Abstract
It has been reported that protein phosphatase, Mg2+/Mn2+ dependent, 1D (PPM1D) plays an important role in cancer tumorigenesis. However, the clinical and functional significance of PPM1D expression has not been characterized previously in non-small cell lung cancer (NSCLC). The purpose of this study was to assess PPM1D expression and to explore its contribution to NSCLC. We examined PPM1D messenger RNA (mRNA) expression in 53 NSCLC tissues and matched adjacent noncancerous tissues by quantitative reverse transcription PCR (qRT-PCR). Furthermore, the PPM1D protein expression was analyzed by immunohistochemistry in 157 NSCLC samples. The relationship between PPM1D expression and clinicopathological features was analyzed by appropriate statistics. Kaplan-Meier analysis and Cox proportional hazards regression models were used to investigate the correlation between PPM1D expression and prognosis of NSCLC patients. The relative mRNA expression of PPM1D was significantly elevated in NSCLC tissues as compared with adjacent noncancerous tissues (P < 0.001). The high expression of PPM1D in NSCLC tissues was significantly correlated with tumor grade (P = 0.006), tumor size (P = 0.017), clinical stage (P = 0.001), and lymph node metastases (P = 0.002). Kaplan-Meier survival analysis revealed that high PPM1D expression correlated with poor prognosis of NSCLC patients (P < 0.001). Multivariate analysis showed that PPM1D expression was an independent prognostic marker for overall survival of NSCLC patients. In conclusion, PPM1D plays an important role in the progression of NSCLC. PPM1D may potentially be used as an independent biomarker for the prognostic evaluation of NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common intrathoracic malignancies throughout the world due to its late clinical presentation and rapid progression [1, 2]. Despite some advances in early detection and radical surgical resection combined with adjuvant therapy over the recent decade, the prognosis of NSCLC patients remain relatively poor [3]. It is generally acknowledged that genetic heterogeneity and environmental factors lead to tumorigenesis of lung cancer simultaneously. Therefore, identification of novel prognostic biomarkers with possible therapeutic and prognostic relevance is important to optimize the treatment of NSCLC.
Protein phosphatase, Mg2+/Mn2+ dependent, 1D (PPM1D) is a member of the protein phosphatase 2C family and is involved in a wide range of physiological functions, including cell signaling, apoptosis, and cell cycle [4]. Overexpression of PPM1D was shown to complement several oncogenes for cell transformation in vitro, indicating that PPM1D possesses obvious oncogenic properties [5, 6]. Recently, PPM1D was also implicated in DNA repair and overexpression of this gene was suggested to lead to increased genomic instability and thereby increased mutation frequency [7]. PPM1D amplification is found in several solid tumors, including medulloblastoma, neuroblastoma, pancreatic adenocarcinoma, ovarian clear cell carcinoma, and breast cancer [8–12]. For breast cancer, ovarian cancer, and lung adenocarcinoma, PPM1D overexpression is associated with poor survival [11, 13, 14]. Recently, Li et al. [15] reported that PPM1D silencing by RNA interference inhibits the proliferation of lung cancer cells. These results suggest that PPM1D might play an important role in promoting tumorigenesis. However, there are few reports concerning the association between PPM1D expression and clinicopathological characteristics or patient survival in NSCLC. In this study, we therefore assessed the expression of PPM1D in a series of NSCLC specimens and investigated its associations with clinicopathologic parameters and overall survival in patients with NSCLC.
Materials and methods
Sample collection
This study was approved by the Research Ethics Committee of Yantai Yuhuangding Hospital Affiliated to Qingdao University. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to ethical and legal standards. The selection criteria for patients with NSCLC were as follows: (1) pathologically confirmed patients with NSCLC and (2) patients who had no previous history of other cancers. All patients were diagnosed and treated at the Yantai Yuhuangding Hospital Affiliated to Qingdao University in Shandong, China, from November 2002 to June 2008. All subjects underwent clinical examination; plain chest radiograph; CT scan of the chest, upper abdomen, and brain; fiber-optic bronchoscopy; and bone scan. Tumor stage was determined according to the 2009 tumor-node-metastasis (TNM) staging classification system. The duration of follow-up was calculated from the date of surgery to death or last follow-up, and patients were excluded if they had incomplete medical records or inadequate follow-up. For quantitative reverse transcription PCR (qRT-PCR), 53 pairs of fresh NSCLC and matched adjacent normal tissue specimens were collected from patients who underwent surgery in the Yantai Yuhuangding Hospital Affiliated to Qingdao University. The fresh tissue specimens were collected and immediately placed in liquid nitrogen and then stored at −80 °C until RNA isolation. For immunohistochemistry, archived and paraffin-embedded samples were obtained from 157 patients with a diagnosis of NSCLC who underwent surgical resection in the Yantai Yuhuangding Hospital Affiliated to Qingdao University.
RNA extraction and real-time quantitative RT-PCR
Total RNA was extracted from tissues lysate using a TRIzol kit (Invitrogen, Carlsbad, CA, USA), and complementary DNA (cDNA) was subsequently synthesized from total RNA using an Omniscript RT kit (Qiagen, Valencia, CA, USA) following the supplier’s instructions. For detecting the messenger RNA (mRNA) level of PPM1D, real-time quantitative RT-PCR was conducted on the Mastercycler ep realplex (Eppendorf 2 S, Hamburg, Germany). A 25-μl reaction mixture contained 1 μl of cDNA from samples, 12.5 μl of 2× Fast EvaGreen™ qPCR Master Mix, 1 μl primers (10 mM), and 10.5 μl of RNase/DNase-free water. PCR procedure conditions are as follows: incubation at 96 °C for 2 min, 40 cycles at 96 °C for 15 s and 60 °C for 1 min. The threshold cycle (Ct) value was defined as the cycle number at which the fluorescence intensity reached a certain threshold where amplification of each target gene was within the linear region of the reaction amplification curves. Relative expression level for each target gene was normalized by the Ct value of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (internal control) using a 2−ΔΔCt relative quantification method. The sequences of the primers for PPM1D are as follows: PPM1D forward, 5′-CTTGCCAGCCATGGCACGG-3′, and PPM1D reverse, 5′-TGGACCTGACTTTAGGCACCGTCG-3′. The GAPDH gene served as an internal control.
Immunohistochemistry analysis
Immunohistochemistry was performed according to standard protocols [16, 17]. In brief, formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated under the regular condition. Subsequently, the sections were heated in ethylene diamine tetraacetic acid (EDTA, pH 8.0) for antigen retrieval. After the inactivation of endogenous peroxidase, the sections were treated with normal goat serum for blocking and then immunostained with anti-PPM1D polyclonal antibody (1:500 dilution; Santa Cruz Biotechnology, USA) overnight at 4 °C. Negative controls using rabbit serum collected before immunization were also incubated in parallel. Then, horseradish peroxidase-conjugated goat anti-rabbit IgG (ZSGB-BIO, China) was added as the secondary antibody. Immunoreactivity was visualized with 3,3′-diaminobenzidine (DAB) (Maixin Biotechnology, China) followed by hematoxylin counterstain. The degree of immunostaining was evaluated by two independent observers who were blind to the clinical data of the patients. The percent of positive cells was scored as ≤10 % = 0, >10 to ≤25 % = 1, >25 to ≤50 % = 2, >50 to ≤75 % = 3, or >75 % = 4. The intensity of nuclear staining was scored as 0 = negative, 1 = weak, 2 = moderate, or 3 = strong. The two scores were then multiplied to calculate the final score. PPM1D expression was considered low if the final score was equal to or less than 4; otherwise, PPM1D expression was considered high.
Statistical analysis
Statistical significance was defined as P < 0.05. Relationships between PPM1D expression and antibody response with clinicopathological parameters were tested by χ 2 test or Fisher’s exact test. Overall survival rates were calculated using the Kaplan-Meier method, and differences in survival curves were compared using the log-rank test. Multivariable regression analysis was performed to detect prognostic factors using Cox proportional hazards regression models, and variables with a two-sided P value of <0.05 were entered into the final model.
Results
Evaluation of PPM1D mRNA expression by qRT-PCR
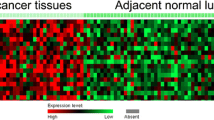
To examine the expression of PPM1D in NSCLC tissues, we firstly detected PPM1D mRNA expression in 53 paired primary NSCLC tissues and corresponding normal tissues using qRT-PCR. In 41 of 53 cases, PPM1D mRNA was upregulated in the primary NSCLC tissues, compared to the adjacent normal tissues. The results showed that the mRNA expression of PPM1D was significantly increased in NSCLC tissues compared with adjacent noncancerous tissues (2.267 ± 0.765 vs. 1.063 ± 0.442, P < 0.001, Fig. 1). Thus, PPM1D may play important roles in the progression of NSCLC.
Association between PPM1D expression and clinicopathological variables
The relationship between high PPM1D expression and clinicopathological variables of 157 NSCLC patients is shown in Table 1. According to the PPM1D immunoreactive intensity, 74 (47.1 %) patients were classified as low-PPM1D group and 83 (52.9 %) patients were classified as high-PPM1D group (Fig. 2). Moreover, the χ 2 test was applied to assess the association between PPM1D protein level and different clinicopathological variables (Table 1). As a result, high PPM1D expression was significantly related to tumor grade (P = 0.006), tumor size (P = 0.017), clinical stage (P = 0.001), and lymph node metastases (P = 0.002). In contrast, no significant association was discovered between PPM1D expression and other clinical parameters, such as tumor histology, age, gender, and smoking history (all P > 0.05).
Survival analysis
As expected, the overexpression of PPM1D protein showed a significant correlation with the decreased 5-year survival rate of 157 NSCLC patients (P < 0.001, Fig. 3). In addition, certain NSCLC clinical prognostic factors, such as differentiation, lymph node metastasis, and advanced TNM stage classification, also showed a statistically significant correlation with the decreased 5-year survival rate based on Cox regression univariate analysis (Table 2). All of the above significant factors were enrolled in a multivariate analysis. The results indicated that PPM1D expression was one of the independent prognostic factors, along with tumor grade, tumor size, clinical stage, and lymph node metastases (Table 2).
Discussion
During the past two decades, due to the histological and phonotypical heterogeneity of NSCLC, the identification of more effective novel prognostic markers has become of vital importance in the selection of high-risk patients with NSCLC [18]. Effective genetic markers can further stratify NSCLC into effective treatment subgroups. Prognosis may improve with a focus on the molecular markers of risk, which may lead to improved detection or treatment strategies [19, 20].
The serine-threonine protein phosphatase PPM1D has been demonstrated to have clear oncogenic properties and to play an important role in tumorigenesis [5, 6]. PPM1D is involved in the regulation of several key cellular processes, such as cell cycle and apoptosis, which are vital for tumor development and progression. Moreover, studies by other researchers have also linked PPM1D upregulation to cancer development in several types of solid tumors, including medulloblastoma, neuroblastoma, pancreatic adenocarcinoma, ovarian clear cell carcinoma, and breast cancer [8–12]. Furthermore, the overexpression of PPM1D protein has now been strongly linked to poor prognosis in cancer, such as breast cancer, ovarian cancer, and lung adenocarcinoma [11, 13, 14]. These results suggest that PPM1D might play an important role in promoting tumorigenesis. However, relatively little is known about the expression and clinical significance of PPM1D in NSCLC. In this study, we therefore assessed the expression of PPM1D in a series of NSCLC specimens and investigated its associations with clinicopathologic parameters and overall survival in patients with NSCLC.
In the present study, we investigated the expression of PPM1D in NSCLC and corresponding normal tissues and then analyzed the relationships between PPM1D expression and other clinicopathologic variables in NSCLC. The findings of our study showed that the relative mRNA expression of PPM1D was significantly elevated in NSCLC tissues as compared with adjacent noncancerous tissues. In addition, high PPM1D expression in NSCLC was significantly correlated with tumor grade (P = 0.006), tumor size (P = 0.017), clinical stage (P = 0.001), and lymph node metastases (P = 0.002). Kaplan-Meier analysis in this study showed that high PPM1D expression significantly predicted decreased 5-year overall survival. To eliminate the impact of mixed factors correlated with prognosis on statistical analysis, the Cox regression multivariate analysis was performed to determine independent prognostic factors. Multivariate analysis showed that PPM1D expression was an independent prognostic marker for the overall survival of NSCLC patients. Our survival analysis demonstrated that the expression of PPM1D protein could be a useful diagnostic marker for patients with NSCLC. As stated above, our results implied that PPM1D protein may have an antitumor effect on carcinogenesis and tumor progression of NSCLC.
In conclusion, our present study demonstrated that the overexpression of PPM1D was positively associated with the progression and poor prognosis in patients with NSCLC, which indicated that PPM1D might serve as a valuable prognosis marker for NSCLC patients. However, the possible underlying mechanisms for its participation in NSCLC progression are still unclear. Therefore, the next step that we will take will be further research of the cell signaling pathway in order to gain a better understanding of molecular mechanism in this field.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi:10.1200/JCO.2005.05.2308.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi:10.3322/caac.20006.
Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25(34):5506–18. doi:10.1200/JCO.2007.14.1226.
Lu G, Wang Y. Functional diversity of mammalian type 2C protein phosphatase isoforms: new tales from an old family. Clin Exp Pharmacol Physiol. 2008;35:107–12. doi:10.1111/j.1440-1681.2007.04843.x.
Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–5. doi:10.1038/ng894.
Li J, Yang Y, Peng Y, Austin RJ, van Eyndhoven WG, Nguyen KC, et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet. 2002;31:133–4. doi:10.1038/ng888.
Lu X, Nguyen TA, Appella E, Donehower LA. Homeostatic regulation of base excision repair by a p53-induced phosphatase: linking stress response pathways with DNA repair proteins. Cell Cycle. 2004;3:1363–6.
Castellino RC, De Bortoli M, Lu X, Moon SH, Nguyen TA, Shepard MA, et al. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol. 2008;86:245–56. doi:10.1007/s11060-007-9470-8.
Saito-Ohara F, Imoto I, Inoue J, Hosoi H, Nakagawara A, Sugimoto T, et al. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res. 2003;63:1876–83.
Loukopoulos P, Shibata T, Katoh H, Kokubu A, Sakamoto M, Yamazaki K, et al. Genome-wide array-based comparative genomic hybridization analysis of pancreatic adenocarcinoma: identification of genetic indicators that predict patient outcome. Cancer Sci. 2007;98:392–400. doi:10.1111/j.1349-7006.2007.00395.x.
Tan DS, Lambros MB, Rayter S, Natrajan R, Vatcheva R, Gao Q, et al. PPM1D is a potential therapeutic target in ovarian clear cell carcinomas. Clin Cancer Res. 2009;15:2269–80. doi:10.1158/1078-0432.CCR-08-2403.
Yu E, Ahn YS, Jang SJ, Kim MJ, Yoon HS, Gong G, et al. Overexpression of the wip1 gene abrogates the p38 MAPK/p53/Wip1 pathway and silences p16 expression in human breast cancers. Breast Cancer Res Treat. 2007;101:269–78.
Rauta J, Alarmo EL, Kauraniemi P, Karhu R, Kuukasjärvi T, Kallioniemi A. The serine-threonine protein phosphatase PPM1D is frequently activated through amplification in aggressive primary breast tumours. Breast Cancer Res Treat. 2006;95:257–63.
Satoh N, Maniwa Y, Bermudez VP, Nishimura K, Nishio W, Yoshimura M, et al. Oncogenic phosphatase Wip1 is a novel prognostic marker for lung adenocarcinoma patient survival. Cancer Sci. 2011;102:1101–6. doi:10.1111/j.1349-7006.2011.01898.x.
Zhang C, Chen Y, Wang M, Chen X, Li Y, Song E, et al. PPM1D silencing by RNA interference inhibits the proliferation of lung cancer cells. World J Surg Oncol. 2014;12:258. doi:10.1186/1477-7819-12-258.
Tu L, Liu Z, He X, He Y, Yang H, Jiang Q, et al. Over-expression of eukaryotic translation initiation factor 4 gamma 1 correlates with tumor progression and poor prognosis in nasopharyngeal carcinoma. Mol Cancer. 2010;9:78. doi:10.1186/1476-4598-9-78.
Liu Z, Li L, Yang Z, Luo W, Li X, Yang H, et al. Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer. 2010;10:270. doi:10.1186/1471-2407-10-270.
Zhong X, Li M, Nie B, Wu F, Zhang L, Wang E, et al. Overexpressions of RACK1 and CD147 associated with poor prognosis in stage T1 pulmonary adenocarcinoma. Ann Surg Oncol. 2013;20:1044–52. doi:10.1245/s10434-012-2377-4.
Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–82. doi:10.1038/nature08136.
Niu Y, Liu T, Tse GM, Sun B, Niu R, Li HM, et al. Increased expression of centrosomal alpha, gamma-tubulin in atypical ductal hyperplasia and carcinoma of the breast. Cancer Sci. 2009;100:580–7. doi:10.1111/j.1349-7006.2008.01075.x.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Hua Yang, Xiao-Yu Gao, and Ping Li contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Yang, H., Gao, XY., Li, P. et al. PPM1D overexpression predicts poor prognosis in non-small cell lung cancer. Tumor Biol. 36, 2179–2184 (2015). https://doi.org/10.1007/s13277-014-2828-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2828-6