Abstract
The poor prognosis, few available treatment options, and multidrug resistance present in hepatocellular carcinoma are major problems, and new early biomarkers are needed to reduce the liver cancer death rate. ATP-binding cassette sub-family C member 3 (Abcc3) is overexpressed in different cancers and is associated with multidrug resistance and a carcinogenic stem cell phenotype. We present evidence for the first time that ABCC3 is a potential sanguine biomarker and anticancer target in hepatocellular carcinoma. Abcc3 mRNA expression was elevated in liver nodules and tumors in rat hepatocarcinogenesis model. Accordingly, the ABCC3 protein was preferentially overexpressed within the nodules and tumors during hepatocellular carcinoma progression and was secreted into the bloodstream of rat hepatocarcinogenesis model. The ABCC3 protein was expressed in human hepatoma cells and, importantly, was also present in HepG2- and Huh7-conditioned media. Furthermore, ABCC3 was overexpressed in human hepatocellular carcinoma. This report is the first to describe liver overexpression of Abcc3 during the cancer initiation, promotion, and progression periods in rat hepatocarcinogenesis model and in human hepatocellular carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide, and the global disease burden is increasing [1]. The risk factors for HCC are chronic hepatitis B and C infection, cirrhosis, non-alcoholic fatty liver disease, alcohol-induced liver disease, and exposure to aflatoxin and others carcinogens [2]. HCC development is a multistep process with concurrent genetic and epigenetic alterations. HCC is a major health problem because of its poor prognosis and few available treatment options [3]. The detection of HCC is based on programmed ultrasound studies and serum alpha-fetoprotein; however, the evidence that this prognostic practice improves survival rates is controversial [4]. The prognosis of HCC following surgery remains dismal because of the rates of recurrence or metastases [5–7]. Therefore, new early biomarkers are needed to reduce the liver cancer death rate.
To find new biomarkers, a transcriptome profile of hepatocellular liver cancer progression would be a helpful tool to uncover proteins participating in its development, but this process is a complex undertaking in human tumors. Animal models are essential to further our understanding of the molecular, cellular, and pathophysiological mechanisms of HCC and for the development of new therapeutic strategies or biomarkers [8]. Previous studies on rat hepatocarcinogenesis models and humans have found similarities between the molecular changes that occur in both species and have described the overexpression of similar proteins [9–11].
HCC is often diagnosed in an advance stage and in the presence of multidrug resistance. Multidrug resistance is associated with the high expression of ATP-binging cassette (ABC) transporters, such as ABCB1, ABCC1, and ABCC3 [12, 13]. ABCC3 belongs to the ABCC family, which contains the greatest number of known drug transporters for organic anions, and in vitro studies have demonstrated that it confers resistance to chemotherapies [14]. ABCC3 localizes in the sinusoidal membrane, and its expression in normal rat hepatocytes is very low. Subsequent studies reported the overexpression of ATP-binding cassette sub-family C member 3 (Abcc3) in the lung, liver, ovary, and breast cancers and leukemia [15–19]. ABCC3 has not yet been investigated as a potential biomarker of hepatocellular carcinoma.
In a previous report, we employed a rat HCC model to study cancer progression. Transcriptome analyses were performed to identify the gene expression profile of rat hepatocarcinogenesis model. Abcc3 was found to be intensely expressed throughout liver cancer progression. This study is the first to assess the sequential expression of Abcc3 in a liver carcinogenesis rat model. Abcc3 was overexpressed throughout liver cancer development with a preferential gradual increase in preneoplastic lesions and tumors. Combined with liver gene expression, we investigated Abcc3 protein expression, Abcc3 secretion into bloodstream in a rat hepatocarcinogenesis model, ABCC3 protein expression in human HepG2 and Huh7 hepatoma cells, and ABCC3 presence in human liver cancer biopsies. Each of these hallmarks provides evidence for Abcc3 as a potential liver cancer biomarker.
Methods
Animals
All experiments were performed in accordance with and approved by the Comité Interno para el Cuidado y Uso de Animales de Laboratorio (CICUAL) of CINVESTAV under the protocol no. 0001-02. Male Fischer 344 rats (180–200 g) were obtained from the Unit for Production of Experimental Laboratory Animals (UPEAL-CINVESTAV, Mexico City, Mexico). The animals had free access to food (PMI Feeds Inc., Laboratory Diet, Richmond, IN, USA) and water. The animals were maintained in a holding room under controlled conditions with a 12-h light/dark cycle, 50 % relative humidity, and a temperature of 21 °C. Animal care followed the institutional guidelines for the use of laboratory animals.
The rats were subjected to the full procedure of the resistant hepatocyte model that consists in summary of a cancer initiator administration, 7 days afterwards three doses of a promoter carcinogen and finally, at the 10th day, a 3/4 hepatectomy as a proliferative stimulus; all rats develop hepatocellular carcinoma by 12 months [20]. The rats were intraperitoneally administered 200 mg/kg of diethyl nitrosamine (DEN). At 7, 8, and 9 days after initiation, 20 mg/kg of 2-acetylaminofluorene (2-AAF) was orally administered to the rats. On day 10 post-initiation, the rats underwent a partial hepatectomy [20]. Finally, the rats were sacrificed at 1, 7, 11, and 16 days and at 1, 5, 9, 12, and 18 months post-initiation. Control, non-treated groups were sacrificed at 0, 9, 12, and 18 months. The livers were removed under ether anesthesia, washed in a physiological saline solution, quickly frozen in 2-methyl butane with liquid nitrogen, and stored at −80 °C until further use. Paraffin-fixed liver samples were also prepared for histochemical and immunohistochemical examination.
Chemicals and reagents
DEN and 2-AAF were purchased from Sigma Chemical Co. (St Louis, Missouri, USA). The Lowry Assay Kit was used to determine protein concentrations and was purchased from BioRad (Richmond, California, USA). Complete protease inhibitor cocktail tablets were purchased from Roche Molecular Biochemicals (Mannheim, Germany). The DAB-Plus Substrate kit and Cas-Block (00-8120) were obtained from Zymed (San Francisco, California, USA). Anti-GST-p and anti-ABCC3 antibodies were purchased from Santa-Cruz (Santa Cruz, California, USA).
Histochemical analysis and tissue selection
Histological analysis of preneoplastic and neoplastic lesions was performed using hematoxylin and eosin and γ-glutamyl transpeptidase (GGT staining) [12]. Images of GGT-positive lesions were acquired with a digital camera (Color View 12, Soft Imaging System GmbH, Germany) and quantified with the AnalySIS software (AnalySIS, Soft Imaging System GmbH). Total liver homogenates were used to analyze specimens obtained at 0 h and 1, 7, 11, and 16 days. Based on GGT histochemical analysis, tissues from preneoplastic lesions corresponding to the persistent nodules, tumors, and the adjacent tissue were selected to be analyzed at 30 day and at 5, 9, 12, and 18 months.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the tissue samples of 40 rats, divided in ten groups, nine of the groups treated according to the hepatocarcinogenesis model and a group not treated; samples were reverse transcribed into cDNA using SuperScript II RT and oligo dTs according to the manufacturer’s instructions. Quantitative real-time PCR assays were performed with gene-specific fluorescent-labeled probes on a 7000 Sequence Detector (Applied Biosystems). The specificity of the ABCC3 primers was designed using the Primer Express software (Applied Biosystems). Endogenous 18S was used to normalize the mRNA data. The PCR reaction mixture contained 1 μL of cDNA, 7.5 μL of 1× TaqMan Universal PCR Master Mix, and 1 μL of the primers and probe. The following cycling protocol was used: 1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 10 min, and 40 cycles at 95 and 60 °C for 15 s and 1 min, respectively. The results were evaluated according to the comparative Ct method.
Immunohistochemistry
Paraffin-fixed liver tissue sections of 120 rats were divided in five groups, four of the groups treated according to the hepatocarcinogenesis model and a group not treated; samples (3 μm) were deparaffinized and gradually hydrated. Hepatic biopsies with hepatocellular carcinoma were diagnosed by expert pathologists. HCC were obtained from archive (years 1993–2008) at the Centro de Especialidades Médicas del Estado de Veracruz (CEMEV), Xalapa, Veracruz, México. The Scientific and Ethical Hospital of CEMEV approved this study (permission number: 005/2011). The following parameters were used for image analysis: number of objects defined as cells in the image, staining intensity (high, medium, low, and negative) and fraction (%) of positive cells in the image area. For all section, of rats or human origin, the antigens were unmasked by immersing the sections in sodium citrate buffer (pH 6) for 15 min in a heated water bath. Endogenous peroxidase activity was blocked with 0.3 % H2O2 in methanol. After a standard staining protocol using the LSAB Plus-kit and chromogenic development, the sections were lightly counterstained with hematoxylin, dehydrated, and mounted. The tissue images were captured using light microscopy (Olympus 1X70, Olympus Europa GmbH, Hamburg, Germany).
Western blot
The liver tissue samples (~50 mg) of 90 rats were divided in ten groups; nine groups of rats were treated according to the hepatocarcinogenesis model and a group not treated. The liver tissue sample were homogenized in a lysis buffer (10 mM Tris-Cl, pH 7.4; 150 mM NaCl; 1 % Triton-X100; and protease inhibitor cocktail) and centrifuged at 15,000g for 30 min. The proteins were separated by SDS-PAGE and transferred to PVDF membranes. The protein of interest was visualized using the indicated antibody and a chemiluminescence system.
ELISA
The sanguine Abcc3 concentration was measured by a quantitative enzyme immunoassay technique acquired from CUSABIO (Hubei, CN) in 40 rats, divided in nine groups of four rats each treated according to the hepatocarcinogenesis model and a group of four not treated. Briefly, Standards and samples were pipetted into the well, and any ABCC3 present bound to the immobilized antibody. Absorbance was measured at 450 nm with the correction wavelength set at 540 nm.
Cells culture and biopsies
Human HepG2 and Huh7 hepatocellular carcinoma cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with the addition of 10 % fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. In quadruplicate independent assays, the cells were processed for immunocytochemistry and western blot. The human hepatocellular carcinoma cell line HepG2 was obtained from ATCC, and Huh7 cells were obtained from Dr. Zentella Dehesa. For immunohistochemistry, human liver cancer samples were processed as described for rat liver samples.
Statistical evaluation
Data analysis was evaluated using a one-way ANOVA with Tukey’s post hoc test. Data are expressed as the mean ± standard deviation. All experiments were performed on at least four animals per treatment group. Differences between groups were evaluated with a Student’s t test, and a P value <0.01 was considered to be significant.
Results
Abcc3 gene expression profile during the liver cancer progression
We selected ten time points to analyze the gene expression profile of Abcc3 in quadruplicate. These gene expression times were representative of the process of chemical carcinogenesis (Fig. 1a). The initiation progression period includes rats at 24 h and 7, 11, and 16 days post-treatment. The preneoplastic progression period includes the maximum appearance of nodular lesions 30 days and 5 and 9 months post-treatment initiation in all rats treated according to the hepatocarcinogenesis model. At 9 months post-initiation, nodules coexisted with early cancers. The cancer progression period is represented by 9, 12, and 18 months of fully grown tumors. During the initiation progression period, whole liver tissue homogenates were the RNA source, and during both the preneoplastic and cancer progression periods, RNA was obtained from dissected nodular lesions, tumors, and adjacent tissues. Hepatocyte nodules and tumor lesions were identified with the GGT marker. Abcc3 gene expression was evaluated by quantitative real-time PCR, and the significant overexpression of Abcc3 is denoted by asterisks (Fig. 1a). Abcc3 was overexpressed during the initial period with high values at 7 and 16 days post-treatment. The nodules at 30 days and 5 and 9 months had increased gene expression compared with the adjacent tissue and with the control group with a statistical significance. During liver cancer progression, Abcc3 was overexpressed in the tumor tissue compared with the adjacent tissue and with the control groups. In nodules 30 days post-initiation, Abcc3 mRNA increased 10.3-fold compared with the control, and at 18 months, tumor tissue mRNA Abcc3 expression increased 21.4-fold compared with the control. These results validate the microarray assays and show significant overexpression of Abcc3 mRNA with a clear behavior during liver cancer progression.
Characterization of ABCC3 expression during liver cancer progression in a rat hepatocarcinogenesis model. The initial progression period includes the initiation stage at 24 h and 7 days and the promotion stage at 11 and 16 days. The preneoplastic progression period includes the evolution of persistent preneoplastic lesions at 30 days and 5 and 9 months, and the cancer progression period includes the growth of hepatocellular carcinomas at 9, 12, and 18 months. a Validation of gene expression by quantitative PCR of Abcc3. b Blood expression of ABCC3 by western blotting. c Densitometric analysis of the western blot signals. d Validation of ABCC3 serum concentrations by enzyme-linked immunosorbent assay. Statistically different from NC, **P < 0.001 and ***P < 0.0001. d day, m month, NN no nodule, N nodule, NT no tumor, T tumor, NC normal control, and CT complete treatment
ABCC3 secretion into plasma during liver cancer progression
To determine whether the changes in gene expression were reflected at the protein level, we evaluated ABCC3 plasma protein expression during liver carcinogenesis progression (Fig. 1b). Our study is the first to show chronological overexpression of ABCC3 in the plasma with values that doubled compared with the control during liver carcinogenesis. Western blot densitometric analysis of plasma samples treated with hepatocellular carcinoma model showed ABCC3 overexpression from 7 days to 18 months post-initiation (Fig. 1c). In the same samples, we determined the ABCC3 serum concentration by enzyme-linked immunosorbent assay. The rats treated according to the hepatocarcinogenesis model overexpressed Abcc3 in the plasma compared with the control group from 7 days to 18 month post-initiation with values of 10 ng/mL or more (Fig. 1d). This result clearly showed the overexpression of Abcc3 in plasma in rats during liver cancer progression.
ABCC3 expression in hepatocellular carcinoma
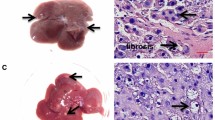
ABCC3 tissue distribution was analyzed by immunohistochemistry during liver cancer progression. Glutathione S-transferase P (GST-p) was used as marker of preneoplastic lesions and tumor. In serial tissue sections, Abcc3 expression clearly coincided with GST-p expression during the initiation period (Fig. 2). In the progression period, ABCC3 expression increased in preneoplastic lesions and tumors. Moreover, we observed the highest ABCC3 overexpression in certain altered hepatocytes with a cytoplasmic stain or highest basolateral ABCC3 expression from 16 days to 18 months post-initiation. These results confirmed ABCC3 protein overexpression during liver carcinogenesis with elevated staining only within the GST-p-positive area.
ABCC3 protein expression in human hepatoma cells and human hepatocellular carcinoma
ABCC3 expression was quantified by western blotting from the total protein extracted from HepG2 and Huh7 cell lines. The results showed that both cell lines expressed similar amounts of ABCC3 (Fig. 3). In addition, ABCC3 was present in the conditioned media of the HepG2 and Huh7 cells. Figure 4 shows a 6.2-fold increase in ABCC3 expression in the conditioned media of Huh7 cells compared with HepG2 cells. In addition, immunocytochemistry revealed ABCC3 protein expression in HepG2 and Huh7 cells, mostly expressed in the cytoplasm. ABCC3 was overexpressed in human hepatocellular carcinoma, as observed with a general stain in altered hepatocytes’ cytoplasm (Fig. 4). Furthermore, these samples showed elevated staining in some tumor cells. These results confirmed Abcc3 protein overexpression during the liver carcinogenesis with elevated staining in human hepatoma cells and human hepatocellular carcinoma.
Expression of ABCC3 in HCC cell lines. a Upper, ABCC3 expression in total protein and conditioned medium from HepG2 and Huh7 cells. Actin was used as a loading control for total protein, lower densitometric analyses of western blot signals of ABCC3 expression in total protein and conditioned media. b Densitometric analyses of western blot signals of ABCC3 expression in total protein and conditioned media. c Positive immunocytochemical staining in HepG2 and Huh7 cells
Discussion
The aim of this study was to investigate Abcc3 gene expression and protein expression during liver cancer progression. Abcc3 stood out from the examined genes because it was intensely expressed throughout liver cancer progression. We found that this gene was highly upregulated, strongly associated with preneoplastic and HCC progression periods, and had little expression in the adjacent tissue, suggesting a direct relationship between ABCC3 gene expression and liver cancer stage. We also showed its behavior at different levels in vivo and in vitro. In vivo, we showed that the ABCC3 protein was secreted into plasma at a higher rate compared with non-treated rats. In vitro, in the human cell lines of liver cancer origin HepG2 and Huh7, this protein was secreted into the extracellular media, which imitated its behavior in the rat. We also showed ABCC3 protein expression in human HCC samples.
Upregulation of this gene has been associated with HCC progression and is negatively regulated by miRNA [21]. Additionally, ABCC3 upregulation has been characterized together with ABCC2 as part of the multidrug resistance phenotype [16], and its upregulation has been confirmed in choriocarcinoma cells and cervical cancer [22]. Protein expression was reported as an HCC tumor-associated antigen recognized by cytotoxic T cells and has been suggested as immunogenic target for HCC immunotherapy [23]. Recently, this observation was confirmed through a comparative analysis of various TAA-specific T-cell responses in 31 HCC patients that were used to select antigens for immunotherapy, and ABCC3 was proposed as an immunogenic target.
In recent years, ABCC3 has been associated as a key protein in chemotherapy-resistant tumor cells [14, 24]. A major problem during cancer therapy is acquiring resistance to multiple structurally unrelated antitumor agents, either by host factors or genetic or epigenetic mechanisms [25]. Human hepatocellular carcinoma is no exception, and cancer relapse and metastasis are associated with high expression multidrug resistance proteins, enhanced DNA repair ability, cell survival, and self-renewal capacity [26, 27].
ABCC3 has been proposed as a contributor to the intrinsic resistance of HCC to a wide variety of chemotherapy agents. Importantly, chemotherapy does not prolong life in most patients and is not a promising option in liver cancer treatment. According to our results, ABCC3 could be a therapeutic marker throughout cancer development. In principle, the reversal of drug resistance in patients with cancer could be possible with the use of inhibitory pharmacological agents, antibodies, and antisense oligonucleotides targeting ABCC3.
One of the main challenges in HCC management is the high recurrence rate. ABCC3 could function as a marker for monitoring post-treated patients, which we extrapolated from our results that demonstrated plasma ABCC3 overexpression in rats with HCC. In addition, cancer stem cells (CSCs) play important roles in cancer initiation and relapse and resistance to most cytotoxic agents related to the overexpression of the ABC transporters [2, 28, 29]. For example, in breast cancer, the cancer stem cells upregulate and overexpress the ABC transporters, including ABCC3 [30]. Human breast CSCs treated with berberine liposomes showed inhibition of ABCC1, ABCC2, ABCC3, and ABCG2 and the activation of intrinsic apoptotic pathways [29]. In human colon cells, Abcc3 mRNA was overexpressed by approximately threefold over various bile salts transporters [31]. Moreover, hypoxia in HepG2 cells increased ABC drug transport genes, including Abcc3, and induced resistance towards curcumin cytotoxicity, a promising anticancer agent [27]. In leukemia cell lines treated with silibinin, a potential antioxidant, cell proliferation was inhibited, and Abcc3 mRNA expression levels were reduced together with other members of the ABC family transporters [32].
In conclusion, we clearly showed Abcc3 mRNA overexpression in the initiation process and during preneoplastic and tumor progression periods and showed protein overexpression in the liver and plasma. Even though this protein has been proposed as a marker in four malignant tumors [15–19] as far as we know, its overexpression is specific of cancers. We have not determined ABCC3 sensitivity but by ELISA a difference of 7 ng/mL can be discriminated as statistically significant. For the first time, we report a chronological gradual secretion of ABCC into the blood in an animal model of liver cancer development. In cell culture with human hepatocellular carcinoma cells, ABCC3 was also overexpressed and secreted into the extracellular media. Additionally, ABCC3 was overexpressed in biopsy samples of human hepatocellular carcinoma with a similar pattern to that observed in the rat liver cancer model. This report is the first to show ABCC3 as a biomarker that is overexpressed in preneoplastic lesions and tumors and secreted into the plasma during the HCC development, outstanding characteristics as an alternative diagnostic molecule.
Abbreviations
- Abcc3:
-
ATP-binding cassette sub-family C member 3
- HCC:
-
Hepatocellular carcinoma
- ABC:
-
ATP-binging cassette
- DEN:
-
Diethyl nitrosamine
- 2-AAF:
-
2-Acetylaminofluorene
- GGT:
-
γ-Glutamyl transpeptidase
- GST-p:
-
Glutathione S-transferase P
- CSCs:
-
Cancer stem cells
References
Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42.
Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–76.
Wang Y, Luo Q, Li Y, Wang H, Deng S, Wei S, et al. Quality assessment of clinical practice guidelines on the treatment of hepatocellular carcinoma or metastatic liver cancer. PLoS One. 2014;9:e103939.
Tsai CL, Koong AC, Hsu FM, Graber M, Chen IS, Cheng JC. Biomarker studies on radiotherapy to hepatocellular carcinoma. Oncology. 2013;84 Suppl 1:64–8.
Malagari K, Pomoni M, Sotirchos VS, Moschouris H, Bouma E, Charokopakis A, et al. Long term recurrence analysis post drug eluting bead (deb) chemoembolization for hepatocellular carcinoma (hcc). Hepato-Gastroenterology. 2013;60:1413–9.
Yang T, Zhang H, Cai SY, Shen YN, Yuan SX, Yang GS, et al. Elevated SHOX2 expression is associated with tumor recurrence of hepatocellular carcinoma. Ann Surg Oncol. 2013;20 Suppl 3:S644–9.
Cho EJ, Lee JH, Yoo JJ, Choi WM, Lee MJ, Cho Y, et al. Serum insulin-like growth factor-I level is an independent predictor of recurrence and survival in early hepatocellular carcinoma: a prospective cohort study. Clin Cancer Res. 2013;19:4218–27.
Lee JS, Grisham JW, Thorgeirsson SS. Comparative functional genomics for identifying models of human cancer. Carcinogenesis. 2005;26:1013–20.
Siveen KS, Ahn KS, Ong TH, Shanmugam MK, Li F, Yap WN, et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget. 2014;5:1897–911.
Ganapathy-Kanniappan S, Kunjithapatham R, Torbenson MS, Rao PP, Carson KA, Buijs M, et al. Human hepatocellular carcinoma in a mouse model: assessment of tumor response to percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists. Radiology. 2012;262:834–45.
Huynh H, Soo KC, Chow PK, Panasci L, Tran E. Xenografts of human hepatocellular carcinoma: a useful model for testing drugs. Clin Cancer Res. 2006;12:4306–14.
Konig J, Hartel M, Nies AT, Martignoni ME, Guo J, Buchler MW, et al. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer J Int du Cancer. 2005;115:359–67.
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4.
Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A. 1999;96:6914–9.
Hong X, Yang ZY, Wang M, Lu L, Li YH, Hao X, et al. Multidrug resistance-associated protein 3 and Bcl-2 contribute to multidrug resistance by vinorelbine in lung adenocarcinoma. Int J Mol Med. 2011;28:953–60.
Nies AT, Konig J, Pfannschmidt M, Klar E, Hofmann WJ, Keppler D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int J Cancer J Int du Cancer. 2001;94:492–9.
Wang XB, Wang SS, Zhang QF, Liu M, Li HL, Liu Y, et al. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep. 2010;23:211–5.
Partanen L, Staaf J, Tanner M, Tuominen VJ, Borg A, Isola J. Amplification and overexpression of the ABCC3 (MRP3) gene in primary breast cancer. Genes Chromosomes Cancer. 2012;51:832–40.
Benderra Z, Faussat AM, Sayada L, Perrot JY, Tang R, Chaoui D, et al. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–72.
A. Marche-Cova, S. Fattel-Fazenda, A. Rojas-Ochoa, E. Arce-Popoca, S. Villa-Trevino, Follow-up of GST-P during hepatocarcinogenesis with DEN-2AAF in F344 rats. Archives of medical research 26 Spec No (1995) S169-173.
Borel F, Han R, Visser A, Petry H, van Deventer SJ, Jansen PL, et al. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55:821–32.
Serrano MA, Macias RI, Briz O, Monte MJ, Blazquez AG, Williamson C, et al. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta. 2007;28:107–17.
Mizukoshi E, Honda M, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Expression of multidrug resistance-associated protein 3 and cytotoxic T cell responses in patients with hepatocellular carcinoma. J Hepatol. 2008;49:946–54.
Zhao Y, Lu H, Yan A, Yang Y, Meng Q, Sun L, et al. ABCC3 as a marker for multidrug resistance in non-small cell lung cancer. Scientific Rep. 2013;3:3120.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–49.
Hoffmann K, Shibo L, Xiao Z, Longerich T, Buchler MW, Schemmer P. Correlation of gene expression of ATP-binding cassette protein and tyrosine kinase signaling pathway in patients with hepatocellular carcinoma. Anticancer Res. 2011;31:3883–90.
Sakulterdkiat T, Srisomsap C, Udomsangpetch R, Svasti J, Lirdprapamongkol K. Curcumin resistance induced by hypoxia in HepG2 cells is mediated by multidrug-resistance-associated proteins. Anticancer Res. 2012;32:5337–42.
Lun SW, Cheung ST, Cheung PF, To KF, Woo JK, Choy KW, et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PLoS One. 2012;7:e52426.
Ma X, Zhou J, Zhang CX, Li XY, Li N, Ju RJ, et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34:4452–65.
Lee SH, Kim H, Hwang JH, Lee HS, Cho JY, Yoon YS, et al. Breast cancer resistance protein expression is associated with early recurrence and decreased survival in resectable pancreatic cancer patients. Pathol Int. 2012;62:167–75.
Inokuchi A, Hinoshita E, Iwamoto Y, Kohno K, Kuwano M, Uchiumi T. Enhanced expression of the human multidrug resistance protein 3 by bile salt in human enterocytes. A transcriptional control of a plausible bile acid transporter. J Biol Chem. 2001;276:46822–9.
Noori-Daloii MR, Saffari M, Raoofian R, Yekaninejad M, Dinehkabodi OS, Noori-Daloii AR. The multidrug resistance pumps are inhibited by silibinin and apoptosis induced in K562 and KCL22 leukemia cell lines. Leukemia Res. 2014;38:575–80.
Acknowledgments
This work was supported by a scholarship (GCT), a grant contribution (178558) from CONACYT, and a postdoctoral scholarship, Multidisciplinary Project 3, from the SVT grant from CINVESTAV. We would like to acknowledge the animal technical support at UPEAL-CINVESTAV, including Rafael Leyva-Muñoz, Ricardo Gaxiola-Centeno, and Dr. Jorge Fernandez.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Carrasco-Torres, G., Fattel-Fazenda, S., López-Alvarez, G.S. et al. The transmembrane transporter ABCC3 participates in liver cancer progression and is a potential biomarker. Tumor Biol. 37, 2007–2014 (2016). https://doi.org/10.1007/s13277-015-3999-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3999-5







