Abstract
Background
The six-transmembrane protein of the prostate 2 (STAMP2) is crucial for damage-induced inflammation. Excessive inflammatory responses in microglia have been discovered as the critical factor in triggering neurological diseases. However, inconclusive evidence suggests that STAMP2 alleviates neuroinflammatory diseases by regulating the inflammatory state of microglia.
Objectives
To ascertain whether the favorable effects of STAMP2 on neuroinflammation act through microglia.
Results
STAMP2 served as an anti-inflammatory modulator to inhibit the production of inflammatory and reactive oxygen species (ROS) factors and attenuated neurotoxicity. Downregulation of STAMP2 increased the levels of IL-1β, IL-6, ROS, and glutamate, and exogenous STAMP2 knock-in rescued both the exacerbated inflammation and oxidative stress induced by STAMP2 knockdown in BV-2 cells. Mechanistic study showed that STAMP2 siRNA accelerated the expression of p-P65 and p-IkBα, whereas STAMP2OE interrupted the NF-kB translocation. In addition, STAMP2 could reduce neurotoxicity by promoting cell survival and suppressing apoptosis.
Conclusion
These findings revealed that STAMP2 is involved in regulating the LPS-NF-kB signal pathway to modulate inflammatory processes in microglia cells, which could represent a possible therapeutic therapy for neurodegenerative disorders.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation is recognized as a key pathogenic process in a variety of neurodegenerative diseases (Floyd 1999), such as Parkinson’s disease (PD) (Kim and Joh 2006), Alzheimer’s disease (AD) (Krause and Muller 2010; Lee et al. 2012), multiple sclerosis (Koning et al. 2007), and amyotrophic lateral sclerosis (ALS) (Casula et al. 2011). As resident macrophages in the central nervous system (CNS), over-activated microglia are the hallmark of neural inflammation in major neurodegenerative disorders (Puigdellivol et al. 2020).
Typically, traditionally activated M1 microglia protect neurons and promote brain recovery by removing protein aggregates and cell debris (Hu et al. 2015). However, alternatively activated M2 microglia trigger the constant release of pro-inflammatory mediators, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, reactive oxygen species (ROS), and excitatory amino acids in response to the continuous LPS stimulation. These inflammatory factors could cause neurotoxicity and lead to cell apoptosis through an inflammation cascade response (Dohi et al. 2010; Wang et al. 2014; Yuan et al. 2016). Therefore, alleviating microglial neuroinflammation and neurotoxicity may be promising treatments for neurodegenerative diseases.
The six-transmembrane protein of the prostate 2 (STAMP2), also referred to as the six-transmembrane epithelial antigen of prostate 4 (STEAP4), has been recognized as an anti-inflammatory regulator of leukocyte-driven inflammation in metabolism (Wellen et al. 2007), atherosclerosis (ten Freyhaus et al. 2012), and pulmonary vascular reconstruction (Batool et al. 2020). Through the Rossman Fold oxidoreductase domain, STAMP2 controls oxidative stress and co-enzyme (NAPDH) (ten Freyhaus et al. 2012; Wellen et al. 2007; Zhou et al. 2013), which is involved in oxidizing NADPH to NADP+, thereby delivering electrons across the plasma membrane to metal ions (Ohgami et al. 2006). NADPH accumulates in the cytosol with STAMP2 dysfunction, exacerbating aggressive inflammatory responses and nuclear factor kappa B (NF-kB) translocation (ten Freyhaus et al. 2012). Furthermore, STAMP2 is involved in the secretion of inflammatory cytokines via the LPS-induced NF-kB signaling pathway, such as IL-1β, IL-6, TNF-α, and MCP-1 (Batool et al. 2020). Moreover, previous research has demonstrated that STAMP2 is associated with the development of neurological diseases. Specifically, the increased morbidity of autoimmune encephalomyelitis is correlated with STAMP2 expression in Th17 cells (Batool et al. 2020). Schwann cells are susceptible to palmitate-induced lipoapoptosis via ROS-mediated downregulation of STAMP2 (Lee et al. 2018). Based on these analyses, further studies are required to determine whether the favorable effects of STAMP2 on neuroinflammation act through microglia.
In this study, we found that the knockdown of STAMP2 exacerbated neural inflammation, oxidative stress, and glutamate release induced by LPS in BV-2 cells via regulating the activity of NF-κB-related proteins. In addition, STAMP2 alleviated microglia neurotoxicity.
Methods
Reagents
LPS (Escherichia coli 055:B5) was supplied by Solarbio (Beijing, China). Moreover, CCK-8 (#CA1210), Reactive Oxygen Species Assay Kit (#CA1410), d-lactate dehydrogenase (LDH) activity Assay Kit (#BC5280), and TUNEL Apoptosis Assay Kit (#T2190) were purchased from Solarbio (Beijing, China), while Glutamate Oxidase Assay Kit (#MAK170) was obtained from Sigma-Aldrich (Darmstadt, Germany). IL-1β (#ab229440) and IL-6 (#ab222503) ELISA kits were obtained from Abcam (Cambridge, the United Kingdom). Dulbecco’s Modified Eagle’s Medium (DMEM, 12800017), fetal bovine serum (FBS, 10099-141), Penicillin–Streptomycin (15140122), and phosphate-buffered saline solution (PBS, 11965092) were acquired from Gibco. Abcam provided antibodies against STAMP2 (#ab140951, 1:1000), IL-1β (#ab234437, 1:1000), IL-6 (#ab290735, 1:1000), p65 (#ab32536, 1:1000), IkBα (#ab32518, 1:1000), p-P65 (#ab76302, 1:1000), p-IkBα (#ab133462, 1:1000), NADPH (#ab154244, 1:1000). From Cell Signaling Technology, anti-mouse (#4410, 1:10000) peroxidase-conjugated secondary antibodies were obtained.
Preparation of STAMP2 knockdown or knock-in
Lipofectamine 2000 reagent (0.5 μL, Invitrogen) was utilized for transfection. Before transfection, 2 × 105 cells were plated in 12-well plates for at least 12 h. 2 μg of STAMP2 SiRNA or negative control (NC) was transfected into BV-2 cells. The subsequent primers were applied for transfection: STAMP2, forward 5′-GCA GCA UCC AAG UCU GAC ATT-3′ and reverse 5′-UGU CAG ACU UGG AUG CUG CTT3-3′; NC, forward 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and reverse 5′-ACG UGA CAC GUU CGG AGA ATT3-3′.
For STAMP2 overexpression, the primers used to amplify STAMP2 were: 5′-CGC GCT CGA GGC CAC CAT GGA GAA AAC TTG TAT AGA-3′ and 5′-CGA AGG CGG CCG CCT AGT GTT TTG AGT TCC TTT CC-3′.
The culture medium should be replaced after 6 h of incubation. Following that, cells were examined using a laser scanning confocal microscope (Leica TCS SP2; Leica) to calculate the transfection efficiency. After 24 h of transfection, cells were cultivated with DMEM (10% FBS) for 16 h and harvested.
Cell lines and culture conditions
The murine microglial cell line BV-2 (GDC0311) was acquired from the China Center for Type Culture Collection (Wuhan, China), and the hippocampal cell line HT22 (SCC129) was purchased from the Cell Culture Center at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Peking, China). BV-2 and HT22 cells were cultured in DMEM (Gibco, 12800017), added with 10% FBS (Gibco, 10099-141), 10,000 U/mL Penicillin–Streptomycin (Gibco, 15140122). All the cells were maintained at 37 ℃ in a humidity chamber with 5% CO2. To induce cell inflammation, we used LPS (1 µg/mL) treated BV-2 cells for 12 or 24 h. Then, the CM from LPS-stimulated BV-2 cells pretreated with STAMP2 knockdown or knock-in was collected and transferred to HT22 cultures.
Western blot analysis
STAMP2, p65, IkBα, p-P65, p-IkBα, and NADPH expressions in BV-2 cells were analyzed by western blotting. After being extracted by RIPA buffer (Beyotime, Shanghai, China), the protein debris was eliminated by centrifuging (Thermo Fisher, 75004061) at 12,000 rpm for 10 min at 4 °C. Then, protein quantification was determined using a bicinchoninic acid kit (BCA; Beyotime, Shanghai, China). After denaturing proteins, equivalent quantities (10 μg) of proteins were separated according to molecular weight by electrophoresis and then transferred to PVDF membranes (LMAI Bio, LM1136). Membranes were blocked for 1 h at room temperature with 5% nonfat dry milk. Then, the membrane was incubated with primary antibodies overnight, washed by TBST, and treated with corresponding secondary antibodies for 1 h. Afterward, specific protein bands in membranes were observed using an ECL detection system (GE Healthcare, Piscataway, USA). All experiments were repeated three times.
Enzyme-linked immunosorbent assay (ELISA)
After LPS treatment for 24 h, the culture media from microglia with STAMP2 knockdown or knock-in were harvested and centrifuged. IL-1β and IL-6 levels were assessed using ELISA kits in accordance with the manufacturer’s instructions (Lai et al. 2005). Absorbance was calculated at 450 nm through a microplate reader (Bio-Rad, Hercules, USA).
ROS measurement
Microglia BV-2 cells were grouped based on the experimental design and placed in 96-well plates (10 wells per group) with 5 × 105 cells/mL for ROS measurement. The cells were treated with 10 µM DCFH-DA in DMEM for 60 min at 37 °C in the dark, then rinsed twice with DMEM. Each group’s fluorescence was measured using a fluorescence microplate reader (BioTek, Vermont, USA) set to 484 nm for excitation and 530 nm for emission. The data were reported as a percentage of the intensity of fluorescence relative to the control.
Measurement of glutamate release
Microglia cells were transfected with STAMP2 knockdown or knock-in, then stimulated with 1 µg/mL LPS to assess the impact of STAMP2 on LPS-induced glutamate release. Glutamate release was measured using a Glutamate assay kit after 24 h of incubation. According to the protocol, add 50 µL of the Amplex Red reagent to each well (10 wells per group), incubating the cells for 30 min at room temperature in the dark, with the untreated cells acting as the control. Measurements of fluorescence were taken with excitation at 530 nm and emission at 590 nm in a microplate reader (BioTek, Vermont, USA), and the amount of glutamate released (µM) was then determined by standard curve.
Cell viability
In 96-well plates with 100 μL of 10% FBS-supplemented DMEM, 2.5 × 104 cells per well were plated. After reaching 70% confluency, cells were incubated with the medium from treated microglia for 24 h. Then, cells were slightly washed twice using PBS after the growth media was discarded. Consequently, the Cell Counting Kit-8 (CCK-8, Abcam, Cambridge, UK) was used to assess cell viability under protocol and using the microplate reader (Bio-Rad, Hercules, USA) to evaluate cell absorbance in each well at 450 nm. The experiment was repeated in triplicate.
Lactate dehydrogenase (LDH) release assay
A 96-well plate containing hippocampal HT22 cells at a density of 1 × 106 cells per mL. After 90% confluence, the medium was discarded, and the cells were washed once with PBS. The stimulation medium from treated BV-2 cells was given to culture HT22 cells for 24 h. Each well was added with 150 µL of PBS-diluted LDH release solution and incubated for one hour. After centrifuging at 1000 rpm for 5 min, 120 µL of supernatant from each well was moved into a new 96-well plate for analysis. Each well then obtained a 60 µL LDH test reagent before being mixed and cultivated in the dark at room temperature for 30 min. Using a microplate reader (BioTek, Vermont, USA), the absorbance was recorded at 490 nm. The data were displayed as a cytotoxicity percentage in relation to the control.
Flow cytometry assay
The Apoptosis Detection Kit (eBioscience, 88-8005-72) was used to quantify apoptosis cells. In 6-well plates, HT22 cells (2 × 105/well) were transplanted, then treated with BV-2 medium from different groups for 24 h. After being resuspended with binding buffer, the cells were supplementary with FITC Annexin V and PI for 20 min and evaluated by the FACS-Calibur flow cytometer (BD, USA). The experiment was done in triplicate.
Statistical analysis
All data were statistically analyzed using SPSS 22.0 (IBM, Armonk, USA) and Prism5.0 software (GraphPad Software, San Diego, USA). Significant differences were determined using the student’s t test. Three distinct experiments’ results were provided as the mean ± standard deviation (SD). P < 0.05, P < 0.01, and P < 0.001 were regarded as statistically significant.
Results
Knockdown of STAMP2 aggravated LPS-induced inflammation in BV-2 cells
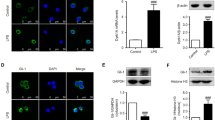
In vitro, LPS-stimulated BV-2 cells are widely used as a neuroinflammation model (Lelakova et al. 2020). The expression of STAMP2 was detected at 0, 12, and 24 h after LPS (1 μg/mL) stimulation in BV-2 microglia cells. In addition, to explore the impact of STAMP2 on BV-2 microglia treated by LPS, small interfering RNA (siSTAMP2) or overexpression vector (STAMP2OE) were transfected into cells before LPS treatment. STAMP2 expression was downregulated in BV-2 cells following treatment with LPS in a time-dependent manner (Fig. 1A). Intriguingly, these effects were aggravated by siSTAMP2 transfection, whereas they were eliminated by STAMP2 overexpression (Fig. 1B).
Knockdown of STAMP2 aggravated LPS-induced inflammation in BV-2 cells. A Stimulated with LPS (1 μg/mL), then measured the expression levels of STAMP2 in BV-2 microglia cells at 0, 12, and 24 h. B STAMP2 siRNA (siSTAMP2), overexpression vector (STAMP2OE), or control-siRNA (si-NC) were transfected into BV-2 and then exposed to LPS (1 μg/mL) for 24 h. The western blot assays were used to analyze the expression of the STAMP2 protein. C The contents of pro-inflammatory factors (IL-1β and IL-6) in the cell supernatant of BV-2 cells were measured using ELISA. *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group. $$$P < 0.001 vs LPS + siSTAMP2-treated groups
Active microglia are significant indication of neuroinflammation and have neurotoxicity (De Caris et al. 2020). In LPS-induced BV-2 cells, we examined the expression of pro-inflammatory cytokines through ELISA and found that LPS stimulation significantly promoted IL-1β and IL-6 production in BV-2 cells (Fig. 1C), demonstrating that LPS stimulation was successful in inducing the inflammatory phenotype of BV-2 microglia. As expected, siSTAMP2 transfection promoted the production of pro-inflammatory cytokines, especially IL-1β and IL-6, induced by LPS activation (Fig. 1C), revealing that siSTAMP2 mediated the escalation of neuroinflammatory processes in LPS-activated microglia.
Knockdown of STAMP2 aggravated LPS-induced oxidative stress and glutamate release in BV-2 cells
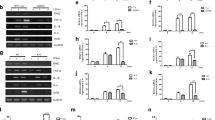
Pro-inflammatory factors, ROS, and glutamate are released by excessively activated microglia, which further contribute to neuron damage (Butovsky et al. 2006; Ding et al. 2021; Miron et al. 2013). The LPS-stimulated BV-2 cells were further studied to examine whether STAMP2 affects the release of ROS and glutamate. We quantified the ROS and glutamate levels in BV-2 microglia with DCFH-DA and ELISA assays, respectively. Indeed, LPS stimulation slightly increased ROS baseline levels and glutamate release compared with control cells (Fig. 2A, B). In addition, a significant increase in ROS and glutamate release were observed in STAMP2 knockdown BV-2 cells, while these were decreased in BV-2 cells transfected with STAMP2OE (Fig. 2A, B), indicating that STAMP2 knockdown is involved in the accumulation of ROS and glutamate-induced by LPS.
Knockdown of STAMP2 aggravated LPS-induced oxidative stress and glutamate release in BV-2 cells. STAMP2 siRNA, STAMP2OE, or si-NC were transfected into BV-2 cells and then exposed to LPS (1 μg/mL) stimulation for 24 h. A In BV-2 microglia, we quantified ROS levels through DCFH-DA fluorescence assay. B The glutamate release was measured with a Glutamate assay kit. **P < 0.01 vs the control group. $P < 0.05, $$$P < 0.001 vs LPS + siSTAMP2-treated groups
STAMP2 inhibited LPS-induced NF-κB activation
Studies have revealed a link between the inflammatory phenotype of microglia and LPS-induced NF-kB activation (Gao et al. 2020). We further assessed the alterations in the LPS-NF-kB pathway. The LPS stimulation enhanced the expression of NF-kB p-P65 and p-IkB in BV-2 cells, detected by Western blot assay (Fig. 3). Simultaneously, the LPS-induced NF-kB activity was further enhanced by siSTAMP2 transfection, which was reflected by dramatically increased p-P65 and p-IkBα (Fig. 3). Nevertheless, the NF-kB pathway activation was suppressed in BV-2 cells transfected with STAMP2OE (Fig. 3). These results suggested that STAMP2 could eliminate LPS-induced neural inflammation by blocking the NF-kB nuclear translocation.
STAMP2 relieves microglial neurotoxicity
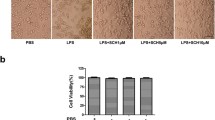
It has been established that activated microglia can mediate neurotoxic effects by producing numerous pro-inflammatory cytokines. To determine whether STAMP2 could protect neurons from neurotoxicity via limiting microglial activation, microglia culture mediums (MCM) treated with LPS (1 µg/mL) for 24 h with or without STAMP2OE plasmid transfection were added to HT22 cells. Neuronal viabilities were evaluated using the CCK-8 assay after HT22 cells were incubated with conditional medium for 24 h. The results showed that the viability of HT22 cells was increased by L-STAMP2-MCM stimulation, while L-MCM caused a significant decrease in HT22 cell viability (Fig. 4A). In addition, the LDH release assay showed that L-MCM markedly enhanced the generation of LDH from HT22 neurons. In contrast, L-STAMP2-MCM reduced LDH compared with L-MCM (Fig. 4B), which suggests that STAMP2 could alleviate the cytotoxic caused by LPS. Similarly, TUNEL results showed that apoptotic rate of HT22 cells was significantly inhibited with MCM from TAMP2OE transfection BV-2 cells, compared with L-MCM (Fig. 4C, D). These results indicated that STAMP2 could attenuate inflammation-related neuronal toxicity in LPS-activated microglia.
STAMP2 relieves microglial neurotoxicity. BV-2 cells were treated with LPS (1 µg/mL) for 24 h, with or without STAMP2OE plasmid transfection. The medium from BV-2 was added to culture HT22 cells. A CCK-8 experiments were carried out to assess cell viability. ***P < 0.001 vs the control group. $$P < 0.01 vs LPS + siSTAMP2-treated groups. B The release of LDH was measured by LDH Assay Kit. ***P < 0.001 vs the control group. $$$P < 0.001 vs LPS + siSTAMP2-treated groups. C, D The cell apoptosis rate of HT22 cells was examined using flow cytometry. ***P < 0.001 vs the control group. $$$P < 0.001 vs LPS + siSTAMP2-treated groups
Discussion
In this study, we discovered that LPS-induced BV-2 cell inflammation and HT22 cell neuronal injury, as demonstrated by the increased production of pro-inflammatory cytokines, ROS, and glutamate, which were restored by STAMP2. Mechanistic studies showed that LPS elicited the activation of the NF-kB signaling pathway. In addition, the LPS-dependent NF-kB activation could be reversed by STAMP2. To gain a deeper understanding, relevant cell experiments were conducted, and the results revealed that LPS-induced microglial inflammation was exacerbated by STAMP2 siRNA, while mitigated by STAMP2OE transfection, as evidenced by the downregulation of IL-1β, IL-6, ROS, and glutamate. These findings indicated that the expression of STAMP2 prevented HT22 cells from LPS-caused cell inflammation and neurotoxicity, which may have significant implications for neuronal degeneration diseases.
Neurodegenerative diseases manifest as pathological changes of apoptosis or necrosis of hippocampal neuronal cells after brain damage caused by various internal and external stimuli, most of which are abnormal stimulation of microglia in the CNS system (Cerbai et al. 2012; Lana et al. 2021). LPS has long been used to construct the in vitro model of neuroinflammatory disease, and LPS stimulation could cause a cascade of inflammatory responses, leading to the influx of inflammatory mediators, excessive ROS responses, and neurotoxicity, thereby resulting in neuronal death (Gui et al. 2021; He et al. 2021; Liu et al. 2021; Sato et al. 2021; Zhuang et al. 2021). Previous research has shown that LPS does not directly harm hippocampus HT22 cells, but instead induces neuroinflammation by triggering abnormally activated microglia (Calvo-Rodriguez et al. 2017). As shown in this study, we successfully developed an inflammation model by treating BV-2 cells with LPS, which triggered the massive release of pro-inflammatory factors, ROS, glutamate, and the phosphorylation of NF-kB-related proteins. Thereafter, we obtained CM from LPS-induced BV-2 cells to culture HT22 cells. Results showed that LPS elicited neuroinflammation in BV-2 cells through upregulating of NF-kB p65 and IkBα nuclear translocation and producing neurotoxicity to HT22 cells, resulting in apoptosis. Therefore, inhibition of LPS-induced cell inflammation may be crucial for the treatment of neurodegenerative diseases.
Accumulating evidence suggests that STAMP2 protects against inflammatory responses. Specifically, as an anti-inflammatory mediator, the lack of STAMP2 expression in mononuclear cells was associated with the advent of obesity and glycemic recognition (Shayo et al. 2022). However, its function in neuroinflammation is unclear. In our study, we noticed a decrease in STAMP2 expression in microglial BV-2 cells over time with LPS stimulation, indicating a role in the regulation of LPS-induced inflammatory responses. To clarify these effects, we transfected BV-2 cells with STAMP2 siRNA or STAMP2OE. The results suggested that downregulation of STAMP2 causes significant increases in IL-1β, IL-6, ROS, and glutamate release, as well as the expression of p-P65 and p-IkBα, which are induced by LPS. In contrast, STAMP2OE attenuated the exaggerated inflammation and oxidative stress of LPS. In addition, CCK-8 and flow cytometry assays indicated that STAMP2 could attenuate microglia inflammation and neurotoxicity, thereby promoting HT22 cell viability and reducing apoptosis. This clarifies the anti-inflammatory effect of STAMP2 on microglia and its protective effect on neuronal cells. Furthermore, studies have reported that certain medicine may impact STAMP2 expression level. For example, cilostazol, as a therapeutic drug, upregulated the expression of STAMP2 in hepatic tissues (Oh et al. 2018). These findings suggested that STAMP2 had the potential to be explored as a new therapeutic target for the prevention and management of neuroinflammatory diseases.
In conclusion, our results revealed that STAMP2 inhibited the NF-kB pathway to reduce the inflammatory response stimulated by LPS in the murine BV-2 cells. Furthermore, STAMP2 relieves the neurotoxicity caused by activated microglia. These findings provide a novel therapeutic target with anti-inflammation and neuroprotective effects on preventing or reducing the development of neurodegenerative disease.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and any raw data can be obtained from the corresponding author upon request.
References
Batool M et al (2020) The six-transmembrane protein Stamp2 ameliorates pulmonary vascular remodeling and pulmonary hypertension in mice. Basic Res Cardiol 115:68
Butovsky O et al (2006) Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31:149–160
Calvo-Rodriguez M et al (2017) Aging and amyloid beta oligomers enhance TLR4 expression, LPS-induced Ca(2+) responses, and neuron cell death in cultured rat hippocampal neurons. J Neuroinflamm 14:24
Casula M et al (2011) Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 179:233–243
Cerbai F et al (2012) The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS ONE 7:e45250
De Caris MG et al (2020) Blueberry counteracts BV-2 microglia morphological and functional switch after LPS challenge. Nutrients 12:1830
Ding L et al (2021) Glutaminase in microglia: a novel regulator of neuroinflammation. Brain Behav Immun 92:139–156
Dohi K et al (2010) Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflamm 7:41
Floyd RA (1999) Neuroinflammatory processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic Biol Med 26:1346–1355
Gao X et al (2020) Beta-naphthoflavone inhibits LPS-induced inflammation in BV-2 cells via AKT/Nrf-2/HO-1-NF-kappaB signaling axis. Immunobiology 225:151965
Gui Y, Sun L, Liu R, Luo J (2021) Pachymic acid inhibits inflammation and cell apoptosis in lipopolysaccharide (LPS)-induced rat model with pneumonia by regulating NF-kappaB and MAPK pathways. Allergol Immunopathol (madr) 49:87–93
He Y et al (2021) Coniferyl aldehyde alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via JAK2-STAT1 pathway in acute pneumonia. Allergol Immunopathol (madr) 49:72–77
Hu X et al (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11:56–64
Kim YS, Joh TH (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med 38:333–347
Koning N, Bo L, Hoek RM, Huitinga I (2007) Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol 62:504–514
Krause DL, Muller N (2010) Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer's disease. Int J Alzheimers Dis 2010:732806. https://doi.org/10.4061/2010/732806
Lai Y, Feldman KL, Clark RS (2005) Enzyme-linked immunosorbent assays (ELISAs). Crit Care Med 33:S433-434
Lana D et al (2021) The emerging role of the interplay among astrocytes, microglia, and neurons in the hippocampus in health and disease. Front Aging Neurosci 13:651973
Lee YJ et al (2012) Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflamm 9:35
Lee SW et al (2018) Palmitate induces lipoapoptosis in Schwann cells through ROS generation-mediated STAMP2 downregulation. Biochem Biophys Res Commun 503:1260–1266
Lelakova V et al (2020) Therapeutic potential of prenylated stilbenoid macasiamenene F through its anti-inflammatory and cytoprotective effects on LPS-challenged monocytes and microglia. J Ethnopharmacol 263:113147
Liu S, Li J, Hu L (2021) MiR-216a-5p alleviates LPS-induced inflammation in the human bronchial epithelial cell by inhibition of TGF-beta1 signaling via down-regulating TGFBR2. Allergol Immunopathol (madr) 49:64–71
Miron VE et al (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218
Oh YJ et al (2018) Cilostazol improves HFD-induced hepatic steatosis by upregulating hepatic STAMP2 expression through AMPK. Mol Pharmacol 94:1401–1411
Ohgami RS, Campagna DR, McDonald A, Fleming MD (2006) The Steap proteins are metalloreductases. Blood 108:1388–1394
Puigdellivol M, Allendorf DH, Brown GC (2020) Sialylation and galectin-3 in microglia-mediated neuroinflammation and neurodegeneration. Front Cell Neurosci 14:162
Sato K, Tatsunami R, Wakame K (2021) Epalrestat suppresses inflammatory response in lipopolysaccharide-stimulated RAW264.7 cells. Allergol Immunopathol (madr) 49:1–8
Shayo SC et al (2022) Dietary obesity and glycemic excursions cause a parallel increase in STEAP4 and pro-inflammatory gene expression in murine PBMCs. Diabetol Int 13:358–371
ten Freyhaus H et al (2012) Stamp2 controls macrophage inflammation through nicotinamide adenine dinucleotide phosphate homeostasis and protects against atherosclerosis. Cell Metab 16:81–89
Wang X et al (2014) Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-kappaB, MAPKs and Akt signaling pathways. Neuropharmacology 79:642–656
Wellen KE et al (2007) Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell 129:537–548
Yuan L et al (2016) Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflamm 13:77
Zhou J et al (2013) Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem 288:30064–30074
Zhuang L et al (2021) Dynamic changes of inflammation and apoptosis in cerebral ischemia-reperfusion injury in mice investigated by ferumoxytol-enhanced magnetic resonance imaging. Mol Med Rep 23:1–13
Acknowledgements
Not applicable.
Funding
No funding was used in this study.
Author information
Authors and Affiliations
Contributions
ZC and JJ designed the study and carried them out, ZC and JJ supervised the data collection, analyzed the data, and interpreted the data, ZC, JJ and QL prepared the manuscript for publication and reviewed the draft of the manuscript. All the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author Zengbian Chen declares that he/she has no conflict of interest; the author Jie Jin declares that he/she has no conflict of interest; the author Qi Lu declares that he/she has no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Jin, J. & Lu, Q. STAMP2 alleviates microglial neurotoxicity by inhibiting LPS-induced NF-κB activation. Mol. Cell. Toxicol. 20, 335–342 (2024). https://doi.org/10.1007/s13273-023-00346-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00346-1