Abstract
Interleukin-3 (IL-3) receptor α chain (CD123) plays an essential role in regulating the proliferation of hematopoietic stem cells. In the hematopoietic malignancies, CD123 expression has been found in acute myeloid leukemia (AML), B-precursor acute lymphoblastic leukemia (B-ALL), as well as dendritic cell malignancies. However, whether CD123 is also expressed in T-acute lymphoblastic leukemia (T-ALL) remains unknown. Using multi-parameter flow cytometry, we analyzed CD123 expression in 160 consecutive diagnostic T-ALL patients, including 88 pediatric T-ALL cases and 72 adult T-ALL cases. The minimal residual disease (MRD) was detected after one course of induction therapy to evaluate the treatment effects. CD123 expression was detected in 24 out of 88 (27 %) pediatric T-ALLs and 30 out of 72 (42 %) adult T-ALLs. Further analysis revealed that CD123 expression is associated with the maturation stage of T-ALLs. The frequencies of CD123-positive cases decreased from 83 to 40 % and 21 % in early T-precursor ALLs, T-precursor ALLs, and mature T-ALLs, respectively. Interestingly, we detected the CD4+CD8+ double-positive leukemic cells in 22 immature and 34 mature T-ALL patients. Of note, only 4 % of these patients expressed CD123. In addition, we found that 79 % of CD33+ and 64 % of CD117+ immature T-ALL patients also expressed CD123. However, CD123 expression did not predict the outcomes of the first course of induction therapy in T-ALL patients. In conclusion, we found that CD123 is preferentially expressed in immature T-ALL. Moreover, CD123 expression is strongly associated with cross-lineage expression of myeloid markers in early T-precursor ALL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute leukemia is characterized by the accumulation of undifferentiated cells in marrow, blood, and other tissues, due to the arrest of cell maturation [1]. The growth factors and cytokines play important roles in regulating the hematopoiesis via modulating cell survival, proliferation, differentiation, and functional activation [2]. In hematopoietic cells, interleukin-3 (IL-3) stimulates cell cycle progression, promotes differentiation, and inhibits cell apoptosis [3]. CD123, an interleukin-3 receptor, has been well described on hematopoietic stem cells in which it plays an important role in cellular proliferation [4]. It has been well documented that CD123 is expressed in acute myeloid leukemia (AML), B-precursor acute lymphoblastic leukemia (B-ALL), and dendritic cell malignancies but rarely expressed in mature B cell disorders [5–7]. However, expression of CD123 in T-acute lymphoblastic leukemia (T-ALL) remains controversial [2, 6, 8]. Using multi-parameter flow cytometry, we analyzed the expression of CD123 in different stages of T-ALL and investigated the correlations between CD123 expression and outcome of the first course of induction therapy.
Materials and methods
Patients and samples
From our flow cytometry (FC) database, we collected a total of 160 consecutive diagnostic samples including 88 newly diagnosed childhood T-ALLs (mean age 10 years, range 0–16) and 72 newly diagnosed adult T-ALLs (mean age 35 years, range 18–63) from August 2011 to February 2013 in the Stem Cell Center of Wuhan Union Hospital, in which CD123 was done as part of a routine FC panel. The diagnosis of T-ALL was based on morphologic features, immunophenotype, clinical history, cytogenetics, and molecular diagnostic testing according to the World Health Organization (WHO) 2008 criteria [8], and the immunophenotype was classified according to the European Group for the Immunological Characterization of Leukemias (EGIL) [9].
FC immunophenotyping
Fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll-protein (Per-CP), or allophycocyanin (APC) conjugated monoclonal antibodies (Abs) were used in this study, including CD45, CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD13, CD15, CD33, CD34, CD38, CD56, CD117, cCD3, TdT, HLA-DR, and isotype control IgGs. The reagents were provided by Becton-Dickinson Bioscience in the USA. As we described previously [10], 100 μl (about 106 cells/ml) well-mixed, EDTA anticoagulated BM or PB samples were incubated at room temperature with Abs (see earlier) which had to be fit together according to the protocol, and then lysed with 1× BD FACS lysing solution. After that, specimens were washed with 2 ml phosphate-buffered saline (PBS) twice and then resuspended in 100 μl PBS for acquiring. From each tube, we acquired at least 104 cells per tube using a FACSCaliburTM flow cytometer (BD Biosciences, San Jose, CA) with CellQuest Pro Software (BD Biosciences). Data were analyzed with Paint-A-Gate Software (BD Biosciences).
For analysis, cell populations were gated by CD45/SSC gating, in conjunction with antigen back-gating. Expression of related antigens was determined by gating on the abnormal cells. We regarded no less than 20 % of cell expression in a tube as positive expression, unless TdT and cCD3 (10 %). The sensitivity of minimal residual disease (MRD) is 0.01 % (abnormal cells were no less than 1 × 10−4).
Statistical method
Comparisons of categorical variables were performed with chi-squared test and Fisher’s exact test. All reported p values are two tailed and are considered statistically significant if p < 0.05. All statistical calculations were performed using the SPSS 18.0 (SPSS Inc., Chicago, IL, USA) software.
Result
CD123 expression in different immunophenotypes of T-ALL
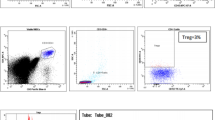
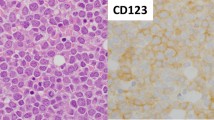
One hundred sixty newly diagnostic T-ALL patients were collected in our study, and the clinical characteristics are presented in Table 1. The CD123 expression was detected in 54 out of 160 (34 %) patients. We compared the expression of CD123 in pediatric T-ALL and adult T-ALL patients at first. While 42 % (30/72) of the adult T-ALL patients were CD123 positive, only 27 % (24/88) of the pediatric T-ALL patients expressed CD123. To further define the relation of CD123 expression with the subtypes of T-ALLs, patients were classified into early T-precursor ALL (CD7+), T-precursor ALL (CD2+ and/or CD5+ and/or CD8+), and mature T-ALL (CD3+), according to EGIL suggestions [8]. As shown in Table 2, 69 % of the CD123-positive cases were found in immature T-ALL patients. More specifically, CD123 expression was detected in 80 % of early T-precursor ALL patients, 40 % of T-precursor ALL patients, and 21 % of mature T-ALL patients, respectively (p < 0.05).
CD123 expression is rare in CD4 and CD8 double-positive T-ALL cases
During the T cell maturation, the double-negative (CD4−CD8−) precursors initially give rise to double-positive (CD4+CD8+) thymocytes, which eventually mature to single-positive CD4+CD8− regulatory T cells or CD4−CD8+ cytotoxic T cells [11]. The patients with CD4+CD8+ leukemic cells may represent an intermediate type between immature and mature T-ALLs. We evaluated CD4 and CD8 expression in all 160 cases. The double-positive (CD4+CD8+) populations were detected in 56 (35 %) patients. Interestingly, the double-positive leukemic T cells were found in both CD3− T-ALLs (22/56) and CD3+ T-ALLs (34/56), suggesting CD3 expression statue alone might not be sufficient to distinguish immature and mature T-ALLs. Of note, CD123 expression was detected in only two cases among these patients (CD4+CD8+), suggesting that CD123 is expressed rarely in CD4+CD8+ T-ALLs when compared with CD4− and/or CD8− T-ALLs (p < 0.05).
CD123 was preferentially expressed among CD33/CD117-positive precursor-T-ALL
The expression of four common myeloid markers (CD13, CD15, CD33, and CD117) was examined in all 160 T-ALL patients. CD33 expression was detected in 47 patients, including 34 immature T-ALLs and 13 mature T-ALLs. Strikingly, in immature T-ALLs, 27 out of 34 (79 %) CD33-positive patients also expressed CD123, while only 4 out of 46 (8 %) CD33-negative cases were positive for CD123 (p < 0.05). Our data suggest a significant association between CD123 and CD33 expression in immature T-ALL patients. In contrast, this association was not observed in mature T-ALL patients. CD117 expression was detected in 33 patients, including 25 immature T-ALLs and 8 mature T-ALLs. A significant correlation between CD123 expression and CD117 expression has also been observed in immature T-ALLs (Table 3) (p < 0.05). CD13- and CD15-positive blast cells were detected in 21 and 23 patients, respectively. However, no significant correlation was observed among the expression of CD13, CD15, and CD123 in all patient groups (data not shown).
CD123 expression is not associated with induction treatment response
Co-expression of CD117 and CD123 in AML has been linked to increased resistance to apoptosis and poor prognosis [12]. So we evaluated whether CD123 expression is correlated with outcome of the first course of induction therapy in T-ALL. The minimal residual diseases (MRD) were evaluated for all 160 patients after one course of induction by flow cytometer (Table 4). We regarded the abnormal cells ≥0.01 % (1 × 10−4) of nucleated cells as MRD positive. In 12 early T-precursor ALLs, all 7 pediatric patients were MRD negative. Two out of 5 adult patients (1 CD123-positive case and 1 CD123-negative case) were MRD positive. MRD can be detected in 3 adult CD123+ cases (3/27, 11 %), 4 pediatric, and 1 adult CD123− cases (5/41, 12 %) after induction in the group of 68 T-precursor ALL patients. In the group of 80 mature T-ALL patients, only 7 pediatric patients under the age of 15 years were MRD positive, including 2 CD123+ cases (2/17, 12 %) and 5 CD123− cases (5/63, 8 %). The p values were all above 0.05 which indicated that there was no statistically significant difference. Overall, our data indicated that CD123 expression did not associate with outcome of the first course of induction treatment in T-ALL patients.
Discussion
CD123 expression has been found in approximately 80 % of AMLs and 92 % of precursor-B ALLs [6]. Several studies with small sample sizes reported that CD123 is rarely expressed in T-ALLs [2, 5, 6]. In contrast to these studies, we found a significant proportion of Chinese T-ALL patients that express CD123. Here, we found that CD123 was expressed less frequently in pediatric than in adult patients in 160 consecutive diagnostic T-ALL samples. Furthermore, using differentiation antigens CD7, CD2/CD5/CD8, and CD3 which represent lymphocytes arrested at various stages, we showed that CD123 expression was more common in early T-precursor ALL either in adults or children which is consistent with the previous study reported by Lhermitte et al. [13]. It is well known that CD123 as an α subunit of a heterodimer IL-3 receptor plays an important role in regulating cellular proliferation [14]. Analogous to the situation in AML, it has been reported that CD123 may provide a proliferative advantage to leukemic blasts [5]. However, the mechanism of CD123 overexpression in early precursor-T-ALL remains unclear.
In T cell maturation stage, pro-T cells that co-express CD4 and CD8 can be detected in a very short time before maturation in the thymic cortex. Leukemia blasts with CD4 and CD8 double-positive immunophenotype were considered as cells blocked in a relatively early stage during maturation. However, in our current results, we found that only 4 % of CD4 and CD8 co-expression leukemic cells expressed CD123 in T-ALL patients, which seems not consistent with the results that CD123 is preferentially expressed in immature T-ALL. It is well recognized that the process of T cell maturation takes place first in the bone marrow, then in the thymus. T-lineage surface markers CD7 together with CD2 and CD5 can be detected in bone marrow, and then CD4 with CD8 co-expressed on T cells after migrating to the thymic cortex before maturation [9]. We think that the leukemic cells with CD4 and CD8 double-positive immunophenotype may represent an intermediate type between immature and mature T-ALLs. Leukemia blasts with corticothymocyte immunophenotype may indicate that this type of T-ALL might originate in the thymus before disseminating back to the bone marrow. A possible explanation is that the bone marrow microenvironment induces CD123 expression in the T-ALL which originates in the bone marrow. Nevertheless, further analysis is necessary to understand the mechanism.
A previous study reported that cross-lineage expression of CD117 and CD123 in AML has been linked to increased resistance to apoptosis and poor prognosis. In our study, CD123 expression is strongly associated with cross-lineage expression of myeloid markers in early T-precursor ALL. It seems reasonable to speculate that CD123 expression may indicate poor treatment outcome in T-ALLs. Interestingly, there is no significant difference of MRD detection rate between the CD123-positive and CD123-negative groups in our 160 Chinese T-ALL patients after the first course of induction therapy by using flow cytometric detection. It was well known that flow cytometric detection of MRD can be reliably used to assess early response to treatment and predict relapse [12]. Patients with more MRD have a greater risk of relapse than those with less MRD. The rapidity with which leukemia cells are eliminated after induction of treatment and the level of residual disease at the end of induction have an effect on long-term prognosis. In addition, prospective studies in large series of patients have demonstrated a strong correlation between MRD levels during clinical remission and treatment outcome. Considering this, we think that CD123 expression did not predict the outcomes of induction therapy in our study. One potential explanation for the inconsistent results might be that the disease we studied is T-ALL, not AML. Several studies including more than 9000 pediatric patients with ALL have failed to demonstrate an association between myeloid-antigen-positive ALL and poor outcome [15–18]. In adult patients, several studies, but not all, reported that the presence of myeloid antigen was associated with a poor outcome. However, recent studies have not adequately taken into account the prognostic importance of myeloid-antigen expression found in adult T-ALL patients [19]. Another potential explanation might be that the level of residual disease at the end of the first induction is one of the important effects on long-term prognosis. The influencing factors of prognosis include the age, sex, clinical manifestation, cytogenetics, immunophenotype, and rapidity with which leukemia cells are eliminated after induction. Furthermore, the limited sample size and lack of long-term clinical observation can preclude definitive evaluation as well. Although it is difficult to get a definite conclusion, our study provides a reference for drawing up a reasonable conclusion that CD123 may not be sufficient as an independent predictor of treatment outcome in T-ALL. Altogether, further prospective studies are needed to elucidate more accurately the correlation between CD123 and prognosis of childhood and adult T-ALL.
References
Lowenberg B, Touw IP. Haemopoietic growth factors in acute myeloblastic and lymphoblastic leukaemia. Baillieres Clin Haematol. 1992;5:599–618.
Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–9.
Moretti S, Lanza F, Dabusti M, Tieghi A, Campioni D, Dominici M, et al. Cd123 (interleukin 3 receptor alpha chain). J Biol Regul Homeost Agents. 2001;15:98–100.
Sato N, Caux C, Kitamura T, Watanabe Y, Arai K, Banchereau J, et al. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood. 1993;82:752–61.
Testa U, Riccioni R, Diverio D, Rossini A, Lo Coco F, Peschle C. Interleukin-3 receptor in acute leukemia. Leukemia. 2004;18:219–26.
Djokic M, Bjorklund E, Blennow E, Mazur J, Soderhall S, Porwit A. Overexpression of cd123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica. 2009;94:1016–9.
Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208:227–34.
Swerdllow SH, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. France: IARC Press; 2008.
Bene MC. Immunophenotyping of acute leukaemias. Immunol Lett. 2005;98:9–21.
Zheng J, Wang X, Hu Y, Yang J, Liu J, He Y, et al. A correlation study of immunophenotypic, cytogenetic, and clinical features of 180 AML patients in China. Cytometry B Clin Cytom. 2008;74:25–9.
Bene MC, Nebe T, Bettelheim P, Buldini B, Bumbea H, Kern W, et al. Immunophenotyping of acute leukemia and lymphoproliferative disorders: a consensus proposal of the European LeukemiaNet Work Package 10. Leukemia. 2011;25:567–74.
Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999;38:139–52.
Lhermitte L, de Labarthe A, Dupret C, Lapillonne H, Millien C, Landman-Parker J, et al. Most immature T-ALLs express Ra-IL3 (CD123): possible target for DT-IL3 therapy. Leukemia. 2006;20:1908–10.
Blalock WL, Weinstein-Oppenheimer C, Chang F, Hoyle PE, Wang XY, Algate PA, et al. Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with anti-neoplastic drugs. Leukemia. 1999;13:1109–66.
Uckun FM, Sather HN, Gaynon PS, Arthur DC, Trigg ME, Tubergen DG, et al. Clinical features and treatment outcome of children with myeloid antigen positive acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 1997;90:28–35.
Putti MC, Rondelli R, Cocito MG, Arico M, Sainati L, Conter V, et al. Expression of myeloid markers lacks prognostic impact in children treated for acute lymphoblastic leukemia: Italian experience in AIEOP-ALL 88-91 studies. Blood. 1998;92:795–801.
Pui CH, Rubnitz JE, Hancock ML, Downing JR, Raimondi SC, Rivera GK, et al. Reappraisal of the clinical and biologic significance of myeloid-associated antigen expression in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16:3768–73.
Ludwig WD, Harbott J, Bartram CR, Komischke B, Sperling C, Teichmann JV, et al. Incidence and prognostic significance of immunophenotypic subgroups in childhood acute lymphoblastic leukemia: experience of the BFM study 86. Recent Results Cancer Res. 1993;131:269–82.
Schabath R, Ratei R, Ludwig WD. The prognostic significance of antigen expression in leukaemia. Best Pract Res Clin Haematol. 2003;16:613–28.
Acknowledgments
Thanks to the patients for their consent in participating in this study. This study was supported by the National Natural Science Foundation of China (No. 81202093 and No. 81100356).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Wen Du and Juan Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Du, W., Li, J., Liu, W. et al. Interleukin-3 receptor α chain (CD123) is preferentially expressed in immature T-ALL and may not associate with outcomes of chemotherapy. Tumor Biol. 37, 3817–3821 (2016). https://doi.org/10.1007/s13277-015-3272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3272-y