Abstract
Purpose
Muscle atrophy is the prominent clinical feature of cancer-induced cachexia. Zhimu and Huangbai herb pair (ZBHP) has been used since ancient China times and have been phytochemically investigated for constituents that might cause anti-cancer, diabetes, and their complication. In this study, the effects and mechanisms of ZBHP on reversal of muscle atrophy were explored.
Methods
C57BL/6 mice implanted with colon-26 adenocarcinoma were chosen to develop cancer cachexia for evaluating the effects of ZBHP on reversal of muscle atrophy. The body weight, survival time, inflammatory cytokines, and pathological changes of muscle were monitored. In addition, IGF-1/Akt and autophagy pathway members were analyzed to interpret the mechanism of drug response.
Results
The function and morphology of skeletal muscle in cachexia model were significantly disturbed, and the survival time was shortened. Consistently, inflammatory cytokines and muscle atrophy-related atrogin-1, MuRF1, and FOXO3 were significantly increased, and IGF-1/Akt and autophagy signal pathways were depressed. Treatment with ZBHP significantly alleviated tumor-free body weight reduction and cachexia-induced changes in cytokines and prolonged survival. ZBHP treatment not only inhibited the muscle atrophy-related genes but also activated the IGF-1/Akt and autophagy signal pathways to facilitate the protein synthesis.
Conclusions
The results revealed that ZBHP treatment could inhibit the muscle atrophy induced by cancer cachexia and prolong the survival time, and ZBHP may be of value as a pharmacological alternative in treatment of cancer cachexia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer cachexia is a complex metabolic disorder characterized by body weight loss, fatigue, weakness, loss of skeletal muscle, and adipose tissue [11]. Muscle protein wasting in cancer cachexia is one of the most critical problem and contributing to increased morbidity in patients. In fact, cachexia accounts for high morbidity and mortality in up to 80 % with advanced cancer patients, and almost 20 % of the death is the direct result of cachexia-induced muscle wasting [31]. Despite the fact that cachexia has been well investigated for decades, the underlying mechanisms of skeletal muscle atrophy in cancer cachexia are poorly understood, and there are currently no effective treatment strategies for it.
Various tumor-derived factors have been reported to influence muscle protein balance through several signal pathways, including pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IFN-γ [2]. TNF-α and IL-6 are the most important regulators of muscle mass loss [5, 31]. Anti-IL-6 and TNF therapy to tumor-bearing rats decreased protein degradation rates in skeletal muscle [8, 28, 34]. Inflammatory cytokine-driven atrophy-related ubiquitin ligases activation is central in muscle atrophy [1, 12], and inhibition of inflammatory cytokines levels has enormous potential for the prevention and treatment of muscle atrophy. Furthermore, FOXO3 signal was seen to be a principal protein degradation pathway in cancer cachexia [23]. The activation of FOXO3 in atrophying muscles would induce the muscle-specific ubiquitin ligases atrogin-1 and muscle ring finger-1 (MuRF1) [3, 23], two important muscle atrophy-related ubiquitin ligases.
Previous studies have demonstrated the importance of the IGF-1 signal transduction pathway in the regulation of skeletal muscle mass. The IGF-1 system is downregulated in cancer cachexia [9]. Low-dose IGF-1 reduced mortality and attenuated loss of body weight as well as muscle mass in the Yoshida hepatoma rat model [24]. Muscle loss is associated with the IGF-1/Akt pathway through its downstream effectors FOXO and mTOR [18]. In skeletal muscle, the biological functions of IGF-I were able to activate the PI3K/Akt pathway, which considered as a master controller of the balance between protein synthesis and degradation [13], and its loss of activity is sufficient to initiate skeletal muscle atrophic program. While IGF-I-induced protein synthesis is mediated by Akt phosphorylation, the activation of the Akt pathway is associated with muscle growth. These results suggested that IGF-1/Akt pathway is involved in the regulation of the protein synthesis in skeletal muscle.
The initiation and regulation of skeletal muscle atrophy involve a number of independent signaling pathways. Recent study has shown that autophagy system plays a critical role for myofiber maintenance, and its inhibition can lead accumulation of toxic proteins and dysfunctional organelles that, in the end, would lead to muscle atrophy [19]. Basal autophagy for homeostasis of skeletal muscle is helpful to prevent accumulation of toxic proteins and dangerous dysfunctional organelles. Previous studies have shown that lack of autophagy does not protect skeletal muscles from atrophy, and muscle atrophy in autophagy inhibition mice is ameliorated by a low-protein diet and by genetic and pharmacologic approaches that activate autophagy [14]. These results indicated that basal autophagy is a vital role for skeletal muscle homeostasis during cancer cachexia.
Herbal medicines have been attracted considerable attention all over the world because of its distinct efficacy. Zhimu and Huangbai herb pair (ZBHP) is composed of Rhizoma Anemarrhena and Cortex Phellodendri, which has been widely used in China to treat various diseases [17, 30]. ZBHP were phytochemically investigated for constituents that might cause anti-cancer, diabetes, etc. [10, 15, 33]. And most importantly, our previous study has demonstrated that ZBHP could reverse muscle atrophy in streptozotocin-induced diabetic mice [35]. However, the effect of ZBHP on cancer cachexia-induced skeletal muscle atrophy is unclear. In the present study, we demonstrated that ZBHP could reverse skeletal muscle atrophy induced by cancer cachexia, thereby dramatically prolonging survival of the tumor-bearing animals, and these marked effects would be seen as downregulated the levels of inflammatory cytokines, and activated the IGF-1/Akt and autophagy signals. Thus, ZBHP appears to have therapeutic potential for treating various types of muscle wasting.
Materials and methods
Chinese herbal extracts
Zhimu (Rhizoma Anemarrhena) and Huangbai (Cortex Phellodendri) were purchased from Anguo herbal medicine market in Hebei Province, China, and were authenticated by Chinese Materia Medica College, Tianjin University of Traditional Chinese Medicine (TJUTCM). The extracts of ZBHP were consistently prepared by following stringent manufacturing practices, Zhimu and Huangbai (1:1) were reflux extracted with 50 % ethanol, and the main components of the extracts were detected by UPLC/Q-TOF-MS system as previously described [35].
Animal model
Adult male C57BL/6 mice (4–6 weeks old) were obtained from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). Mice were housed in a climate-controlled room (12-h light/dark cycle with a constant room temperature), with food and water ad libitum. Experimental procedures were conducted in accordance with recommendations of the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (TCM-2012-010-E01). The C26 colon adenocarcinoma (2 × 105)-implanted mice model was used as previously described [29]. Mice were divided into weight-matched groups: (1) normal control receiving water (C), (2) C26-bearing administered with water (M), and (3) C26-bearing treated with ZBHP (ZBHP). Mice were daily given orally either water or ZBHP extract (104 mg/kg, equivalent dose to the clinical) 1 days after implant until sacrificed. All animals were sacrificed 19 days after C26 implanted.
Grip force assessment
Skeletal muscle strength was quantified by the grip-strength test [6, 21]. The grip-strength device (YLS-13A, China) was used to measure limb grip strength of the mice. Basically, the grip-strength meter was positioned horizontally, the mice are held by the tail by which allowed to grasp the wire of the grip-strength device, then mice were pulled backward in the horizontal plane, and the strength of the limb would be recorded. The test was repeated three measurements per mouse, and the results were averaged for analysis.
ELISA assay
The blood and gastrocnemius muscles homogenate samples were centrifuged at 3000 rpm for 15 min to separate serum. The levels of TNF-α, IL-6, and IGF-1 in animals were detected by commercially available mouse ELISA kits purchased from R&D Systems (USA), used according to the manufacturer’s instructions, and the serum from each animal (10 μl) was assayed in duplicate.
RNA extraction and real-time PCR
Total muscle RNA was extracted with TRIzol (Roche), and complementary DNA (cDNA) was synthesized from 0.4 μg of total RNA using Reverse Transcriptase (Takala) Random primer as described in the manufacturer’s instructions. After synthesis, 5 μl of cDNA was used in PCR reaction with gene-specific primers, and RT-PCR analysis was carried out in 25 μl reaction systems. Values were normalized to Actin messenger RNA (mRNA) amount. The sequences of the PCR primer pairs (5′ to 3′) that were used for each gene are as follows:
-
Atrogin-1
-
F: 5′-TGTCCTCTCACATCCGATTTTTG-3′
-
R: 5′-CGGTTTACTCGGCAGATCTTG-3′
-
MuRF1
-
F: 5′-CAAGTGCCAAGCAGCTAATCAA-3′
-
R: 5′-TCTCAAAGCCTTGCTCTGTCTTC-3′
-
β-Actin
-
F: 5′-TGCGTGACATCAAAGAGAAG-3′
-
R: 5′-GATGCCACAGGATTCCATA-3′
Muscle protein content
After 19 days of C-26 implanted, the gastrocnemius muscles were quickly harvested from hind limb, and fresh muscle weights were recorded. Then, the muscles were homogenized, and the protein contents were determined with a Bradford protein assay kit (Bio-Rad) according to the manufacturer’s instructions.
Histological analysis
For the morphometric analysis, after 19 days of C-26 implanted, the gastrocnemius muscles were collected. The tissues samples dehydrated and embedded in paraffin. Serial sections of 5-μm thickness were cut. Three sections were stained with hematoxylin-eosin and examined by optical microscopy (Nikon). Mean muscle fiber diameters were measured. Mean fiber diameters were measured in ten randomly selected microscope fields.
Protein extraction and western blotting
The procedures are almost the same with previous [37, 38]. Gastrocnemius muscle samples were lysed with RIPA lysis buffer at 4 °C. An equal amount of protein (40 μg) was fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto PVDF membrane. Anti-FOXO3 (1:500), Sirt1 (1:500), LC3B (1:500), Akt (1:500), Akt (p-Ser473) (1:500), or β-actin (1:1000) antibody (Cell Signaling Technology, Inc.) were incubated for overnight at 4 °C, and the secondary antibody was incubated for 1 h at room temperature in PBS-T containing 2 % BSA. Protein signals were detected using ECL plus western blotting detection system according to the manufacturer’s specifications (Millipore). Images were scanned, and band intensities were quantified by densitometry (Bioquant Image Analysis, Nashville, TN).
Statistical analysis
Data were expressed as means ± standard deviation. Statistical significance was compared using Student’s t test. Survival rate difference was determined by chi-square test. Significance was accepted at the level of P < 0.05 (*) and P < 0.01 (**).
Results
ZBHP reverses body weight loss and prolongs survival in C26 Model
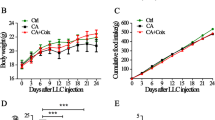
The C26 tumor-implanted mice model was employed in the present study to analyze whether ZBHP might reverse muscle atrophy. For cancer cachexia treatment, mice received ZBHP or water after 1 day of tumor implantation. At the termination of the experiment, the body and tumor weight were analyzed. As shown in Fig. 1a, weight loss in these tumor-bearing mice began around day 3 after implantation. After 19 days of tumor implantation, tumor-free body weights in model group were 10 % lower than those in the control. However, the body weight was significantly increased with the administration of ZBHP (Fig. 1b). To monitor possible effects of ZBHP on growth of the C26 tumor, we measured tumor weights. As shown in Fig. 1c, ZBHP treatment had no effect on C26 tumor weight.
Changes in body, tumor weights, and survival rates in treated or untreated C26-bearing mice. a The body weight of the mice was measured every 2 days. b, c The weight of tumor-free body and tumors was checked 19 days after tumor transplantation. c Survival plots for mice, comparing model mice to those admistreated ZBHP in the C26-bearing mice, and age-matched normal control mice were treated with water (n = 20). Data represent the mean ± S.D. **P < 0.01 as compared with control, ##P < 0.01 and # P < 0.05 as compared with model
Since the C26 tumor implanted could induce chronic loss of muscle that most often disrupts the physiological functions of the mice and culminating in significantly shortened survival time [29, 36], the treatment of the drugs on the survival of mice was checked. Chi-square test indicated that the survival curves were significantly different among model and control groups (P < 0.01), and the ZBHP treatment group had a significantly longer survival time than the C26-bearing mice group (P < 0.01, Fig. 1d).
Taken together, the reversal of body weight loss of ZBHP-treated mice were associated with a profound prolongation of survival in the tumor-bearing animals. Thus, ZBHP was able to reverse weight loss completely and to prolong animal survival dramatically, and most importantly, ZBHP was able to prolong the survival by reversing cachexia-induced muscle loss even without inhibiting tumor growth.
ZBHP suppresses tumor-induced muscle atrophy
Grip strength reflects the extent of the damage in skeletal muscle, as evidenced by a voluntary grip-strength test, the grip strength of the untreated C26-bearing mice declined in parallel with the muscle weights. C26-bearing mice develop a force 20 % lower than control, and ZBHP treatment almost restored grip strength to the normal levels (Fig. 2a). Thus, ZBHP treatment caused a buildup of functional muscle in the tumor-bearing animals to levels resembling control.
Grip strength in each groups and the wasting of gastrocnemius. a Grip strength of the limbs were monitored every 5 days and shown. Gastrocnemius muscle weight (b) and gastrocnemius muscle protein (c) (n = 8). Data represent the mean ± S.D. **P < 0.01 or *P < 0.05 as compared with control, ##P < 0.01 and #P < 0.05 as compared with model
To assess the contribution of ZBHP in muscle atrophy on C26-bearing mice in vivo, gastrocnemius muscle mass was evaluated. The gastrocnemius were weighed and taken homogenate. As shown in Fig. 2b, c, the levels of muscle mass and the protein content were reduced by nearly 15 % in tumor-bearing mice relative to control mice (P < 0.01 or P < 0.05), indicating that C26-bearing did cause muscle atrophy in C57BL/6 mice. Gastrocnemius muscle from C26-bearing mice treated with ZBHP was significantly higher than untreated tumor-challenged mice (P < 0.01 or P < 0.05).
To determine a more general effect on skeletal muscle, the gastrocnemius was also subjected to histological examination. The results showed that muscles from mice-bearing C-26 tumors were severely atrophic, exhibiting reduction in mean fiber diameter in gastrocnemius, and mean diameter reductions of these fibers were 40 % (P < 0.01 Fig. 3). In contrast, the mean diameter reduction was rather mild in the ZBHP-treated mice, and the mean diameter was closer to normal in the samples administrated with ZBHP (Fig. 3). These results indicated that ZBHP could suppress tumor-induced muscle atrophy.
ZBHP reduces the elevated levels of proinflammatory cytokines in C26 model
As reported previously, TNF-α and IL-6 were elevated in the circulation in C26-bearing mice [2, 5]. To test whether ZBHP treatment might be achieving its anti-cachexia effect by attenuating inflammatory cytokines, these major cytokines in gastrocnemius homogenate and serum were measured by ELISA assay. The levels of TNF-α and IL-6 were significantly increased in C26-bearing mice than normal (P < 0.01, Fig. 4a–d). However, C26-bearing mice treated with ZBHP had significantly lower levels of TNF-α and IL-6 than untreated mice (P < 0.01 or P < 0.05, Fig. 4a–d), and thus, they can, at least in part, account for the reversal of muscle atrophy seen upon ZBHP treatment.
The levels of inflammatory cytokines and IGF-1 in serum and gastrocnemius homogenate. The level of TNF-α and IL-6 in gastrocnemius homogenate (a, b) and serum (c, d). The level of IGF-1 in gastrocnemius homogenate (e) and serum (f) (n = 8). Data represent the mean ± S.D. **P < 0.01 or *P < 0.05 as compared with control, ##P < 0.01 and #P < 0.05 as compared with model
ZBHP attenuates the accelerated muscle protein catabolism in C26 model
Since the inflammatory cytokines were elevated in the circulation in C26-bearing mice, which could interact with their cognate receptors on muscle to mediate the hypercatabolic response [13]. To determine whether the ZBHP might regulate these critical components during cancer cachexia, a semiquantitative RT-PCR analysis was employed to evaluate the levels of atrogin-1 and MuRF1 (two muscle-specific atrophy-related ubiquitin ligases) expression in gastrocnemius. The results showed that mRNA levels for these atrophy-related genes in the gastrocnemius were markedly increased above control levels in the cachexia model mice (P < 0.01, Fig. 5), but ZBHP treatment completely abolished their induction (P < 0.05, Fig. 5).
Expression of MuRF1 and atroghy-1 in gastrocnemius muscles of control and tumor-bearing mice. a The mRNA levels of MuRF1 and atroghy-1 were analyzed by RT-PCR. b The expression levels were semiquantified by densitometric measurements, normalized with β-actin internal control (n = 3). Data represent the mean ± S.D. **P < 0.01 as compared with control, #P < 0.05 as compared with model
FOXO3 activation has been shown to stimulate proteolysis and cause atrophy. In the present study, FOXO3 expression was investigated in the gastrocnemius tissues. As expected, western blot analysis revealed an increase in the gastrocnemius of C26-bearing mice (P < 0.01, Fig. 6), ZBHP treatment significantly reduced the FOXO3 expression (P < 0.05, Fig. 6). Collectively, these observations provide strong direct evidence that enhanced FOXO3 signaling was inhibited by ZBHP and helps to explain the profound inhibition of atrogin-1 and MuRF1 induction and these findings suggested one mechanism by which ZBHP could prevent the activation of key components of the ubiquitin-proteasome pathway.
The expression of IGF-1/Akt and autophagy pathway members. Histograms represent the change in each members normalized to anti-actin antibodies (n = 3). The expression of Akt and activated Akt (a), Sirt1 (b), LC3B (c), and FOXO (d). Data represent the mean ± S.D. **P < 0.01 as compared with control, #P < 0.05 as compared with model
ZBHP actives IGF-1/Akt signal in C26 Model
Besides increased protein degradation through ubiquitin-proteasome pathway, inflammatory cytokines could inhibit IGF-I, the main positive regulators of muscle growth [4, 25], to stimulate protein synthesis. Low circulating IGF-1 levels also were reported in cachectic cancer patients [26]. To further explore potential mechanisms for the effect of ZBHP on reversal muscle wasting, the level of IGF-1 was checked. Consistent with the previous reports, our results showed that serum and gastrocnemius muscle IGF-1 was markedly reduced in the 19 days after tumor transplantation and ZBHP treatment could increase the level of IGF-1 significantly (Fig. 4e, f). Since IGF-1 is able to activate the PI3K/Akt pathway, and the activity of the Akt kinase is crucially required to stimulate protein synthesis and keep low degradation. The activation of Akt was checked in the present study. Indeed, western blotting results showed that the level of Akt phosphorylated form was diminished in the cachexia model mice compared to normal control mice, and ZBHP treatment induced an increase in Akt activity in the gastrocnemius (Fig. 6). These results indicated that ZBHP treatment not only inhibited the skeletal muscle protein degradation but also promoted the stimulated protein synthesis.
ZBHP induces autophagy in C26 model
Autophagy is essential for cellular survival by clearing damaged proteins and organelles. Previous study suggested that atrophic muscles suffer from lack of autophagy [19]. Thus, pharmacological reactivation of autophagy may ameliorate muscle myopathy. To clarify the activity of autophagy system in C26-bearing mice, the expression of LC3B and Sirt1 proteins, which were accepted as markers of autophagy, has been assessed in the gastrocnemius muscle on day 19 after tumor transplantation, representing the advanced stage of muscle wasting. As Fig. 6 showed, Sirt1 and LC3B expression was significantly downregulated in C26-bearing mice, whereas the expression of LC3B and sirt1 was markedly increased in response to ZBHP treatment. These results indicated that ZBHP treatment could activate the autophagy system in the advanced stage of cancer cachexia.
Discussion
Muscle atrophy occurs inappropriately in many diseases, for example during disuse, or systemic diseases such as cancer, diabetes, and renal failure. The maintenance of skeletal muscle mass is important to ensure the quality of life and even prolong survival time of patients. Thus, to looking for efficiency and low toxicity of the drug turn out to be the common goals in contemporary cachexia therapeutic research studies. The present study demonstrated that ZBHP treatment could target different pathogenic mechanisms to ameliorate skeletal muscle atrophy caused by cancer cachexia in a mouse model, so ZBHP may be an excellent candidate for developing therapeutic strategies for the tumor-induced muscle atrophy.
The C26-bearing mice showed a lethal wasting syndrome characterized by progressive weight loss and death. In the present study, weight loss in these tumor-bearing mice began around day 3 after implant. However, the total weight of untreated C26-bearing mice was higher than control and ZBHP treated after 15 days of tumor inoculation; this was mainly because the speed of tumor growth was too fast. On day 19 after tumor inoculation, although there was no statistical difference of the tumor weight between each group (Fig. 1c), there were significant differences of the tumor-free weight among each group. The tumor-free body weights of the mice treated with ZBHP was significantly higher than the model group. This suggests that ZBHP treatment partially reverses the weight loss induced by cachexia. Previous study has demonstrated that prevents and reverses skeletal muscle loss could dramatically prolong survival of the tumor-bearing animals [36]. As expected, our results showed that ZBHP treatment significantly prolonged the survival time of C26-bearing mice. These results indicated that ZBHP treatment dramatically prolonged survival, even of animals in which tumor growth was not inhibited.
The pathogenetic mechanisms by which cancer mediates skeletal muscle atrophy are complex and only partially identified, and a number of independent signaling pathways and multifactor were involved to initiate and regulate skeletal muscle atrophy. Initial studies of the C26-bearing mouse model identified a prominent role of a variety of cytokines, especially TNF-α, IL-6, and IL-1, and seemed to be the direct mediators that induce and aggravate muscle atrophy [2, 5]. These inflammatory cytokines are potent inducers of the ubiquitin ligase atrogin-1 and MuRF1, which play a partial role in mediating skeletal muscle atrophy in vivo. The results obtained in the present study showed that ZBHP administration to C26-bearing mice significantly reduced the levels of TNF-α and IL-6. Furthermore, FOXO3 transcription factor could activate the muscle-specific ubiquitin ligase atrogin-1 and MuRF1 and cause marked skeletal muscle atrophy. As expected, ZBHP treatment downregulated the expression of FOXO3, atrogin-1, and MuRF1, and the results were consistent with the reduction of cytokines. Otherwise, NF-κB is an important element of muscle atrophy during cancer cachexia. To better delineate the impact of ZBHP on the ubiquitin-proteasome pathway, further study should focus on the NF-κB activation.
Several reports suggested that impairment of IGF-1 signaling may result in muscle atrophy by enhancing the muscle-specific ubiquitin ligases atrogin-1 and MuRF1 [16, 27]. Consistent with the previous findings, our results showed that IGF-1 levels were significantly downregulated in C26-bearing mice, and ZBHP treatments increased the levels of IGF-1 and consequently led to significant reduction of atrogin-1 and MuRF1 expression. Moreover, the reduction of FOXO activity indicated activation of the AKT signal [7, 27]. While activation of the AKT pathway is associated with muscle growth and protein synthesis, protein degradation mediated by FOXO is suppressed. Therefore, the phosphorylation levels of Akt are a good prognostic marker of the muscle protein synthesis. Our results showed that the levels of Akt phosphorylated form were reduced in the cachexia model compared to normal mice; consistent with the previous studies, the present study showed an impairment of the Akt signaling pathway during the cachexia-induced skeletal muscle atrophy. Accordingly, western blot analysis demonstrated an increase in Akt activity in the gastrocnemius of the ZBHP-treated mice. In short, mice treated with ZBHP had significantly better survival times, stimulated protein synthesis, and inhibited the protein degradation. Crosstalk and feedback loops between the cancer-induced cytokines and IGF-1/Akt signal pathways may be responsible for these effects. However, there is a controversial debate, and activation of IGF-1/Akt signaling could promote protein accumulation in muscle. It also has the potential to promote tumor growth. Further study should focus on the balance of regulation of the drugs.
Autophagy system is emerging as another crucial system that controls muscle mass during cancer cachexia [31, 32]. Autophagy is required to allow the cell survival by clearing of damaged proteins and altered organelles. And recent data showed that autophagy inhibition induces atrophy and myopathy in adult skeletal muscles [20]. Since autophagy system inhibition has been demonstrated to be an important stimulator of muscle atrophy, the markers of autophagy (LC3B and sirt1) were examined. The results obtained in the present work confirmed the decline of autophagy in the skeletal muscle of C26-bearing mice. The result was in contrast with a number of reports demonstrating a negative role of autophagy system in mice cachexia model; this is mainly because of the different stage of the disease. The decrease in IGF-1/Akt signaling is accompanied by a paradoxical decrease in autophagy in the advanced stage of muscle wasting, despite an increase in FOXO signaling. Therefore, the role of IGF-1/Akt signal in autophagy regulation, at least in skeletal muscle, is much less important and does not mediate the negative effect on the autophagy pathway [22]. Besides, myostatin and activin are also important to regulate the muscle atrophy or hypertrophy [36]. We need a more precise understanding of the signaling pathways and its interrelation that control the muscle atrophy in the further study.
Collectively, the findings in the present study demonstrate that ZBHP protects against cancer-induced muscle atrophy by activating IGF-1/Akt and autophagy signal pathways, suggesting one or more mechanisms by which it is the reversal muscle atrophy effect. ZBHP treatment would be satisfactory in ameliorating cancer cachectic symptoms. The findings may be important for the future therapeutics, and this formula would be an ideal starting point for developing more effective treatments against cancer cachexia.
References
Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, Thierse HJ, Witt CC, Linke A, Schuler G, Labeit S (2008) Induction of MuRF1 is essential for TNF-alpha-induced loss of muscle function in mice. J Mol Biol 384:48–59
Argiles JM, Lopez-Soriano FJ (1999) The role of cytokines in cancer cachexia. Med Res Rev 19:223–248
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW (2003) Cytokine-hormone interactions: tumor necrosis factor alpha impairs biologic activity and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. Endocrinology 144:2988–2996
Carson JA, Baltgalvis KA (2010) Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 38:168–176
Chambon C, Duteil D, Vignaud A, Ferry A, Messaddeq N, Malivindi R, Kato S, Chambon P, Metzger D (2010) Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci U S A 107:14327–14332
Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Derijard B (2010) Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 30:470–480
Costelli P, Carbo N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argiles JM, Baccino FM (1993) Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 92:2783–2789
Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano FJ, Doglietto GB, Argiles JM, Baccino FM, Rossi Fanelli F (2006) IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul, Integr Comp Physiol 291:R674–R683
Diogo CV, Machado NG, Barbosa IA, Serafim TL, Burgeiro A, Oliveira PJ (2011) Berberine as a promising safe anti-cancer agent - is there a role for mitochondria? Curr Drug Targets 12:850–859
Fearon KC (2008) Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 44:1124–1132
Frost RA, Nystrom GJ, Jefferson LS, Lang CH (2007) Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab 292:E501–E512
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P (2010) Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med 16:1313–1320
Iizuka N, Hazama S, Yoshimura K, Yoshino S, Tangoku A, Miyamoto K, Okita K, Oka M (2002) Anticachectic effects of the natural herb Coptidis rhizoma and berberine on mice bearing colon 26/clone 20 adenocarcinoma. Int J Cancer 99:286–291
Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ (2005) Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280:2737–2744
Ma C, Fan M, Tang Y, Li Z, Sun Z, Ye G, Huang C (2008) Identification of major alkaloids and steroidal saponins in rat serum by HPLC-diode array detection-MS/MS following oral administration of Huangbai-Zhimu herb-pair Extract. Biomed Chromatogr 22:835–850
Mammucari C, Schiaffino S, Sandri M (2008) Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4:524–526
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy is required to maintain muscle mass. Cell Metab 10:507–515
Masiero E, Sandri M (2010) Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6:307–309
Pandey SN, Cabotage J, Shi R, Dixit M, Sutherland M, Liu J, Muger S, Harper SQ, Nagaraju K, Chen YW (2012) Conditional over-expression of PITX1 causes skeletal muscle dystrophy in mice. Biol Open 1:629–639
Sandri M (2010) Autophagy in skeletal muscle. FEBS Lett 584:1411–1416
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, Springer J, Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, Springer J (2011) IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J Cachex Sarcopenia Muscle 2:105–109
Schulze PC, Gielen S, Adams V, Linke A, Mobius-Winkler S, Erbs S, Kratzsch J, Hambrecht R, Schuler G (2003) Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulinlike growth factor-I in chronic heart failure. Basic Res Cardiol 98:267–274
Simons JP, Schols AM, Buurman WA, Wouters EF (1999) Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin Sci 97:215–223
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Strassmann G, Fong M, Kenney JS, Jacob CO (1992) Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 89:1681–1684
Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T (1990) Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res 50:2290–2295
Tang YH, Sun ZL, Fan MS, Li ZX, Huang CG (2012) Anti-diabetic effects of TongGuanWan, a Chinese traditional herbal formula, in C57BL/KsJ-db/db mice. Planta Med 78:18–23
Tisdale MJ (1997) Biology of cachexia. J Natl Cancer Inst 89:1763–1773
Tolkovsky AM (2010) Autophagy thwarts muscle disease. Nat Med 16:1188–1190
Wang N, Feng Y, Zhu M, Siu FM, Ng KM, Che CM (2013) A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim Biophys Acta 1833:2890–2899
Zaki MH, Nemeth JA, Trikha M (2004) CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer 111:592–595
Zhang J, Zhuang P, Wang Y, Song L, Zhang M, Lu Z, Zhang L, Wang J, Alemu PN, Zhang Y, Wei H, Li H (2014) Reversal of muscle atrophy by Zhimu-Huangbai herb-pair via Akt/mTOR/FoxO3 signal pathway in streptozotocin-induced diabetic mice. PLoS One 9:e100918
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142:531–543
Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, Liu Y, Yang X, Isaiah AO, Lin Y, Jiang Y (2012) Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One 7:e35636
Zhuang PW, Cui GZ, Zhang YJ, Zhang MX, Guo H, Zhang JB, Lu ZQ, Isaiah AO, Lin YX (2013) Baicalin regulates neuronal fate decision in neural stem/progenitor cells and stimulates hippocampal neurogenesis in adult rats. CNS Neurosci Ther 19:154–162
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81403213) and Program for Changjiang Scholars and Innovative Research Team in University (“PCSIRT”, IRT 14R41).
Conflict of interest
We do not have any financial relationship with the organization that sponsored the research and the authorship. Besides, we have full control of all primary data, and we agreed to allow the journal to have the data if requested by the reviewer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pengwei Zhuang and Jinbao Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhuang, P., Zhang, J., Wang, Y. et al. Reversal of muscle atrophy by Zhimu and Huangbai herb pair via activation of IGF-1/Akt and autophagy signal in cancer cachexia. Support Care Cancer 24, 1189–1198 (2016). https://doi.org/10.1007/s00520-015-2892-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2892-5