Abstract
Autophagy is a process that involves lysosomal degradations of cellular organelles and closely related to tumor occurrence and progression. However, its importance in hepatocellular carcinoma (HCC) was still controversial. Therefore, this study is aimed to address the clinicopathologic effect of microtubule-associated protein 1 light chain 3B (LC3B) and Beclin-1, as autophagic markers, in HCC patients. Tissue microarray-based immunohistochemistry was used to examine the expression of LC3B and another autophagy key regulator (Beclin-1) in 156 operable HCC patients. Kaplan-Meier analysis, chi-square test, and Spearman’s correlation analysis were used to analyze correlation of LC3B and Beclin-1 and their influence on clinical characteristics and prognosis. We found that the expression level of LC3B was significantly associated with vascular invasion (P = 0.008), lymph node metastasis (P < 0.001), and Beclin-1 expression level (P < 0.001). However, LC3B was not related to other clinicopathological features, including hepatitis B virus infection, liver cirrhosis, tumor number, tumor size, pathology grade, and tumor-node-metastasis (TNM) stage. Besides, correlation between the expression of Beclin-1 and clinicopathological features were not identified. Survival analysis showed that patients with high LC3B expression had a poorer 5-year overall survival (OS) rate than those with low LC3B expression (high vs. low: 79.5 % vs. 20.5 %, P = 0.026). And high LC3B expression tended to be related with shorter progression-free survival (PFS) (P = 0.074), whereas the expression level of Beclin-1 did not show statistically significant association with OS or PFS. Further multivariate analysis revealed that lymph node metastasis (P = 0.047) and LC3B expression level (P = 0.047) were independent factors to predict the prognosis of OS in all patients. Our study demonstrated that high expression of LC3B, correlated with vascular invasion and lymph node metastasis, might be a novel prognostic biomarker and would be a potential therapy target for HCC, especially in operable patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), as one of the main digestive system cancers, is the sixth most common malignancy and the third leading cause of cancer-related deaths worldwide [1]. Surgical resection and liver transplantation were considered to be the curative modalities for HCC [2–4]. However, despite resection with curative intent, the clinical course is variable and the long-term prognosis is still poor with reported 5-year survival rates ranging from 17 to 53 % [5]. Although there are several diagnostic and prognostic markers for HCC, they lack specificity, hampering the management of this disease [6]. Thus, to identify more novel molecular markers that not only predict the prognosis individually but also provide promising therapy targets will be of great benefit for HCC patients.
Autophagy, a type of non-apoptotic cell death, is an intracellular pathway: cytoplasmic constituents are enveloped in double membrane vesicles, called autophagosomes, which deliver the contents to lysosomes for bulk degradation [7–12]. In cancer, autophagy has two sides function, both as a tumor suppressor and as a tumor promoter [13–16]. Autophagy can maintain homeostasis and viability during periods of metabolic stress, and it is also associated with many physiological pathway, such as differentiation, development, neurodegenerative diseases, infection, and cancer [17]. Until now, nearly 30 genes have been recognized to regulate autophagy in yeasts, with 16 homologues in humans [11]. Among these, microtubule-associated protein light chain 3 (LC3) and Beclin-1 (BECN-1) play an important role in autophagy in humans [18]. They are essential for autophagosome formation and can monitor autophagic activity. LC3 has three isoforms (including LC3A, LC3B, and LC3C). LC3B localizes to the autophagosome membrane which was the key regulator involved in autophagosome formation. Therefore, it is widely used as a marker of autophagic activity [15, 19, 20]. Aberrant expression of LC3B has been reported in several solid tumors, including brain cancer [21], pancreatic cancer [22], prostate cancer [23], breast cancer, and melanoma [24]. However, the role of LC3B in HCC patients has not been yet clear.
Therefore, in the present study, we investigated the expression of LC3B and Beclin-1 in HCC and adjacent paracancrous tissues and further evaluated the relationship between expression of these markers and clinicopathological features and survival outcome in HCC patients. We found that high expression of LC3B correlated with vascular invasion and lymph node invasion predicted unfavorable prognosis of HCC. LC3B was expected to be the new therapeutic targets for hepatocellular carcinoma treatment.
Material and methods
Cohorts
A total of 156 consecutive patients diagnosed with HCC by pathological examination were recruited in our study, who received surgical resection in the Third Affiliated Hospital of Sun Yat-sen University from September 2003 to September 2010. Patients were included with the following inclusion criteria: pathologically confirmed as HCC; previously without oncological surgery, chemotherapy, and radiotherapy; all patients had the completed follow-up information and paraffin-embedded specimens. Moreover, patient would be excluded for any of the following reasons: received any other anticancer therapy before or after surgery, prior malignancy, pregnancy, and the cholangiocarcinoma. We used serum markers detection, such as hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antibody (HBeAb), hepatitis B core antibody (HBcAb), and HBV DNA to diagnose HBV infection. Liver cirrhosis was diagnosed according to the international guidelines by comprehensive consideration of liver biopsy, imaging examination, clinical symptoms, cirrhosis-associated complications, physical signs, laboratory tests, medical history, and progress notes. The tumor-node-metastasis (TNM) stages were defined according to 2010 AJCC staging system for HCC. This study was approved by the Human Ethics Committee of the Third Affiliated Hospital, Sun Yat-sen University. A written informed consent was obtained from all the patients at the time of admission, with which the blood, tissue, and other samples were authorized to scientific purpose.
Tissue microarray (TMA) construction
Prior to TMA construction, we re-reviewed hematoxylin and eosin (H&E)-stained slides and selected the typical tumor zone in the paraffin-embedded specimens for further TMAs design. Tissue microarrays were constructed as described in our earlier study [25]. Briefly, for each case, two cores taken from the selected tumor area. Firstly, a hollow needle was utilized to punch the cylinders tissue cores (1.0 mm in diameter) from selected donor tissues. Secondly, the punched tissue was inserted into a recipient paraffin core in a precisely spaced, array pattern, using an automatic TMA instrument (Beecher Instruments, Silver Spring, MD, USA) [26, 27].
Semi-quantitative assessment of immunohistochemical (IHC) staining
IHC staining was performed as we previously described. Briefly, the TMA blocks were cut into 4-μm sections, deparaffinized three times in xylene for 30 min, and rehydrated with graded alcohols. Sections were then incubated in H2O2 for 10 min, then heated in antigen retrieval solution (sodium citrate, pH 6.0) in microwave for 30 min, and blocked with 1 % bovine serum albumin (diluted in phosphate-buffered saline (PBS) buffer) for 10 min at room temperature. The TMA sections were then incubated at 4 °C overnight with primary rabbit anti-LC3B antibody (sigma USA, L7543) that was diluted at 1:400 and primary rabbit anti-Beclin-1 antibody (Santa Cruz, SC-11427) that was diluted at 1:100. The brown granules in cytoplasm or nuclei were regarded as positive staining. A negative control was used by replacing the specific primary antibody with PBS.
LC3B and Beclin-1 expression level were evaluated by integrating the percentage of positive tumor cells and the intensity of positive staining. The intensity of staining was scored as follows: negative (score 0), bordering (score 1), weak (score 2), moderate (score 3), and strong (score 4). According to the percentage of positive staining cells in the whole core, the staining extent was categorized into 5 grades: negative (score 0), 0–25 % (score 1), 26–50 % (score 2), 51–75 % (score 3) and 76–100 % (score 4). The sum of the intensity and extent score was considered as the overall IHC score. Immunohistochemical staining was evaluated and scored by two independent pathologists, Min Dong and Na Cheng, without knowledge of the clinicopathological and follow-up information of these patients.
Selection of cutoff scores for LC3B and Beclin-1 “positive” expression
The receiver operating characteristic (ROC) curve analysis was applied to select the rational LC3B and Beclin-1 IHC cutoff score. Briefly, the sensitivity and specificity for the patient outcome being studied at each score was plotted to generate a ROC curve. The IHC score localized closest to the point at both maximum sensitivity and specificity was selected as the cutoff score that leads to the greatest potential to determine having or not having the outcome. To facilitate ROC curve analysis, the survival features were dichotomized: death vs. others (censored, alive, or death from other causes).
Clinical outcome assessment
All patients were followed up until the date of death or when censored at the latest date (May 13, 2013). Overall survival (OS) was defined as the time from diagnosis to the date of death or when censored at the latest date if patients still alive. Progression-free survival (PFS) was defined as the time from diagnosis to the date of local failure/distant metastasis; if the patients have no local failure or distant metastasis at the latest date, PFS was defined as the date of death or when censored at the latest date. Local failure means local recurrence and local metastasis.
Statistical analysis
The correlations between LC3B and Beclin-1 expression levels and clinicopathological features, including gender, age, tumor surgery, hepatitis B virus (HBV) infection, liver cirrhosis, α-fetal protein (AFP) level, alkaline phosphatase (ALP) level, bilirubin level, tumor size and number, vascular invasion, tumor stage, and TNM stage, were evaluated by chi-square test. The Spearman rank test was applied to demonstrate clinicopathological correlations. Correlations of LC3B and Beclin-1 were determined by Spearman’s correlation analysis. The relationships between the LC3B and Beclin-1 expression level and OS as well as PFS were determined by the Kaplan-Meier analysis. The log-rank tests were performed to assess the difference in survival probabilities between patient subgroups. The multivariate Cox proportional hazards regression model was used to assess the potential independent prognostic factors and 95 % confidence intervals (CI) of hazard ratios (HR). All P values quoted were two sided and P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v. 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
In our study, 156 HCC cases that received surgical resection were recruited. The clinical features of these patients, including gender, age, surgery, T classification, N classification, TNM stage, WHO pathological type, the level of AFP, hepatitis, and liver cirrhosis were summarized in Table 1. Among of the 156 HCC cases, 39 HBV negative HCC cases have no other virus infection or other basic diseases. And, the median survival time was 82.7 months among all the patients. The 1, 3, 5 years postoperative cumulative survival rate were 94.6, 74.5, and 69.7 %, respectively.
Immunohistochemistry staining and ROC analysis
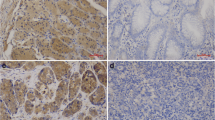
Immunohistochemistry staining of LC3B was overexpressed in the HCC tumor nests area compared to the normal adjacent epithelium (Fig. 1a), Beclin-1 was lowly expressed in 53.2 % HCC specimen, especially in the tumor nest zone whereas the normal adjacent tissues showed moderate or strong staining (Fig. 1b). For LC3B, we found that ROC analysis revealed that a score of 4 was the cutoff point to distinguish the patients as high or low LC3B expression. Similarly, for Beclin-1, we selected the median expression score of 6 (>6 vs. ≤6) as the rational cutoff point for survival analysis (data not shown).
LC3B and Beclin-1 expression in HCC. Immunohistochemistry analysis of LC3B expression (a) and Beclin-1 expression (b) in HCC. LC3B was highly or moderately expressed in cancer cell cytoplasma, whereas lowly expressed in the well-differentiated tissue (original magnification, ×100). The lower panel displayed the enlarged view (original magnification, ×400). Beclin-1 is the same
LC3B and Beclin-1 expression and their correlation with clinical features
To elucidate the biologic significance, we correlated the expressions of LC3B and Beclin-1 with clinicopathological characteristics of HCC. As shown in Table 1, LC3B expression in HCC was associated with vascular invasion (P = 0.016), lymph node metastasis (P = 0.042), and TNM stage (P = 0.037). However, Beclin-1 expression was not related with any clinicopathological features (all P value >0.05), such as age, gender, tumor size, HBV infection, liver cirrhosis, AFP level, and pathology grade. Furthermore, we compared the LC3B IHC scores in the subgroup with dichotomized clinicopathological features (Fig. 2). We found that LC3B IHC score in N1 subgroup (5.97 ± 0.79) was higher than N0 subset (4.74 ± 1.85, P < 0.001, Fig. 2d), and the similar results were also found in these subgroups: vascular invasion (with vs. without: 5.41 ± 1.18 vs. 4.70 ± 1.93, P = 0.008, Fig. 2e), Beclin-1 (high vs. low: 5.55 ± 1.28 vs. 4.25 ± 1.98, P < 0.001, Fig. 2i). However, close association between LC3B IHC score and other clinicopathological features were not identified, including HBV infection (negative vs. positive: 4.51 ± 2.01 vs. 4.96 ± 1.74, P = 0.427, Fig. 2a) and liver cirrhosis (with vs. without: 4.74 ± 1.85 vs. 5.16 ± 1.67, P = 0.195, Fig. 2b). T number (>1 vs. 1: 4.78 ± 1.80 vs. 4.90 ± 1.82, P = 0.695, Fig. 2c), tumor size (>5 cm vs. ≦5 cm: 4.96 ± 1.69 vs. 4.78 ± 1.90, P = 0.534, Fig. 2f), histological grade (poor and moderate vs. well 4.82 ± 1.83 vs. 5.11 ± 1.65, P = 0.528, Fig. 2g), TNM stage (1 + 2 vs. 3 + 4: 4.63 ± 2.00 vs. 5.05 ± 1.61, P = 0.154, Fig. 2h).
LC3B levels were compared in the subgroups with dichotomized clinicopathological features. The features were dichotomized: a HBV infection (with vs. without), b liver cirrhosis (with vs. without), c tumor number (>1 vs. =1), d N stage (N1 vs. N0), e vascular invasion (with vs. without), f tumor size (≥5 cm vs. <5 cm), g pathology grade ((poor and moderate vs. well), h TNM stage (1 + 2 vs. 3 + 4), i Beclin-1 level ((low vs. high)
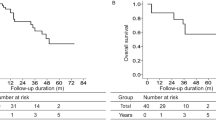
Beclin-1, LC3B expression, and survival analysis
Next, we explored the prognostic value of LC3B and Beclin-1 expression in HCC. As shown in Fig. 3, LC3B overexpression strongly predicted an inferior 5-year OS rate (high vs. low: 79.5 vs. 20.5 %, P = 0.026) in all patients. And lower LC3B expression tended to be related with longer PFS (P = 0.074), although it did not show a statistical significance. Meanwhile, Beclin-1 high or low expression had the similar 5-year OS rate (P = 0.757) for the HCC patients. We cannot find the PFS difference in both Beclin-1 and LC3B expression. Because former correlation analysis showed that LC3B and Beclin-1 expression levels positively correlated with each other (P < 0.001), we analyzed the prognostic value of LC3B and Beclin-1 co-expression. The results showed that for LC3B overexpressed patients, Beclin-1 high or low expression had the similar OS, and for LC3B low-expressed patients, Beclin-1 high or low also had the similar OS (data not shown).
Overall survival and progression-free survival by the Kaplan-Meier analysis, comparing the patients with high or low LC3B and Beclin-1 expression in HCC. a LC3B lowly expressed patients had a favorable overall survival. b LC3B high and low expression had a similar progression-free survival for HCC patients. c, d Beclin-1 high and low expression had a similar overall survival and progression-free survival for HCC patients
Univariate and multivariate analysis
Further univariate analyses showed the clinicopathological variables that might be potential prognostic predictors (Table 2). Multivariate analysis summarized in Table 3 displayed that lymph node metastasis (P = 0.047) and LC3B expression level (P = 0.047) were indeed independent factors to predict the prognosis of OS in all patients. However, TNM stage and tumor size failed to independently predict HCC patients’ prognosis.
Discussion
Autophagic-gene was widely used as the prognosis biomarkers in different tumor types. Accumulating studies indicate that the inhibition of autophagy-related protein might be an efficient strategy for cancer therapy. LC3B, a reliable autophagy factor and frequently used autophagic biomarker, was revealed to have an important role for the development of cancer and to be associated with patient survival. Currently, the role of LC3B in HCC is rarely reported. Hence, in our study, we assessed LC3B expression in operable HCC patients’ samples by IHC and in other pathophysiological contexts, such as HBV infection, liver cirrhosis, vascular invasion, and lymph node metastasis. We found that LC3B was lowly expressed in tumor zone compared with normal adjacent tissues (Fig. 1a), and the expression level of LC3B was positive relatively with vascular invasion and lymph node metastasis. Moreover, we found that the overexpression of LC3B predicted an inferior OS. Our results were consistent with Toshima’s study, which showed that overexpressed LC3 was correlated with tumor size, and LC3 was an independent predictor of HCC recurrence after surgery [28]. In another research, 71 pancreatic cancer tissues were used to evaluate the expression of LC3 by immunohistochemistry. And they got the similar results that autophagy was activated in pancreatic cancer cells, and high LC3 expression was correlated with poor survival [29]. In oral squamous cell carcinoma, high LC3 expression that demonstrated by immunohistochemistry was significantly associated with poorer overall survival [30]. Also, research on gastric cancer has indicated that positive LC3B immunofluorescence histochemical reactivity correlates with lower invasion [31]. By contrary, in a study consisting of 190 HCC patients, it was reported that expression of LC3B in advance stages of TNM was correlated with longer survival but not in the early stage patients [32]. And experimental evidence demonstrated that autophagy was decreased in HCC, which indicated that autophagy malfunction linked to tumorigenesis [33]. These results indicated that even sharing the similar biological behavior, the prognostic phenotype of LC3B might be distinguished among the different tumor stages.
In addition, we analyzed the relationship between LC3B expression and clinical features of HCC patients. The results revealed that LC3B expression level was significantly associated with vascular invasion and lymph node metastasis. The HCC patients with vascular invasion had a high level of LC3B than that without vascular invasion (IHC score: 5.41 ± 1.18 vs. 4.70 ± 1.93, Fig. 2e). Likewise, the expression of LC3B in lymph node metastasis (N1 stage) group was also higher than N0 stage group (IHC score: 5.97 ± 0.79 vs. 4.74 ± 1.85, Fig. 2d). These findings indicated that activation of LC3B was a risk factor for tumor invasive biologic behavior (vascular invasion and lymph node metastasis) and suggested that LC3B might play an important role in cancer progression. Indeed, the relationship between LC3B and tumor invasion or metastasis was cleared in a variety of cancers. Lazova reported that high LC3B was associated with tumor proliferation, invasion, and metastasis, and predicted worse patient outcome [24]. Similarly, in triple-negative breast cancer, LC3B high expression was significantly correlated with tumor size, lymph node metastasis, and Ki-67 expression. The patients with high expression of LC3B showed poorer PFS and OS rates compared with LC3B low-expression group [34]. Based on our and previous reports, we can conclude that the increased autophagy activity (high LC3B expression) were significantly benefited for tumor proliferation, invasion, or metastasis and further confirmed the essential predictive value of LC3B in tumor development, especially in invasion and metastasis. Moreover, in our study, we also found that expression of LC3B was positively correlated with Beclin-1. However, compared to the prognostic efficacy and positive association with clinic characteristics (vascular invasion and lymph node metastasis) of LC3B, Beclin-1 expression did not display any effect on patients’ survival. For the underlying mechanism, we supposed that autophagy is a complex, multistep, and highly dynamic process. Autophagy-related proteins (LC3B and Beclin-1) were involved in different autophagic stage and interconnected with each other under a certain condition [35]. Just as other studies report, not all expressions of autophagy-related protein (LC3B and Beclin-1) were associated with the tumor patients outcome [36], because detecting levels of a single autophagy-related marker did not precisely determine complete autophagy flux. This review explained the paradox of conflicting prognostic values (positively or negatively) of Beclin-1 and LC3B in different tumor types.
Taken together, our study demonstrated that LC3B was an independent prognostic biomarker of OS for early HCC. LC3B high expression might be a novel therapeutic molecular target for HCC.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- OS:
-
Overall survival
- IHC:
-
Immunohistochemistry
- LC3B:
-
Microtubule-associated protein light chains 3B
- Beclin-1:
-
Associated autophagy-related key regulator
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–917.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Shukla PJ, Barreto SG. Surgery for malignant liver tumors. J Cancer Res Ther. 2009;5:154–60.
Said A, Lucey MR. Liver transplantation: an update 2008. Curr Opin Gastroenterol. 2008;24:339–45.
Tsoulfas G, Mekras A, Agorastou P, Kiskinis D. Surgical treatment for large hepatocellular carcinoma: does size matter? ANZ J Surg. 2012;82:510–7.
Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1:593–8.
Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–7.
Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–97.
Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009;14:376–91.
Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009;5:442–50.
Cheong H, Klionsky DJ. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008;451:1–26.
Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–908.
Mann SS, Hammarback JA. Molecular characterization of light chain 3. A microtubule binding subunit of map1a and map1b. J Biol Chem. 1994;269:11492–7.
He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. Lc3, a mammalian homologue of yeast apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8.
Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–41.
Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88.
Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–73.
Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395–8.
Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, et al. Dissection of autophagosome formation using apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–68.
Huang X, Bai HM, Chen L, Li B, Lu YC. Reduced expression of lc3b-ii and beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J Clin Neurosci. 2010;17:1515–9.
Ko YH, Cho YS, Won HS, Jeon EK, An HJ, Hong SU, et al. Prognostic significance of autophagy-related protein expression in resected pancreatic ductal adenocarcinoma. Pancreas. 2013;42:829–35.
Giatromanolaki A, Sivridis E, Mendrinos S, Koutsopoulos AV, Koukourakis MI. Autophagy proteins in prostate cancer: relation with anaerobic metabolism and gleason score. Urol Oncol. 2014;32:11–39.
Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate lc3b expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18:370–9.
Wu XY, Chen J, Cao QH, Dong M, Lin Q, Fan XJ, et al. Beclin 1 activation enhances chemosensitivity and predicts a favorable outcome for primary duodenal adenocarcinoma. Tumour Biol. 2013;34:713–22.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7.
He JH, Luo RZ, Cai MY, Li M, Lu JB, Yuan ZY. Decreased expression of light chain 3 (lc3) increased the risk of distant metastasis in triple-negative breast cancer. Med Oncol. 2013;30:468.
Toshima T, Shirabe K, Matsumoto Y, Yoshiya S, Ikegami T, Yoshizumi T, et al. Autophagy enhances hepatocellular carcinoma progression by activation of mitochondrial beta-oxidation. J Gastroenterol 2014;49:907–16.
Fujii S, Mitsunaga S, Yamazaki M, Hasebe T, Ishii G, Kojima M, et al. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci 2008;99:1813–19.
Tang JY, Hsi E, Huang YC, Hsu NC, Chu PY, Chai CY. High LC3 expression correlates with poor survival in patients with oral squamous cell carcinoma. Hum Pathol 2013;44:2558–62.
He Y, Zhao X, Gao J, Fan L, Yang G, Cho WC, et al. Quantum dots-based immunofluorescent imaging of stromal fibroblasts caveolin-1 and light chain 3B expression and identification of their clinical significance in human gastric cancer. Int J Mol Sci 2012;13:13764–80.
Lee YJ, Ha YJ, Kang YN, Kang KJ, Hwang JS, Chung WJ, et al. The autophagy-related marker LC3 can predict prognosis in human hepatocellular carcinoma. PLoS One 2013;8:e81540.
Wada Y, Sun-Wada GH, Kawamura N, Aoyama M. Role of autophagy in embryogenesis. Curr Opin Genet Dev. 2014;27C:60–6.
Zhao H, Yang M, Zhao J, Wang J, Zhang Y, Zhang Q. High expression of lc3b is associated with progression and poor outcome in triple-negative breast cancer. Med Oncol. 2013;30:475.
Wang TT, Cao QH, Chen MY, Xia Q, Fan XJ, Ma XK, et al. Beclin 1 deficiency correlated with lymph node metastasis, predicts a distinct outcome in intrahepatic and extrahepatic cholangiocarcinoma. PLoS One. 2013;8:e80317.
Kuma A, Matsui M, Mizushima N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: Caution in the interpretation of LC3 localization. Autophagy 2007;3:323–28.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81372374), the Combination Project of Production, Education and Research from Guangdong Province and Ministry of Education (No. 2012B091100460), and the Science and Technology Planning Project of Guangdong Province 2011B031800076 (to Q. Lin), 2011B031800014 (to M. Dong), and 2012B031800259 (to J. Wen).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dong-Hao Wu and Chang-Chang Jia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, DH., Jia, CC., Chen, J. et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumor Biol. 35, 12225–12233 (2014). https://doi.org/10.1007/s13277-014-2531-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2531-7