Abstract
Hepatocellular carcinoma (HCC) is an aggressive cancer with a poor prognosis. Autophagy and hypoxia have been involved in HCC tumorigenesis. In the present study, we examined the relationship between Beclin-1 expression and hypoxia-inducible factor (HIF)-1α expression in HCC by immunohistochemistry on 65 tumor specimens. Their correlations with clinicopathological features were also explored. There was a loss of Beclin-1 protein expression in 49.2 % of HCC. Beclin-1 expression was only significantly correlated with virus infection status (p = 0.025) and marginally associated with HCC grade (p = 0.057). Forty-two tumors (64.6 %) showed high HIF-1α expression, and it was significantly associated with large tumor size (p = 0.003), multifocal tumors (p = 0.038), and advanced stage (p = 0.043). Beclin-1 expression was significantly associated with HIF-1α expression (p = 0.001). HCC cases were further stratified according to their hypoxia status into hypoxic and normoxic groups. In the hypoxic group, Beclin-1 expression was negatively correlated with HCC high tumor grade (p < 0.001), advanced stage (p = 0.013), large size (p = 0.002), and multifocal tumors (p = 0.047). In the normoxic group, no significant relations between Beclin-1 expression and any of the clinicopathological parameters were identified. Our findings that reduced Beclin-1 and high HIF-1α expression are associated with the development and progression of HCC may provide molecular therapeutic targets toward inhibiting HCC development and progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), a global health problem, is the sixth most common malignancy and the third most common cause of cancer-related deaths worldwide and is commonly secondary to hepatitis B and C infections [1]. In Egypt, HCC ranks as the first cancer in males and the second in females constituting 33.63 and 13.54 % of cancer incidence, respectively [2]. Over the last decade, a considerable increase, from 4.0 to 7.2 %, was observed in the proportion of chronic liver disease Egyptian patients with HCC [3]. HCC is characterized by poor prognosis despite improved diagnostic and treatment strategies [4]. Although several clinicopathological parameters and molecular factors have been reported to be associated with the prognosis of HCC [5], more effective biomarkers are necessary to predict the clinical course and outcome of patients with HCC. Recently, autophagy has become a critical focus in cancer research. Autophagy has emerged as a homeostatic mechanism recycling intracellular constituents. Autophagy is believed to be a nonapoptotic form of programmed cell death; most of the evidences support autophagy as a survival pathway required for cellular viability. Simultaneous defects in autophagy and apoptosis activate the DNA damage response-promoted gene amplification and accelerate tumorigenesis in various organs [6]. Beclin-1 (BECN1), the mammalian orthologue of yeast Atg6/Vps30, has been mapped to a tumor susceptibility locus ∼150 kb centromeric to BRCA1 on human chromosome 17q21 [7]. It is considered as a haploinsufficient tumor suppressor gene [8]. Loss of BECN1 leads to reduction in autophagic vacuole formation and an unpredicted increase in several malignancies, including HCC [9]. HCC as a solid tumor shows the character of tissue hypoxia, especially when the tumor grows rapidly and angiogenesis fails to catch up with the speed of tumor growth. Hypoxia induces tumor necrosis which limits the size of HCC. However, severe hypoxia leads to prosurvival reactions, elevated angiogenesis, tumor invasion, and metastasis [4]. Hypoxia-inducible factor (HIF)-1 is the first identified mediator of cell response to hypoxia in mammalian cells cultured under reduced oxygen tension. This transcription factor is a heterodimer composed of α and β subunits [10]. There was a growing body of evidence that linked HIFs and pathogenesis of HCC [11]. Under hypoxic conditions, HIF-1α induces the autophagy cascade, removes damaged mitochondria, and limits reactive oxygen species (ROS) damage in order to protect the cell from apoptotic death [12]. However, the role of autophagy and hypoxia is still controversial in cancer, especially in tumor progression. Recent studies had confirmed that hypoxia-induced autophagy-related gene Beclin-1 expression might be important for disease progression and be correlated with patient outcome in several tumors [13]. The present study performed immunohistochemical analyses to determine the expression of the Beclin-1 and HIF-1α proteins in HCC cases and non-neoplastic hepatic tissues, to evaluate a potential association between Beclin-1 and HIF-1α expression, and to correlate the expressions of both markers to the clinicopathological factors.
Material and methods
Sixty-five cases of primary hepatocellular carcinoma specimens were collected from the files of the Department of Pathology, Minia University Hospital, and Minia Oncology Center, El-Minia, Egypt, during the period from 2009 to 2014. Clinical and pathological characteristics (Table 1), including age, sex, tumor size, focality, histological grade, stage, and hepatitis virus-associated infection, were obtained from the patients’ files. All hematoxylin and eosin (H&E)-stained slides were reviewed to revise histological grade. The clinicopathological stage was determined according to the International Union Against Cancer (UICC) TNM classification system [14]. The patients were 52 males and 14 females with age ranges from 40 to 74 years (median 55 years).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues (4 μm thick) were dewaxed in xylene and rehydrated in gradient ethanol solutions. Antigenic retrieval was performed in sodium citrate (pH = 6.0) with a microwave. Endogenous peroxidase activity was blocked by hydrogen peroxide 0.3 % for 30 min. Sections were then incubated with primary rabbit monoclonal antibody for Beclin-1 (1:100, Abcam, EPR1733Y) and mouse monoclonal antibodies for HIF-1α (1:200, Abcam, 1A3) at 4 °C overnight, followed with secondary antibody for 30 min. Finally, the sections were processed to develop color with diaminobenzidine (DAB) and were counterstained with Mayer’s hematoxylin. Sections of normal breast and lung carcinoma tissues were served as a positive control for Beclin-1 and HIF-1α, respectively. For a negative control, the specific primary antibody was replaced with phosphate-buffered saline (PBS).
Evaluation of IHC staining
Immunohistochemical staining level was assessed and scored by two independent pathologists, who were blind to the clinicopathological and follow-up information. Expression of Beclin-1 was defined as positive if distinct moderate or strong immunoreactivity was present in >10 % of the cells [15]. Regarding HIF-1α scoring, low expression was defined as <10 % of cells exhibiting nuclear staining and/or cytoplasmic staining. High expression was determined when ≥10 % of cells exhibited nuclear staining and/or distinct cytoplasmic staining [16].
Statistical analysis
Statistical evaluation was performed using the SPSS software package (SPSS 16.0). The chi-square test was used to analyze the association between Beclin-1 and HIF-1α expression and their association with clinicopathological features. The level of statistical significance was set at p ≤ 0.05.
Results
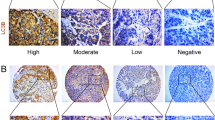
Positive cytoplasmic Beclin-1 expression was found in 18/21 (85.7 %) of adjacent non-neoplastic hepatic tissues and in 33/65 (50.8 %) of HCC cases (Fig. 1a, b), while it was negative in 32/65 (49.2 %) of HCC cases (Fig. 1c). The expression of the Beclin-1 protein in the HCC tissue was significantly lower than that of the adjacent non-neoplastic tissues (p = 0.005). Conversely, HIF-1α staining was observed in the nucleus as well as cytoplasm of tumor cells in 64.6 % of HCC (Fig. 1e, f), compared to the negligible expression in the non-neoplastic hepatic tissues (Fig. 1d) (p < 0.001). Among HCC cases, 42/65 tumors displayed hypoxia (defined by HIF-1α high expression) compared to 23/65 tumors exhibiting normoxia (defined as HIF-1α low or deficient expression).
a–f Immunohistochemical expression of Beclin-1 and HIF-1α in primary HCC and adjacent nonneoplastic hepatic tissues. a Positive Beclin-1 expression in adjacent nonneoplastic hepatic tissue with hepatitis. b Positive Beclin-1 expression in low-grade HCC. c Negative Beclin-1 expression in high-grade HCC. d HIF-1α expression in adjacent nonneoplastic hepatic tissue with hepatitis. e HIF-1α expression in low-grade HCC. f HIF-1α expression in high-grade HCC. Immunohistochemistry: diaminobenzidine (DAB) chromogen and hematoxylin counterstaining. Original magnification ×400 for all figures except for c ×200
The correlation of Beclin-1 expression with the clinicopathological characteristics of HCC is shown in Table 2. Reduced Beclin-1 expression was marginally associated with HCC grade (p = 0.057). A significant positive association was found between Beclin-1 expression and virus infection status (p = 0.025). Among HBV-associated tumors and HCV-associated tumors, 76.9 and 50 % were Beclin-1 positive, respectively, while 80 % of HCC negative for hepatitis virus infection showed negative Beclin-1 expression. No significant differences in Beclin-1 expression with respect to other characteristics were found.
HCC cases were further stratified according to their hypoxia status into hypoxic and normoxic groups. The relationship between Beclin-1 expression and different clinicopathological parameters was separately studied in these groups (Table 2). In the hypoxic group, Beclin-1 expression was negatively correlated with HCC high tumor grade (p < 0.001), advanced stage (p = 0.013), large size (p = 0.002), and multifocal tumors (p = 0.047). In the normoxic group, no significant relations between Beclin-1 expression and any of the clinicopathological parameters were identified.
Table 3 demonstrates the association of HIF-1α expression and clinicopathological features. High HIF-1α immunoreactivity was significantly associated with large tumor size (p = 0.003), multifocal tumors (p = 0.038), and advanced stage (p = 0.043), while no statistically significant associations were detected in relation to other clinicopathological variables.
Relationship between Beclin-1 and HIF-1α expression in HCC cases
The present work found that Beclin-1 expression rates were positively associated with hypoxic conditions (p = 0.001). A significant positive association was found between Beclin-1 and HIF-1α expression (Table 4). In the 33 HCC cases marked as Beclin-1-positive tumors, 5/33 (15.2 %) had low HIF-1α and 28/33 (84.8 %) had high HIF-1α. Whereas, in the 32 HCC cases classified as Beclin-1-negative tumors, the low HIF-1α and high HIF-1α tumors were 18/32 (56.3 %) and 14/32 (43.8 %), respectively.
Discussion
The role of autophagy in carcinogenesis is double sided and dependent on the cellular context. Autophagy exerts an inhibitory role in the initial stage of tumorigenesis, via suppression of inflammation and preserving genome stability. On the other hand, autophagy acts as a prosurvival mechanism to protect cancer cells against cell death induced by cellular stress. However, as cellular stress continues to result in excessive and sustained autophagy, tumor cell death would follow [17, 18]. Direct evidence linking autophagy to tumor suppression comes from the fact that one of its key regulators, Beclin-1, exhibits a tumor suppressor function and has been found to be monoallelically deleted in several tumors [17, 19]. The present study demonstrated deficient Beclin-1 expression in 49.2 % of HCC. As previously reported in various tumors [13, 15, 20, 21], a significantly decreased Beclin-1 expression in HCC tissues compared to the adjacent nonneoplastic tissues was found in this series. These findings support the hypothesis that a defective autophagy can contribute to carcinogenesis of various tumors.
In this study, Beclin-1 expression was more frequently associated with well-differentiated HCC than poorly differentiated tumors, although this difference was marginally significant. Recently, several studies reported that Beclin-1 expression was inversely correlated with poor clinicopathological features and unfavorable prognosis in HCC [15, 21] as well as in other tumors [20, 22–24], suggesting that Beclin-1 expression might reverse the aggressive phenotypes as a tumor suppressor in several types of cancers. Several mechanisms underlying the tumor suppressor role of Beclin-1 could be considered. Beclin-1 delays cell cycle progression [25], suppresses tumor angiogenesis and proliferation [26], limits chromosomal instability and reduces the frequency of additional mutation in tumors [27]. By contrast, other studies demonstrated its association with aggressive features and poor outcome in nasopharyngeal carcinoma [28] and oral squamous cell carcinoma [29]. This may be due to variations in intrinsic properties of these tumor types.
Here, a significant positive association was found between Beclin-1 expression and hepatitis virus infection status, where 76.9 % of HBV-associated tumors and 50 % of HCV-associated tumors were Beclin-1 positive while the great majority of HCC negative for hepatitis virus infection showed negative Beclin-1 expression. Consistent with this finding, previous studies demonstrated that hepatitis B virus x protein (HBx) upregulates endogenous Beclin-1 mRNA and protein levels in the liver and hepatoma cell lines via transactivation of Beclin-1 promoter activity [30]. Also, HCV induces autophagy by upregulation of Beclin-1 at the transcriptional level [31].
Recent studies highlighted the role of HIFs and hepatic oxygen levels in the pathogenesis of chronic liver disease and HCC that may provide new therapeutic opportunities for treatment [11, 32–34]. This study demonstrated high HIF-1α expression in 64.6 % of HCC cases with deficient expression in adjacent nonneoplastic tissue. High HIF-1α was significantly associated with large tumor size, multifocal tumors, and advanced stage. These findings were in agreement with other studies that considered HIF-1α as a negative prognostic indicator in HCC [35–37]. High HIF-1α expression was also noted in 57.1 and 92.3 % of hepatitis C- and hepatitis B-positive HCC cases, respectively. Previous studies suggested that both HBV and HCV induce a cellular hypoxic response and stabilize HIF-1α [38, 39]. Moreover, hypoxia promotes HBV and HCV replication, suggesting a role of HIF-1α in hepatitis virus pathogenesis and oncogenicity that may provide a novel therapeutic target to limit viral replication and associated pathologies [34].
In order to investigate whether a hypoxic status influences Beclin-1 expression and function, cases were stratified according to HIF-1α expression into hypoxic and normoxic groups. Beclin-1 expression was investigated in relation to clinicopathological features in both groups separately. Interestingly, the association between reduced Beclin-1 and the aggressive clinicopathological features including high tumor grade, large size, advanced stage, and multifocal tumors was only significant in the hypoxic group. On the contrary, Beclin-1 expression failed to demonstrate any correlation with clinicopathological features in the normoxic group of HCC. Based on this finding, we propose that the autophagic Beclin-1-induced clinicopathological effect was driven by hypoxia in HCC and that the cumulative effects induced by reduced Beclin-1 and high HIF-1α expression may promote a high-grade malignant phenotype and aggressive behavior in HCC. A previous experimental study, using a primary mouse melanoma tumor model, demonstrated that Beclin-1 deficiency was significantly associated with aggressive growth phenotype and increased angiogenesis in tumors under a hypoxic condition compared to those with a normoxic condition [26]. Another study demonstrated that reduced Beclin-1 expression predicted worse overall survival among patients with HIF-1α-overexpressing breast cancers [13]. This raises a concern regarding the role of autophagy and hypoxia in the progression of malignant tumors.
Furthermore, this study demonstrated a significant association between the autophagic Beclin-1 expression and high HIF-1α in this series where tumors with high HIF-1α expression more frequently developed Beclin-1 upregulation than those with low HIF-1α expression. This finding was similar to that found by previous studies for squamous cell carcinoma of the esophagus [20] nasopharyngeal [39] and breast [13] carcinomas, supporting the critical role of hypoxia in the activation of autophagy. On the contrary, Wu et al. [24] failed to find any correlation between HFI-1α and Beclin-1 expression.
In conclusion, the close association between Beclin-1 and HIF-1α identified in the current study confirms the critical role of hypoxia in the activation of autophagy. Moreover, reduced Beclin-1 and elevated HIF-1α expression were significantly associated with aggressive clinicopathological features, supporting their pivotal role in the development and progression of HCC. Therefore, both markers could be considered as useful targets for new molecular therapies besides conventional treatments against HCC.
References
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:e1264–73.
Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014(437971):1–18.
Abdel-Hamid NM, Nazmy MH, Mahmoud AW, Fawzy MA, Youssof M. A survey on herbal management of hepatocellular carcinoma. World J Hepatol. 2011;3:175–83.
Wong CC, Kai AK, Ng IO. The impact of hypoxia in hepatocellular carcinoma metastasis. Front Med. 2013;8:33–41.
Scaggiante B, Kazemi M, Pozzato G, Dapas B, Farra R, Grassi M, et al. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol. 2014;20:1268–88.
Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–55.
Sun Q, Fan W, Zhong Q. Regulation of Beclin 1 in autophagy. Autophagy. 2009;5:713–6.
Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422–4.
Kang KF, Wang XW, Chen XW, Kang ZJ, Zhang X, Wilbur RR, et al. Beclin 1 and nuclear factor-κBp65 are upregulated in hepatocellular carcinoma. Oncol Lett. 2013;5:1813–8.
Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol. 2007;22:559–72.
Wilson KG, Tennant AD, McKeating AJ. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J Hepatol. 2014;61:1397–406.
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903.
Dong M, Wan X, Yuan ZY, Wei L, Fan XJ, Wang T, et al. Low expression of Beclin 1 and elevated expression of HIF-1 α refine distant metastasis risk and predict poor prognosis of ER-positive, HER2-negative breast cancer. Med Oncol. 2013;30:355–65.
Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer. American Joint Committee on Cancer staging manual. 7th ed. New York: Springer; 2010. p. 175.
Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;15:9167–75.
Xiang ZL, Zeng ZC, Fan J, Tang ZY, He J, Zeng HY, et al. The expression of HIF-1α in primary hepatocellular carcinoma and its correlation with radiotherapy response and clinical outcome. Mol Biol Rep. 2012;39:2021–9.
Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–41.
Cui J, Gong Z, Shen HM. The role of autophagy in liver cancer: molecular mechanisms and potential therapeutic targets. Biochim Biophys Acta. 1836;2013:15–26.
Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;12:2891–906.
Chen Y, Lu Y, Lu C, Zhang L. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1alpha expression. Pathol Oncol Res. 2009;15:487–93.
Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao XL, et al. The expression of beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer. 2014;9:327–40.
Yao Q, Chen J, Lv Y, Wang T, Zhang J, Fan J, et al. The significance of expression of autophagy-related gene Beclin, Bcl-2, and Bax in breast cancer tissues. Tumour Biol. 2011;32:1163–71.
Won KY, Kim GY, Lim SJ, Kim YW. Decreased Beclin-1 expression is correlated with the growth of the primary tumor in patients with squamous cell carcinoma and adenocarcinoma of the lung. Hum Pathol. 2012;43:62–8.
Wu X, Chen J, Cao Q, Dong M, Lin Q, Fan XJ, et al. Beclin 1 activation enhances chemosensitivity and predicts a favorable outcome for primary duodenal adenocarcinoma. Tumor Biol. 2013;34:713–22.
Koneri K, Goi T, Hirono Y, Katayama K, Yamaguchi A. Beclin 1 gene inhibits tumor growth in colon cancer cell lines. Anticancer Res. 2007;27:1453–7.
Lee SJ, Kim HP, Jin Y, Choi AM, Ryter SW. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy. 2011;7:829–39.
Mathew R, Kongara S, Beaudoin B, Karp M, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81.
Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, Guo L, et al. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6:395–404.
Tang JY, Fang YY, Hsi E, Huang YC, Hsu NC, Yang WC, et al. Immunopositivity of Beclin1 and ATG5 as indicators of survival and disease recurrence in oral squamous cell carcinoma. Anticancer Res. 2013;33:5611–6.
Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, et al. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology. 2009;49:60–71.
Shrivastava S, BhanjaChowdhury J, Steele R, Ray R, Ray RB. Hepatitis C virus upregulates Beclin1 for induction of autophagy and activates mTOR signaling. J Virol. 2012;86:8705–12.
Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, et al. Hepatocyte specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–37.
Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, et al. Hypoxia inducible transcription factor 2alpha promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472–83.
Wilson GK, Brimacombe CL, Rowe IA, Reynolds GM, Fletcher NF, Stamataki Z, et al. A dual role for hypoxia inducible factor-1alpha in the hepatitis C virus life cycle and hepatoma migration. J Hepatol. 2012;56:803–9.
Dai CX, Gao Q, Qiu SJ, Ju MJ, Cai MY, Xu YF, et al. Hypoxia-inducible factor-1alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer. 2009;9:418–29.
Xiang ZL, Zeng ZC, Fan J, Tang ZY, Zeng HY, Gao DM. Gene expression profiling of fixed tissues identified hypoxia-inducible factor-1alpha, VEGF, and matrix metalloproteinase-2 as biomarkers of lymph node metastasis in hepatocellular carcinoma. Clin Cancer Res. 2011;17:5463–72.
Zheng SS, Chen XH, Yin X, Zhang BH. Prognostic significance of HIF-1alpha expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8(e65753):1–7.
McFarlane S, Nicholl MJ, Sutherland JS, Preston CM. Interaction of the human cytomegalovirus particle with the host cell induces hypoxia-inducible factor 1 alpha. Virology. 2011;414:83–90.
Darekar S, Georgiou K, Yurchenko M, Yenamandra SP, Chachami G, Simos G, et al. Epstein-Barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect. PLoS One. 2012;7(e42072):1–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osman, N.A.A., Abd El-Rehim, D.M. & Kamal, I.M. Defective Beclin-1 and elevated hypoxia-inducible factor (HIF)-1α expression are closely linked to tumorigenesis, differentiation, and progression of hepatocellular carcinoma. Tumor Biol. 36, 4293–4299 (2015). https://doi.org/10.1007/s13277-015-3068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3068-0