasma glucose (h) and CgA-score (i). Box-plots showing the difference in serum CgA levels in patients divided based on the presence of chronic renal failure (j), use of proton pump inhibitors (k) and the presence of arterial hypertension (l)
The diagnostic performance of CgA in the entire study population divided by CgA-score
A CgA-score was calculated by multiplying the z-statistic values, which are provided in Table 2 and ranged from 0 to 31.3. There was no significant difference in CgA-score between controls and patients with NENs [12.9 (7.6–16.8) vs. 13.4 (9.7–17.7), P = 0.436]. Moreover, there was no significant difference in CgA-score between patients with G1, G2 and G3 NENs [G1 13.8 (11.3–18.7), G2 10.9 (5.1–16.0) and G3 14.7 (11.8–20.3), P = 0.105].
“A CgA-score cut-off of >12.0 had the highest Youden index in predicting increased serum CgA levels with sensitivity of 77.8% and specificity of 75.3% (AUC 0.835, 95% CI 0.766–0.905, P < 0.001) in non-NEN patients.” In a cohort of patients with CgA-score <12.0, serum CgA >156.5 ng/ml had 77.8% sensitivity and 91.5% specificity for detecting NENs (AUC 0.841, 95% CI 0.713–0.969, P < 0.001). On the other hand, serum CgA had no diagnostic value in detecting NENs in patients with CgA-score >12.0 (AUC 0.554, 95% CI 0.405–0.702, P = 0.430) (Fig. 4). We have also performed a subgroup analysis that included only patients with localized NENs and all non-NEN subjects. In this subgroup analysis, patients with CgA-score <12 had the diagnostic accuracy of CgA in detecting patients with NENs of 78% (AUC 0.779, 95% CI 0.598–0.959, P = 0.008). However, in patients with CgA-score >12, CgA had no diagnostic value in detecting localized NENs (AUC 0.397, 95% CI 0.201–0.593, P = 0.264).
Further subgroup analysis was performed, when entire study population was arbitrarily divided into five subgroups according to the CgA-score: 0-8 (interval [I] 1), 8–12 (I2), 12-16 (I3), 16-22 (I4) and >22 (I5). AUC for CgA in the entire population was 0.656 and it increased to 0.905 in I1, 0.788 in I2 and 0.745 in I4 (Table 3, Fig. 2). On the other hand, CgA did not have any diagnostic value in subjects with CgA-score in I3 and I5 (Table 3). Patients in I3 had the lowest prevalence of metastatic disease. CgA cut-off values were substantially different between the groups. Overall, the CgA-score increased the diagnostic accuracy of CgA in detecting patients with NENs in 124 (68.9%) subjects.
Comparison between CgA-score and detailed medical history in increasing the diagnostic performance of CgA
In order to elucidate the impact of CgA-score in routine clinical setting, we compared the change in diagnostic accuracy of CgA in subgroups of patients divided based on CgA-score and number of comorbidities that were found to be associated with increased CgA in previous studies (arterial hypertension, diabetes, heart failure, liver cirrhosis, renal disease, other malignant disease, atrophic gastritis, inflammatory bowel disease and infectious disease). Serum CgA correlated positively both with the number of comorbidities (ρ = 0.406, P < 0.001) and CgA score (ρ = 0.588, P < 0.001). However, when ROC analysis was performed in patients divided into subgroups based on CgA-score intervals and number of comorbidities, CgA-score was far more efficient in both increasing and decreasing the diagnostic accuracy of CgA in detecting patients with NENs (Fig. 5).
Discussion
To the best of our knowledge, this is the first study that has attempted to incorporate all comorbidities that may affect serum CgA into a scoring system. Our study showed that the CgA-score increases the diagnostic accuracy of CgA in detecting patients with NENs, which may be useful in routine clinical practice.
The overall diagnostic accuracy of CgA in our study was 66%, which is similar to recent studies assessing the diagnostic accuracy of a novel biomarker—blood-based multi-transcriptome assay (NETest) [30, 31]. NETest showed excellent diagnostic accuracy when compared with single CgA measurement, but due to its high costs and limited availabilities its wide-spread routine use in clinical practice is unlikely in the near future.
As reported previously, we also showed the association of CgA with arterial hypertension, renal insufficiency, diabetes and the use of PPI. As one would expect, CgA was also associated with serum creatinine, BUN and plasma glucose. We have not found any association with bilirubin or liver enzymes, but previous studies have demonstrated increased CgA only in patients with liver cirrhosis and concomitant hepatocellular carcinoma a clinical confounder that as a relevant differential diagnosis to NEN disease can easily be ruled out in clinical settings [32].
Since CgA has a protective effect on endothelial cells and it furthermore counteracts some effects of proinflammatory cytokines, the association between the CgA and biochemical markers of systemic inflammation is not surprising. Both infectious and noninfectious diseases are associated with increased RDW, ESR, decreased hemoglobin, albumins and thrombocyte count [33,34,35,36,37], which is in accordance with our study results.
However, we found some novel interesting associations. For instance, the strongest correlation of CgA was the one with urinary phosphate, although the urinary phosphate levels were available only in small number of patients. This association could be explained by the presence of renal failure, which is associated with decreased urinary phosphorus levels [38]. Moreover, it might be associated with the degree of vitamin D deficiency, which correlates with severity of the underlying disease [39]. Unfortunately, vitamin D status was available for only five patients and therefore it has not been analyzed in our study. We also found very good correlation between CgA and urinary leukocytes and erythrocytes, which might also reflect the degree of chronic kidney disease, but may also suggest the presence of urinary tract infection, both of which may be associated with increased serum CgA. Finally, the last and previously non-reported association found in our study, is the positive correlation between alpha2-macroglobulin and CgA. Increased alpha2-macroglobulin is found in patients with nephritic syndrome, liver failure and diabetes [40], all of which are known to be associated with increased serum CgA.
The association between a CgA elevation and numerous confounding parameters suggests a relevant heterogeneity among the control subjects of the study cohort, which is an important advantage of our study. Hence, CgA-score has encompassed a wide range of comorbidities that may be encountered in everyday clinical practice. These comorbidities have been included into a scoring system which uses routine biochemical parameters available world-wide. Thus, application of our CgA-score in a given clinical situation puts each patient into a different interval, in which CgA has different diagnostic accuracy. We demonstrated that the CgA does not have any diagnostic accuracy in patients with CgA score >12. This may be very useful in patients with high serum CgA levels in the absence of malignant disease, in order to avoid costly and often invasive and harmful diagnostic procedures particularly in health care systems with significant financial limitations [41]. On the other hand, it can enhance the detection of NENs in some patients, while the early diagnosis may improve treatment outcomes. We observed several interesting findings in a subgroup analysis that divided patients in five intervals. CgA had the best diagnostic accuracy in subjects in I1, as might be expected. These subjects do not have significant comorbidities that would influence serum CgA levels. Further, the diagnostic accuracy of similar serum CgA cut-off was slightly lower in I2, but still higher than reported in the entire study population. However, when subdividing patients with CgA score >12, we came to unexpected results. In subjects categorized into I3 interval, CgA did not have any diagnostic accuracy, probably due to substantial comorbidities leading to falsely increased CgA levels. Moreover, the presence of metastatic disease was the lowest in I3 interval, which could also explain poor diagnostic performance in this subgroup of patients. When we performed a subgroup analysis that included only patients with localized NENs, diagnostic accuracy slightly decreased in I1 and I2, while CgA did not have any diagnostic accuracy in I3, I4 and I5. This is in accordance with previously mentioned explanation why CgA had no diagnostic accuracy in I3. Surprisingly, the diagnostic performance of CgA increased in I4 subgroup, but with two times higher cut-off for serum CgA level (310 μg/L in I4 vs. 150 μg/L in I1 and I2). This phenomenon may be explained by the fact that both patients with NENs and controls have similar comorbidities and similar CgA-score. Patients with NENs in I4 are patients who have significant comorbidities, so it makes sense that they have higher CgA cut-off. This observation also emphasizes the role of CgA-score for quantify the impact of comorbidities in the following way: one should not compare patients with NENs without comorbidities and patients without NENs but with comorbidities. However, it seems feasible to use serum CgA in patients with similar burden of comorbidities, but with a different serum CgA cut-off. Although the serum neuron-specific enolase is routinely used as a biomarker in patients with G3 NENs, serum CgA levels were similar between patients with G2 and G3 NENs in our study. We have tried to explain this observation with an assumption that patients with G3 NENs have rapidly progressive disease, which could lead to more pronounced systemic inflammatory response that might explain increase in serum CgA. Indeed, patients with G3 NENs had almost two times higher CgA-score than patients with G2 NENs. However, patients with G1 and G3 NENs had similar CgA-score indicating the same burden of comorbidities between these two groups, arguing against our previously mentioned theory. Relatively good diagnostic performance of serum CgA in patients with G3 NENs was reported in previous studies as well [42, 43]. However, in the context of heterogeneity of patients with G3 NENs, the role of serum CgA as a biomarker in this subgroup of patients needs further assessment.
Nevertheless, our study showed the superiority of CgA-score over the standard assessment of comorbidities associated with increased CgA. Interestingly, in patients who had no previously defined comorbidities, the diagnostic accuracy of CgA in detecting patients with NENs decreased (Fig. 4). This highlights two important things which must be taken into consideration when discussing falsely elevated CgA levels: i) there are far more diseases associated with increased CgA than we are currently aware of; ii) the stage of the disease is more important than the presence of the disease itself. CgA-score has taken into consideration both premises and consequently increased the diagnostic accuracy of CgA.
Our study has several limitations and the results of this study should not be used in everyday clinical practice. Retrospective design, selection bias of the cohort and limited number of laboratory test decrease the power of this study. Larger sample size of both controls and patients ideally in a multi-centric setting would certainly overcome these limitations. This would also allow multivariate analysis, which could potentially reduce the number of parameters in CgA-score. Moreover, larger sample size of patients with NENs would allow subgroup analyses regarding stage, grade and primary site of NENs. Finally, CgA-score performance should be validated in a different cohort of patients. However, this is a first “proof of concept” study that assessed the possibility for quantifying the burden of comorbidities in relation to serum CgA levels, which may improve the design of future validation studies.
In conclusion, this study has demonstrated a novel scoring system that encompasses a wide range of comorbidities that influence serum CgA levels and could serve in a daily routine practice. CgA-score stratifies each subject into a certain category, in which CgA has different diagnostic performance in detecting patients with NENs. In our study population, CgA-score delineated almost 70% of the study population with increased diagnostic performance of serum CgA and showed the superiority over the standard assessment of comorbidities associated with increased CgA. It may also offer a cost-efficient alternative to molecular analysis-based test for health care systems with restricted resources. However, its application to everyday clinical practice needs to be evaluated.
References
L. Taupenot, K.L. Harper, D.T. O’Connor, The chromogranin–secretogranin family. N. Engl. J. Med. 348, 1134–1149 (2003)
Y.P. Loh, Y. Cheng, S.K. Mahata, A. Corti, B. Tota, Chromogranin A and derived peptides in health and disease. J. Mol. Neurosci. 48, 347–356 (2012)
D. Belloni, S. Scabini, C. Foglieni, L. Veschini, A. Giazzon, B. Colombo et al. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. Faseb. J. 21, 3052–3062 (2007)
E. Ferrero, S. Scabini, E. Magni, C. Foglieni, D. Belloni, B. Colombo et al. Chromogranin A protects vessels against tumor necrosis factor-induced vascular leakage. FASEB J. 18, 554–556 (2004)
B. Tota, S. Gentile, T. Pasqua, E. Bassino, H. Koshimizu, N.X. Cawley et al. The novel chromogranin A-derived serpinin and pyroglutaminated serpinin peptides are positive cardiac β-adrenergic-like inotropes. FASEB J. Fed. Am. Soc. Exp. Biol. 26, 2888–2898 (2012)
V. Sánchez-Margalet, C. González-Yanes, S. Najib, J. Santos-Álvarez, Metabolic effects and mechanism of action of the chromogranin A-derived peptide pancreastatin. Regul. Pept. 161, 8–14 (2010)
M.M. Fung, R.M. Salem, P. Mehtani, B. Thomas, C.F. Lu, B. Perez et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin. Exp. Hypertens. 32, 278–287 (2010)
D. Zhang, T. Lavaux, A.-C. Voegeli, T. Lavigne, V. Castelain, N. Meyer et al. Prognostic value of chromogranin a at admission in critically ill patients: a cohort study in a medical intensive care unit. Clin. Chem. 54, 1497–1503 (2008)
G. Di Comite, C.M. Rossi, A. Marinosci, K. Lolmede, E. Baldissera, P. Aiello et al. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF- -elicited endothelial activation. J. Leukoc. Biol. 85, 81–87 (2008)
V. Sciola, S. Massironi, D. Conte, F. Caprioli, S. Ferrero, C. Ciafardini et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 15, 867–871 (2009)
A. Zissimopoulos, S. Vradelis, M. Konialis, D. Chadolias, A. Bampali, T. Constantinidis et al. Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand. J. Gastroenterol. 49, 942–949 (2014)
P. Gut, A. Czarnywojtek, J. Fischbach, M. Bączyk, K. Ziemnicka, E. Wrotkowska et al. Chromogranin A—unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch. Med. Sci. Terme. Publ. 12, 1–9 (2016)
P.R. Bech, R. Ramachandran, W.S. Dhillo, N.M. Martin, S.R. Bloom, Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaine and amphetamine-regulated transcript. Clin. Chem. 58, 941–943 (2012).
C. Ceconi, R. Ferrari, T. Bachetti, C. Opasich, M. Volterrani, B. Colombo et al. Chromogranin A in heart failure. A novel neurohumoral factor and a predictor for mortality. Eur. Heart J. 23, 967–974 (2002)
M.E. Estensen, A. Hognestad, U. Syversen, I. Squire, L. Ng, J. Kjekshus et al.Prognostic value of plasma chromogranin A levels in patients with complicated myocardial infarction. Am. Heart J. 152, 927.e1–927.e6 (2006)
M.A. Takiyyuddin, R.J. Parmer, M.T. Kailasam, J.H. Cervenka, B. Kennedy, M.G. Ziegler et al., Chromogranin A in human hypertension. Hypertension 26, 213–220 (1995).
M. Peracchi, C. Gebbia, G. Basilisco, M. Quatrini, C. Tarantino, C. Vescarelli et al. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis, enterochromaffin-like cell lesions and gastric carcinoids. Eur. J. Endocrinol. 152, 443–448 (2005)
I. Pregun, L. Herszényi, M. Juhász, P. Miheller, I. Hritz, A. Patócs et al. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 84, 22–28 (2011)
K. Oberg, A. Couvelard, G. Delle Fave, D. Gross, A. Grossman, R.T. Jensen et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Biochemical Markers. Neuroendocrinology 105, 201–211 (2017)
S. Welin, M. Stridsberg, J. Cunningham, D. Granberg, B. Skogseid, B. Eriksson et al. Elevated plasma chromogranin a is the first indication of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology 89, 302–307 (2009)
J.C. Yao, C. Lombard-Bohas, E. Baudin, L.K. Kvols, P. Rougier, P. Ruszniewski et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J. Clin. Oncol. 28, 69–76 (2010)
B. Lawrence, B.I. Gustafsson, M. Kidd, M. Pavel, B. Svejda, I.M. Modlin, The clinical relevance of chromogranin a as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. North Am. 40, 111–134 (2011)
V. Marotta, V. Nuzzo, T. Ferrara, A. Zuccoli, M. Masone, L. Nocerino et al. Limitations of Chromogranin A in clinical practice. Biomarkers 17(2), 186–191 (2012)
S. Nölting, A. Kuttner, M. Lauseker, M. Vogeser, A. Haug, K.A. Herrmann et al. Chromogranin A as serum marker for gastroenteropancreatic neuroendocrine tumors: a single center experience and literature review. Cancers (Basel) 4, 141–155 (2012)
A. Tirosh, G.Z. Papadakis, C. Millo, S.M. Sadowski, P. Herscovitch, K. Pacak et al. Association between neuroendocrine tumors biomarkers and primary tumor site and disease type based on total 68Ga-DOTATATE-Avid tumor volume measurements. Eur. J. Endocrinol. 176, 575–582 (2017)
M. Stridsberg, B. Eriksson, K. Oberg, E.T. Janson, A comparison between three commercial kits for chromogranin A measurements. J. Endocrinol. 177, 337–341 (2003)
P. Glinicki, R. Kapuścińska, W. Jeske, Improved diagnostic accuracy for neuroendocrine neoplasms using two chromogranin A assays: the importance of protein matrix effects. Clin. Endocrinol. (Oxf.). 79, 295–296 (2013)
M. Pavel, E. Baudin, A. Couvelard, E. Krenning, K. Öberg, T. Steinmüller et al. ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 95, 157–176 (2012)
C.A. Elisa, Instructions for use Chromogranin A ELISA DEE9000 (2016), Accessed 11 Sep 2017, http://www.ibl-international.com/en/chromogranin-a-elisa
I.M. Modlin, I. Drozdov, D. Alaimo, S. Callahan, N. Teixiera, L. Bodei et al. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr. Relat. Cancer 21, 615–628 (2014)
I.M. Modlin, M. Kidd, L. Bodei, I. Drozdov, H. Aslanian, The clinical utility of a novel blood-based multi-transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am. J. Gastroenterol. 110, 1223–1232 (2015)
S. Massironi, M. Fraquelli, S. Paggi, A. Sangiovanni, D. Conte, V. Sciola et al. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig. Liver. Dis. 41, 31–35 (2009)
S. Doğan, N. Atakan, Red blood cell distribution width is a reliable marker of inflammation in plaque psoriasis. Acta Dermatovenerol. Croat. 25, 26–31 (2017)
V. Veeranna, S.K. Zalawadiya, S. Panaich, K.V. Patel, L. Afonso, Comparative analysis of red cell distribution width and high sensitivity C-reactive protein for coronary heart disease mortality prediction in multi-ethnic population: Findings from the 1999–2004 NHANES. Int. J. Cardiol. 168, 5156–5161 (2013)
Z.-D. Hu, Y. Chen, L. Zhang, Y. Sun, Y.-L. Huang, Q.-Q. Wang et al. Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Clin. Chim. Acta 425, 202–205 (2013)
N.S. Ku, H. Kim, H.J. Oh, Y.C. Kim, M.H. Kim, J.E. Song et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock 38, 123–127 (2012)
J.H. Lee, H.J. Chung, K. Kim, Y.H. Jo, J.E. Rhee, Y.J. Kim et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am. J. Emerg. Med. 31, 72–79 (2013)
T. Isakova, P. Wahl, G.S. Vargas, O.M. Gutiérrez, J. Scialla, H. Xie et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011)
J. Silver, T. Naveh-Many, Phosphate and the parathyroid. Kidney Int. 75, 898–905 (2009)
R.F. Ritchie, G.E. Palomaki, L.M. Neveux, O. Navolotskaia, T.B. Ledue, W.Y. Craig, Reference distributions for alpha2-macroglobulin: a practical, simple and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 18, 139–147 (2004)
Healthcare statistics—statistics explained, http://ec.europa.eu/eurostat/statistics-explained/index.php/Healthcare_statistics. Accessed 11 Sept 2017
Y. Wang, Q. Yang, Y. Lin, L. Xue, M. Chen, J. Chen, Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine. (Baltim.). 93, e247 (2014)
S. Massironi, R.E. Rossi, G. Casazza, D. Conte, C. Ciafardini, M. Galeazzi et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology 100, 240–249 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Previous presentations of this work: This work has been presented in a form of poster presentation at 14th Annual ENETS Conference, March 2017, Barcelona, Spain.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kruljac, I., Vurnek, I., Maasberg, S. et al. A score derived from routine biochemical parameters increases the diagnostic accuracy of chromogranin A in detecting patients with neuroendocrine neoplasms. Endocrine 60, 395–406 (2018). https://doi.org/10.1007/s12020-018-1592-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1592-6