Abstract
Background
Benign prostatic hyperplasia (BPH) is a prevalent and chronic progressive disease in aging males with lower urinary tract symptoms. Shikonin is known as traditional herb extracts, which have the capacity of anti-oxidation, anti-inflammation, and antitumor. However, little is known about the effect of shikonin on BPH.
Objective
The BPH animal model was established by orchiectomy and testosterone propionate injection. After the end of animal model, the weight of prostate and the histologic structure of prostate tissues were examined. ELISA and immunoblotting were performed to detect the levels of steroid hormones, inflammatory cytokines, and oxidative factors as well as proteins. TUNEL was utilized to detect apoptotic cells in prostate tissues.
Results
The administration of shikonin in BPH rats reduced the weight gain of prostate tissues, decreased the levels of DHT, testosterone, and PSA, and also restored the histologic change of prostate tissues. Also, in BPH rats, the IL-6, IL-1β, TNF-α, and MDA levels of prostate tissues were higher than control rats, while shikonin treatment reduced these biochemical changes. There were a lower apoptosis rate and lower expression of Bcl-2 in BPH rats, which was reversed by shikonin treatment. An increase in Nrf2, NQO1, and HO-1 of BPH prostate tissues was induced by shikonin supplement. The NF-κB pathway was activated in BPH prostate tissues, which was then inhibited by shikonin treatment.
Conclusion
Our data revealed that shikonin treatment had a beneficial effect on the inflammation response, apoptotic process, and oxidative stress of BPH via the Nrf2-ARE and NF-κB pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostate hyperplasia (BPH) is one of the most prevalent urologic illnesses in men especially in the elderly (Robert et al. 2018; Madersbacher et al. 2019; Chughtai et al. 2016) and is defined by non-malignant hyperproliferation of stromal and epithelial cells in the prostate (Jiang et al. 2019; Chauhan et al. 2020). Overexpression of growth factors and inflammatory factors leads to an imbalance of prostatic cell growth and death, which contributes to the development of BPH (Chauhan et al. 2020). Furthermore, BPH affects the majority of aging men, who have bothersome lower urinary tract symptoms (Kim et al. 2016; Cornu 2020; Lloyd et al. 2019; Mobley et al. 2015). These symptoms affect quality of life and sleeping patterns, which cause a huge burden on public health. The exact mechanisms underlying BPH are not yet fully elucidated, thereby further study is needed.
It is well known that DNA damage induced by oxidative stress (OS) and OS is prominent in the elderly (Luo et al. 2020; Kudryavtseva et al. 2016), which might be vital for the pathogenesis of BPH as well as other male genital tract disorders. Prostate hyperplasia is connected with prostate cell death and proliferation (Jiang et al. 2019; Chauhan et al. 2020). It has been evidenced that OS has an effect on the progression of BPH (Ercan et al. 2019; Zabaiou et al. 2016). B cell lymphoma-2 (Bcl-2) functions as an anti-apoptotic protein, preventing this programmed cell death, and inhibiting the activation of Bcl-2 associated x protein (Bax) (McDonnell et al. 1996; Ashkenazi et al. 2017). Previous researches reported that Bcl-2 was found to be abundant in BPH, whereas Bax levels were low, resulting in a decrease in apoptosis and hyperplasia of the prostate tissues (Li et al. 2018).
Shikonin is an active substance that is obtained from the roots of Lithospermum erythrorhizon and exhibits a variety of capabilities, including antioxidant, anti-inflammatory, and antitumor functions (Guo et al. 2019a). Several lines of evidence have shown that shikonin treatment alleviates various kinds of diseases, including organ injury (Guo et al. 2019b), autoimmune diseases (Liu et al. 2020), and cancers (Wang et al. 2021a). Although several biological and pharmacological effects of shikonin have been identified, the probable mechanisms of shikonin on BPH are yet unknown.
In this study, the animal model of BPH was applied to explore the effect of shikonin on the treatment of BPH. We found that BPH rats showed hyperproliferation of prostatic cells, overproduction of inflammatory cytokines as well as oxidation products. And high dose of shikonin treatment alleviated the progression of BPH by inhibiting the inflammation and hyperplasia via the nuclear factor erythroid 2-related factor-2 (Nrf2)-antioxidant response element (ARE) and nuclear factor-kappa B (NF-κB) pathway.
Materials and methods
Animal model
Male Sprague–Dawley (SD) rats were supplied from Beijing Huafukang Bioscience Co. Inc (Beijing, China). All procedures performed in the animal trials were approved by the Institutional Animal Care Committee, Suzhou Ninth Hospital affiliated to Soochow University.
Thirty male SD rats were randomly divided into five groups, including one control group, one BPH group, and three BPH groups with shikonin treatment. A bilateral orchiectomy was undergone in BPH groups and two days later testosterone propionate (5 mg/kg; Sigma-Aldrich, St Louis, MO, USA) was injected subcutaneously daily for 4 weeks to establish BPH model (Rho et al. 2020; Song et al. 2021; Zhang et al. 2021). During the testosterone propionate injection period, three groups were treated with three different oral doses (5, 10, and 20 mg/kg) of shikonin. After the establishment of rat model, the prostate tissue and blood of rats were collected.
Hematoxylin and eosin (H and E) and apoptosis staining
The prostate tissues of rats were fixed with 4% paraformaldehyde, decalcification by 0.5 M EDTA, embedded in paraffin blocks, and sectioned at a 4–5 μm thickness. Then, H&E staining was performed on the sections as previous reported (Hata et al. 2020). TUNEL staining was performed on section using a commercial kit (Sigma) as instructed by the manufacturer. Then tissue section was stained with DAPI reagent for nuclear staining. Staining was observed by microscopic (Olympus, Japan) and photographed. Apoptotic cells were positive for TUNEL reagent and counted by the Image J software.
ELISA
The levels of serum steroid hormones (dihydrotestosterone (DHT) and testosterone) and prostate-specific antigen (PSA) were measured using the DHT and testosterone commercial kits (JunYu Biotechnology, Shanghai, China) as directed by the manufacturer. The levels of interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), malonaldehyde (MDA) and superoxide dismutase (SOD) in prostatic tissue homogenates were detected by the IL-6, IL-1β, TNF-α, MDA and SOD commercial kits (JunYu Biotechnology, Shanghai, China) as instructed by the manufacturer.
Western blotting
The tissue homogenates were lysed in RIPA buffer with protease and phosphatase inhibitors. The concentrations of supernatant were determined by BCA (bicinchoninic acid) protein detection kit (Servicebio, Wuhan, China). Protein was loaded in equal amounts, separated by 10% SDS–PAGE, and then transferred to PVDF membranes. The membrane was blocked and then reacted with primary antibodies. The detail information of primary antibodies involved in western blotting experiment is presented in Table 1
Statistical analysis
All data analysis and visualization were carried out using GraphPad Prism software (version 9.0). The mean and standard deviation are used to express the data. A Shapiro–Wilkinson test was carried out to confirm the normality of data. If the data is normally distributed, ANOVA with a Tukey’s post hoc test for multiple comparisons were applied. Otherwise, a Kruskal–Wallis test with a Dunn’s post hoc test for multiple comparisons was performed. Data were considered significant at P < 0.05.
Results
Shikonin has an effect on pathological changes of BPH prostate tissue
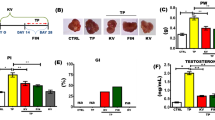
To evaluate the effect of shikonin on BPH rats, the weight of prostate gland was calculated (Fig. 1A). BPH rats had a heavier weight of prostate gland than that of control rats. After treatment with shikonin in BPH rats, the weight of prostate gland was decreased particularly in the 10, and 20 mg/kg dose groups. We further explored the histologic alteration of prostate gland among these groups. H&E staining showed that the prostate epithelial tissue thickness in the BPH group was thicker than that of the control group (Fig. 1B). We found that shikonin treatment at a 10 or 20 mg/kg doses significantly reduced the thickness of prostate epithelial tissue (Fig. 1B). Taken together, these results suggested that shikonin had a suppressive effect on prostate enlargement in BPH rats.
Effect of shikonin on pathological changes of prostate tissue in BPH rats. A Bar graphs showing the weight of prostate gland in the control, BPH, BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. B Bar graph showing the thickness of prostate tissues and representative H and E staining showing the structure of prostate tissue in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. BPH, benign prostate hyperplasia; H and E staining, Hematoxylin and eosin staining
Effect of shikonin on the levels of serum DHT, testosterone, and PSA in BPH rats
The serum levels of DHT, testosterone, and PSA are widely used to evaluate the progression of BPH (Laguna and Alivizatos 2000; Izumi et al. 2013). The BPH group presented the higher levels of DHT, testosterone, and PSA than the control group (Fig. 2). We found that shikonin supplementation downregulated the serum levels of steroid hormones in BPH rats in a dose-dependent manner (Fig. 2). Thereby, these data implied that shikonin treatment ameliorated the progression of BPH rats.
Shikonin inhibits inflammation and oxidative stress of prostate tissue in BPH rats
It is thought that oxidative stress and inflammation play crucial roles in the development of BPH by gradually affecting the structure and function of the prostate (Bostanci et al. 2013; Fibbi et al. 2010). We further examined the levels of inflammatory factors and oxidative factors in the BPH rat model. Shikonin treatment inhibited an increase in IL-6, IL-1β, and TNF-α levels in BPH prostate tissues (Fig. 3A).
Shikonin inhibits inflammation and oxidative stress of prostate tissue in BPH rats. A Bar graphs showing the levels of IL-6, IL-1β, and TNF-α of prostate tissues in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. B Bar graphs showing the levels of MDA and SOD of prostate tissues in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. BPH, benign prostate hyperplasia; MDA, malonaldehyde; SOD, superoxide dismutase; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α
The MDA level of prostate tissues was significantly elevated in the BPH group, while the SOD level of prostate tissues was markedly decreased in the BPH group, indicating enhanced oxidative stress in the pathogenesis or the development of BPH (Fig. 3B). Moreover, after shikonin treatment, the reduced MDA level and the elevated SOD level of prostate tissues in the BPH group was observed (Fig. 3B).
Thus, the alterations of inflammatory factors and markers associated with oxidative stress suggested that shikonin had an effect on the treatment of BPH by regulating inflammation and oxidative stress in prostate tissues.
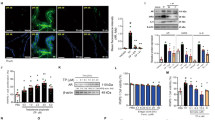
Shikonin promotes apoptosis of prostatic cells in BPH rats
In BPH, apoptosis and growth of male prostatic cells are imbalanced, leading to hyperplasia of the prostate cells (Chauhan et al. 2020). There was a lower apoptosis rate of prostatic cells in BPH than those in controls. Also, the decrease in apoptosis rate in the BPH group was reversed by shikonin treatment (Fig. 4A). Furthermore, an increase in Bcl2 protein levels in prostate tissues was found in the BPH group, which was decreased by shikonin supplementation. Also, shikonin supplement increased the levels of Bax in the BPH group. Overall, these findings indicated that shikonin treatment restored the balance between proliferation and apoptosis in BPH prostatic cells.
Shikonin promotes apoptosis of prostatic cells in BPH rats. A TUNEL test showing the apoptosis rate in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. B Immunoblotting showing the protein levels of Bcl-2 and Bax in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. C Representive graphs of immunoblotting showing the protein levels of Bax and Bcl-2 in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. D BPH, benign prostate hyperplasia; Bax, Bcl-2 associated x protein. Bcl-2, B cell lymphoma-2
Shikonin regulates the Nrf2-ARE and NF-κB pathways in BPH rats
It has been evidenced that oxidative stress has been implicated in the progression of BPH (Ercan et al. 2019; Zabaiou et al. 2016). Consistent with previous findings, we also found the increased protein levels of Nrf2, NAD(P)H Quinone Dehydrogenase-1 (NQO1), and heme oxygenase 1 (HO-1) in BPH prostate tissues, suggesting that the activation of Nrf2-antioxidant response element (ARE) pathway in BPH. Furthermore, it was discovered that the higher levels of Nrf2, NQO1, and HO-1 in prostate tissues following shikonin supplementation counterbalance oxidative stress in BPH (Fig. 5A).
Shikonin regulates the Nrf2-ARE and NF-κB pathways in BPH rats. A Immunoblotting showing the protein levels of Nrf2 and HO-1 in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. B Immunoblotting showing the protein levels of p-p65 and p65 in the control, BPH, and BPH with different dose (5, 10, and 20 mg/kg) shikonin treatment groups. BPH, benign prostate hyperplasia; Nrf2, nuclear factor erythroid 2-related factor-2; ARE, antioxidant response element; NF-κB, nuclear factor-kappa B
As described previously (Bostanci et al. 2013), the disorder of apoptosis and inflammatory responses was found in BPH. NF-κB is a vital transcription factor, which is associated with cell apoptosis and inflammatory responses (Mitchell et al. 2016). We found the activation of NF-κB pathway in BPH prostate tissues, which then was inhibited by shikonin treatment (Fig. 5B).
Altogether, these data supported that shikonin controlled oxidative stress and inflammatory processes through the Nrf2-ARE and NF-κB pathways.
Discussion
Benign prostatic hyperplasia (BPH) is a prevalent and chronic progressive illness that causes lower urinary tract symptoms in aged men (Kim et al. 2016; Cornu 2020; Lloyd et al. 2019; Mobley et al. 2015). Shikonin is known as traditional medicine herb extracts, which have the capacity of anti-oxidation, anti-inflammation, and antitumor (Guo et al. 2019a). However, little is documented about the effect of shikonin on BPH. In this report, by establishment of the BPH rat model, we found that shikonin had a regulatory role in anti-inflammation and anti-oxidation in BPH prostate tissues via the Nrf2-ARE and NF-κB pathways.
BPH is featured by hyperplasia of prostatic epithelial cells. As previously reported, (Rho et al. 2020; Song et al. 2021; Zhang et al. 2021) we build the BPH animal model by testosterone propionate injection. Histologic study had proved that the BPH animal model in this study was established successfully. The anti-apoptotic protein levels such as Bcl-2 were also increased in BPH rats, which was decreased by shikonin treatment. We then found that shikonin could markedly improve the enlargement of prostate tissues resulted by prostatic cells hyperproliferation in BPH. Increasing evidence reported that a change in the steroid hormone levels in the prostate can lead to BPH, since it regulates the growth and death of prostatic cells (Rastrelli et al. 2019; Wang et al. 2021b; Asiedu et al. 2017). Androgens and androgen receptors play a major role in the process of BPH (Izumi et al. 2013). Overproduction of steroid hormones (DHT, testosterone, and PSA) was found in the BPH group, which was inhibited by shikonin treatment. Overall, the administration of shikonin in BPH rats ameliorated the increased prostate weight, biochemical changes including DHT, testosterone, and PSA, and also restored the histologic characteristics of prostate tissues. Thereby, these data indicated that shikonin had a role in the treatment of BPH.
Several researches have evidenced that BPH tissue is prone to inflammation (Bostanci et al. 2013; Fibbi et al. 2010). Shikonin inhibited the upregulation of proinflammatory cytokines (IL-6, IL-1β, and TNF-α), which were found to be elevated in BPH prostate tissues. It is well-established that oxidative stress is implicated in the development of BPH (Ercan et al. 2019; Zabaiou et al. 2016). In BPH rats, there was an imbalance between oxidative stress and anti-oxidation, as shown by the increased synthesis of oxidation products like MDA and the decreased production of antioxidants like SOD in prostate tissues. The supplement of shikonin in BPH rats inhibited the generation of oxidation products and promoted anti-oxidation production. Altogether, these results implied that shikonin had a beneficial effect on the inflammation and oxidative stress of BPH.
We further explored the function of shikonin on the treatment of BPH. The Nrf2 transcription factor plays regulatory role in oxidative stress (Ma 2013). In response to oxidative stress, Nrf2 accumulates in the nucleus and then binds to ARE to activate the transcription of oxidative stress-related genes, like HO-1 and SOD (Zhang et al. 2015). We also found the activation of the Nrf2-ARE pathway in BPH rats. However, this activation could not counterbalance the excessive oxidative stress in BPH. After shikonin supplement, the Nrf2-ARE pathway was enhanced. The transcription factor NF-κB plays a key role in producing inflammatory cytokines and proliferation factors, regulating the transcriptional activation of many fundamental genes (Mitchell et al. 2016). In line with prior reports, the NF-κB pathway was also found to be activated in BPH rats. Furthermore, our results revealed that shikonin treatment suppressed NF-κB pathway activation. As a result, we proposed that shikonin modulated oxidative stress and inflammatory processes through the Nrf2-ARE and NF-κB pathways.
Conclusion
In conclusion, we found the hyperproliferation of prostatic cells, overproduction of inflammatory cytokines, and an imbalance between oxidative stress and anti-oxidation in BPH. Subsequently, our data revealed that shikonin treatment had a beneficial effect on the inflammation response, apoptotic process, and oxidative stress of BPH via the Nrf2-ARE and NF-κB pathways. Thereby, our report presented a novel perspective on the management of BPH.
References
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16:273–284
Asiedu B et al (2017) The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male 20:17–22
Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B (2013) Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol 23:5–10
Chauhan G, Mehta A, Gupta S (2020) Stromal-AR influences the growth of epithelial cells in the development of benign prostate hyperplasia. Mol Cell Biochem 471:129–142
Chughtai B et al (2016) Benign prostatic hyperplasia. Nat Rev Dis Primers 2:16031
Cornu JN (2020) Benign prostatic hyperplasia and urinary incontinence. Prog Urol 30(2):3S10-3S20
Ercan M, Alp HH, Kocaturk H, Bakan N, Gul M (2019) Oxidative stress before and after surgery in benign prostatic hyperplasia patients. Andrologia 51:e13326
Fibbi B, Penna G, Morelli A, Adorini L, Maggi M (2010) Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl 33:475–488
Guo C et al (2019a) Pharmacological properties and derivatives of shikonin-a review in recent years. Pharmacol Res 149:104463
Guo H et al (2019b) Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother 112:108704
Hata J et al (2020) Morphological change and characteristics of myofibroblasts during the growth process of benign prostatic hyperplasia. Int J Urol 27:676–683
Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C (2013) Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol 182:1942–1949
Jiang S, Song CS, Chatterjee B (2019) Stimulation of prostate cells by the senescence phenotype of epithelial and stromal cells: implication for benign prostate hyperplasia. FASEB Bioadv 1:353–363
Kim EH, Larson JA, Andriole GL (2016) Management of benign prostatic hyperplasia. Annu Rev Med 67:137–151
Kudryavtseva AV et al (2016) Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 7:44879–44905
Laguna P, Alivizatos G (2000) Prostate specific antigen and benign prostatic hyperplasia. Curr Opin Urol 10:3–8
Li F et al (2018) BCL-2 and BCL-XL expression are down-regulated in benign prostate hyperplasia nodules and not affected by finasteride and/or celecoxib. Am J Clin Exp Urol 6:1–10
Liu C et al (2020) Anti-angiogenic effect of shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol 260:113039
Lloyd GL, Marks JM, Ricke WA (2019) Benign prostatic hyperplasia and lower urinary tract symptoms: what is the role and significance of inflammation? Curr Urol Rep 20:54
Luo J, Mills K, le Cessie S, Noordam R, van Heemst D (2020) Ageing, age-related diseases and oxidative stress: what to do next? Ageing Res Rev 57:100982
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426
Madersbacher S, Sampson N, Culig Z (2019) Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology 65:458–464
McDonnell TJ, Beham A, Sarkiss M, Andersen MM, Lo P (1996) Importance of the Bcl-2 family in cell death regulation. Experientia 52:1008–1017
Mitchell S, Vargas J, Hoffmann A (2016) Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med 8:227–241
Mobley D, Feibus A, Baum N (2015) Benign prostatic hyperplasia and urinary symptoms: evaluation and treatment. Postgrad Med 127:301–307
Rastrelli G, Vignozzi L, Corona G, Maggi M (2019) Testosterone and benign prostatic hyperplasia. Sex Med Rev 7:259–271
Rho J et al (2020) Asteris Radix et Rhizoma suppresses testosterone-induced benign prostatic hyperplasia in rats by regulating apoptosis and inflammation. J Ethnopharmacol 255:112779
Robert G, De La Taille A, Descazeaud A (2018) Epidemiology of benign prostatic hyperplasia. Prog Urol 28:803–812
Song KH et al (2021) Extracts of phyllostachys pubescens leaves represses human steroid 5-alpha reductase type 2 promoter activity in bhp-1 cells and ameliorates testosterone-induced benign prostatic hyperplasia in rat model. Nutrients 13(3):884
Wang Q, Wang J, Wang J, Ju X, Zhang H (2021a) Molecular mechanism of shikonin inhibiting tumor growth and potential application in cancer treatment. Toxicol Res (camb) 10:1077–1084
Wang YY, Xia K, Wang ZX, Xie H, Xu R (2021b) Osteocyte exosomes accelerate benign prostatic hyperplasia development. Mol Cell Endocrinol 531:111301
Zabaiou N, Mabed D, Lobaccaro JM, Lahouel M (2016) Oxidative stress in benign prostate hyperplasia. Andrologia 48:69–73
Zhang H, Davies KJA, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88:314–336
Zhang J et al (2021) Animal models of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis 24:49–57
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
ZM designed the study, completed the experiment, and supervised the data collection. ZW analyzed and interpreted the data. CX and MJ prepared the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Zheng Ma declares that he has no conflict of interest. Zhenfan Wang declares that he has no conflict of interest. Chen Xu declares that he has no conflict of interest. Minjun Jiang declares that he has no conflict of interest.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Suzhou Ninth Hospital affiliated to Soochow University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Z., Wang, Z., Xu, C. et al. Shikonin alleviates testosterone-induced benign prostatic hyperplasia in rats via the Nrf2-ARE and NF-κB pathway. Mol. Cell. Toxicol. 20, 1–7 (2024). https://doi.org/10.1007/s13273-022-00307-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-022-00307-0