Abstract
Background
Tuberculosis (TB) is an infectious disease caused by infection with Mycobacterium tuberculosis (Mtb), and it remains one of the major threats to human health worldwide. To our knowledge, the polarization of M1/M2 macrophages were critical innate immune cells which play important roles in regulating the immune response during TB progression.
Objective
We aimed to explore the potential mechanisms of M1/M2 macrophage polarization in TB development.
Methods
THP-1 macrophages were treated with early secreted antigenic target of 6 kDa (ESAT-6) protein for an increasing time. The polarization profiles, apoptosis levels of M1 and M2 macrophages were detected by RT-qPCR, immunofluorescence, Western blot and flow cytometry.
Results
ESAT-6 initially promoted the generation of pro-inflammatory M1-polarized macrophages in THP-1 cells within 24 h, which were suppressed by further ESAT-6 treatment at 30–42 h. Interestingly, ESAT-6 continuously promoted M2 polarization of THP-1 cells, thereby maintaining the anti-inflammatory response in a time-dependent manner. In addition, ESAT-6 promoted apoptotic cell death in M1-polarized macrophages, which had little effects on apoptosis of M2-phenotype of macrophages. Then, the potential underlying mechanisms were uncovered, and we verified that ESAT-6 increased the protein levels of TLR4, MyD88 and NF-κB to activate the TLR4/MyD88/NF-κB pathway within 24 h, and this signal pathway was significantly inactivated at 36 h post-treatment. Interestingly, the following experiments confirmed that ESAT-6 TLR4/MyD88/NF-κB pathway-dependently regulated M1/M2 polarization and apoptosis of macrophage in THP-1 cells.
Conclusion
Our study investigated the detailed effects and mechanisms of M1/M2 macrophages in regulating innate responses during TB development, which provided a new perspective on the development of treatment strategies for this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is one of the oldest known human infectious diseases in the world that primarily affects the lungs (Gopalaswamy et al. 2020). TB, caused by infection with Mycobacterium tuberculosis (Mtb), results in millions of deaths annually (Bussi and Gutierrez 2019; Coppola et al. 2017). TB is one of the top ten causes of death worldwide, caused more deaths than any other single infectious diseases (Bussi and Gutierrez 2019). Many TB patients have chronically infected and usually exhibit decreased lung function and respiratory failure (Powers et al. 2020; Shneerson 2004). Despite having effective treatment options, the cure rate remains low, so the treatment of this disease still faces a huge challenge (Chai et al. 2020). Macrophages, as the main host cells against Mtb, play a crucial role in the fight against TB infection (Zhang et al. 2021). Infection of macrophages by Mtb induces macrophage differentiation into different phenotypes (Mily et al. 2020). In addition, macrophages also play an irreplaceable role as antigen-presenting cells in the innate and acquired immune response (C et al. 2021; Zhang et al. 2021). Therefore, it is important to understand the specific mechanisms by which Mtb infection leads to macrophage polarization in order to completely cure this disease.
Early secreted antigenic target of 6 kDa protein (ESAT-6), the main virulence factor of Mbt, is mainly secreted in the blood and sputum of TB, and has been identified as a biomarker for the rapid diagnosis of TB (Araujo et al. 2021; Omar et al. 2021; Poulakis et al. 2016). Recombinant BCG vaccine secreting ESAT-6 enhances the defense against TB in mice (Khanna et al. 2021). And the N-terminally formylated ESAT-6 protein alone or in combination with anti-TB drugs improve moderate therapeutic effect against experimental TB (Mir and Sharma 2022). Besides, ESAT-6 has been reported to be involved in the immune responses (Saba et al. 2020; Zhao et al. 2021), T cell differentiation (Clemmensen et al. 2020), Antigen presentation (Sreejit et al. 2014), iron uptake (Jha et al. 2020), autophagy (Yabaji et al. 2020; Zhang et al. 2012), apoptosis (Grover and Izzo 2012; Yang et al. 2015), and necrosis (Welin et al. 2011). Here, we explored potential changes in the functional state of macrophages infection with the Mycobacterium tuberculosis virulent protein ESAT-6.
Toll-like receptors (TLRs) belong to the class of pattern recognition receptors (PRRs), an important class of protein molecules involved in the innate immune system (Satoh and Akira 2016; Wang et al. 2021). The TLRs expressed in macrophages include TLR1/2, TLR3, TLR4, TLR7 and TLR9 (Hsieh et al. 2020). TLR2 and TLR4 are involved in inflammatory responses (Korbecki and Bajdak-Rusinek 2019; Swanson et al. 2020), and Mtb infection (Faridgohar and Nikoueinejad 2017). For example, TLR2-mediated inflammation of macrophages promoted the progression of hepatic fibrosis (Xie et al. 2021). Inhibition of TLR4 can alleviate inflammation in human hepatocellular carcinoma cells (Sawai et al. 2022). Several specialized intracellular proteins including myeloid differentiation factor 88 (MyD88), tumor necrosis factor (TNF) receptor-associated factor and interleukin-1 receptor-associated kinase can deliver TLR4 signaling, and a cellular response network that triggers activation of the NF-κB cascade and induction of inflammatory factor secretion (Chen et al. 2021; Wang et al. 2018). Previous researches have demonstrated that blocking the TLR4/MyD88/NF-κB signaling pathway reduces inflammation and apoptosis, which then alleviates the LPS-induced acute lung injury (Ju et al. 2018). Suppression of inflammation-related cognitive deficits through inhibition of the TLR4/MyD88/NF-κB signaling pathway while ameliorating experimental vascular dementia (Wang et al. 2020a). Therefore, it is necessary to investigate the role of the TLR4/MyD88/NF-κB signaling pathway in the function of macrophages.
In our study, we explored the effects of ESAT-6 on macrophage function, and we indicated that ESAT-6 regulated macrophage polarization and promoted apoptosis in M1-type macrophages. Mechanically, the TLR4/MyD88/NF-κB signaling pathway participated in M1/M2 macrophage switch and macrophage apoptosis. Our findings suggested that understanding the role of ESAT-6 in macrophages would help elucidate the mechanisms of chronic TB and provide new insight for optimal treatment.
Materials and methods
Cell culture and differentiation
Human monocyte cell line THP-1 was cultured in Roswell Park Memorial Institute-1640 (RPMI-1640, Invitrogen) medium supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin solution (100 U/mL) (Sigma, St. Louis, MO, USA). The THP-1 cells were incubated with phorbol 12-myristate 13-acetate (PMA) (100 ng/mL) for 24 h to obtain macrophage-like THP-1 cells. Transfection was performed using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
MTT assays
THP-1 macrophages were seeded in 96 well plates with various concentrations of ESAT-6 (abcam, Cambridge, UK). After incubation for 24 h, THP-1 macrophages were cultured with 10 μL MTT solution (5 mg/mL) in culture media for 4 h. Then, 100 μL of dimethyl sulfoxide (DMSO) was added to fully dissolve the crystals. The optical density (OD) value of each well was detected at 570 nm. Cytotoxicity was calculated as a percentage of cellular viability using the following formula:
RT-qPCR analysis
Total RNA was isolated from the macrophages and the mRNA was converted to cDNA using Prime Taq Premix (Genet Bio, Daejeon, South Korea). Real-time qPCR for all gene mRNA levels were performed using the SYBR Green Master Mix (QIAGEN, Venlo, The Netherlands). The relative gene expression was calculated using the comparative 2−(∆∆Ct) method and was normalized to GAPDH. The primer sequences used in our study were as follows: IL-6 forward: GCC AGA GCT GTG CAG ATG AGT and reverse: TGG CAT TTG TGG TTG GGT CAG; iNOS forward: TGA CCA TCA TGG ACC ACC AC and reverse: ACC AGC CAA ATC CAG TCT GC; IL-4 forward: CAA GCA GCT GAT CCG ATT CC and reverse: GGA ATT CAA GCC CGC CA; Arg-1 forward: GTG GAA ACT TGC ATG GAC AAC and reverse: AAT CCT GGC ACA TCG GGA ATC; TLR4 forward: AAG CCG AAA GGT GAT TGT TG and reverse: CTG AGC AGG GTC TTC TCC AC.
Immunofluorescence staining assays
After treatment, THP-1 macrophages were washed three times with PBS, fixed with 4% formaldehyde at room temperature, permeabilized with 0.2% Triton X-100, then incubated with monoclonal antibodies against CD86 and CD163 (Cell Signaling) overnight at 4 °C, washed twice with PBS, incubated with fluorescent secondary antibodies, mounted in 95% glycerol, and pictures were acquired using a fluorescent microscopy.
Apoptosis assays
As previously described, IFN-γ/LPS and IL-4/IL-13 induced M1 and M2 macrophages, respectively (Refai et al. 2018; Zhu et al. 2014). Subsequently, M1 and M2 macrophages were grouped and treated accordingly, and the cells were collected and washed, then harvested with trypsin /EDTA and stained using the Apoptosis Detection Kit (BD company, Massachusetts, USA). Apoptotic cells were analyzed by flow cytometry (FACSCalibur, BD).
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay (RIPA) lysis buffer. Protein content was measured by the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein extracts were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. Then PVDF membranes were blocked with 5% skim milk and incubated with primary and secondary antibodies according to the manufacturer’s instructions, followed by chemiluminescence detection. The antibodies used in our study were: Caspase-3, Bax, Bcl-2, TLR4, MyD88, NF-κB p65 and GAPDH (Cell Signaling). All antibodies were diluted at a concentration of 1:1000.
Statistical analysis
All data statistical analysis was performed using GraphPad Prism 8 software for t-test and one-way analysis of variance (ANOVA). Data are presented as mean ± SD. P values of 0.05 or less are considered significant.
Results
The regulating effects of ESAT-6 on M1/M2 polarization in macrophages are dependent on treatment duration
To study the effect of ESAT-6 on the cell viability of THP-1 macrophages, we pretreated THP-1 cells with ESAT-6 at concentrations ranging from 0 to 40 μg/mL. The results showed that the viability of THP-1 cells was unchanged in the presence of low-dose ESAT-6 protein (0–10 μg/mL), however, high-concentrations of ESAT-6 (20 and 40 μg/mL) exerted its cytotoxic effects to significantly suppressed the viability of THP-1 cells (Fig. 1A). Given that 10 μg/mL of ESAT-6 did not alter cell viability of THP-1 cells, we selected this concentration for subsequent experiments. Next, we considered the regulating effect of ESAT-6 on M1/M2 polarization in THP-1 macrophages by applying ESAT-6 (10 μg/mL) to THP-1 cells for 42 h with 6 h-intervals. Interestingly, the Real-Time qPCR analysis results showed that ESAT-6 protein increased the expression levels of IL-6 and iNOS to promote the enrichment of pro-inflammatory M1-polarized macrophages in THP-1 cells from 0 to 24 h, but the expression levels of M1-associated biomarkers were gradually decreased in 24–42 h post-treatments (Fig. 1B, C). In addition, we noticed that ESAT-6 continuously increased the expression levels of IL-4 and Arg-1 to facilitate the generation of M2-polarized THP-1 cells from 0 to 42 h (Fig. 1D, E). Moreover, the expression status of M1 macrophage marker CD86 and M2 macrophage marker CD163 were analyzed by the following immunofluorescence staining assay. As shown in Fig. 1F, the expression levels of CD86 protein were increased by ESAT-6 treatment within 24 h, which were apparently suppressed by further ESAT-6 treatment at 36 h. Also, we confirmed that ESAT-6 time-dependently increased the expression levels of CD163 protein to promote M2-polarzied macrophages (Fig. 1G). These results indicated that ESAT-6 initially promoted M1 polarization of macrophages triggering pro-inflammatory reaction in the host immune system. However, long period of ESAT-6 treatment caused enrichment of M2-polarized macrophages to restrain further immune reactions.
The regulating effects of ESAT-6 on M1/M2 polarization in macrophages are dependent on treatment duration. (A): THP-1 macrophage cells were incubated with ESAT-6 at range from 0 to 40 μg/mL for 24 h. Cell viability was determined by MTT assay. (B-E): The effect of ESAT-6 treatment for different time on the expression of IL-6, iNOS, IL-4 and Arg1 in THP-1 cells. (F-G): Immunofluorescence detection of M1 macrophages (CD86) and M2 macrophages (CD163) in THP-1 cells after 12, 24, and 36 h of ESAT-6 treatment. (H) The ratio of M1/M2 macrophages in THP-1 macrophages. *P < 0.05; **P < 0.01; ***P < 0.001
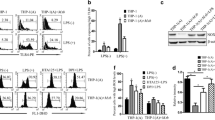
Continuous ESAT-6 treatment particularly promotes apoptotic cell death in M1 macrophages
To investigate the effect of ESAT-6 on macrophage apoptosis, the THP-1 cells were respectively treated with IFN-γ/LPS and IL-4/IL-13 to induce the M1 and M2-polarized macrophages, and the following Real-Time qPCR analysis confirmed that the M1/M2 type of macrophages were successfully obtained (Figure S1A, B). Then, M1 and M2-polarized macrophages were subjected to ESAT-6 protein treatment for 42 h with 6 h-intervals, and the apoptosis ratio of the cells were examined by flow cytometry. Interestingly, the results showed that ESAT-6 protein did not influence cell apoptosis of M1-macrophages at 0–24 h, which were significantly promoted at 30–42 h post-treatment (Fig. 2A, B). However, the apoptosis ratio of M2-polarzied macrophages was not altered by ESAT-6 treatment from 0 to 42 h (Fig. 2A, B). The above results were supported by the following Western Blot analysis, which showed that the expression levels of cleaved Caspase-3 and Bax were significantly increased and Bcl-2 was suppressed in M1 polarized macrophages after 36 h of ESAT-6 treatment, thereby triggering apoptosis (Fig. 2C), but there was no change in the expression levels of the above apoptosis-related proteins in M2 polarized macrophages after ESAT-6 treatment. (Fig. 2D). Those data suggested that ESAT-6 specifically induced apoptotic cell death in the M1-polarized macrophages instead of the M2-phenotypes.
Continuous ESAT-6 treatment particularly promotes apoptotic cell death in M1 macrophages. (A-B): Flow cytometric analysis of apoptosis in THP-1 M1/M2 type macrophages treated with ESAT-6. (C): The effect of EAST-6 on the protein expression of caspase-3, Bax and Bcl-2 in M1 macrophages. (D): The effect of EAST-6 on the protein expression of caspase-3, Bax and Bcl-2 in M2 macrophages. *P < 0.05; **P < 0.01; ***P < 0.001
The TLR4/MyD88/NF-κB pathway is regulated by ESAT-6 in macrophages
We next investigated the underlying mechanisms by which ESAT-6 exerts its biological functions in THP-1 cells. According to recent publications, the TLR/MyD88/NF-κB signaling pathway plays an important role in a variety of life activities and is widely present in various tissues and cells, and is involved in the development and regulation of many diseases (Ju et al. 2018; Li et al. 2021a). Hence, we considered whether ESAT-6 exerts its biological functions through this signal pathway. To achieve this goal, the THP-1 cells were subjected to ESAT-6 treatment for 42 h, and the Real-Time qPCR analysis results showed that ESAT-6 increased the mRNA expression of TLR4 at 0–18 h post-treatments. However, the expression levels of TLR4 were significantly attenuated at 24–42 h post-treatment (Fig. 3A). Consistent with the above findings, our Western Blot analysis confirmed that ESAT-6 initially increased the protein levels of TLR4, MyD88 and NF-κB, which activated the TLR4/MyD88/NK-κB pathway at 0–18 h post-treatment, whereas this signaling pathway was suppressed at 24–36 h post-treatment (Fig. 3B, C). Therefore, these results highlighted that ESAT-6 regulated the activation of the TLR4/MyD88/NF-κB pathway in a time-dependent manner.
ESAT-6 protein regulated M1 polarization of the THP-1 macrophages via regulating the TLR4/MyD88/NF-κB pathway
Based on the fact that the TLR4/MyD88/NF-κB pathway could be modulated by ESAT-6 in a time-dependent manner, and the time points for ESAT-6 to regulate this pathway were in accordance with its biological functions in regulating M1 polarization of THP-1 cells. We next considered whether ESAT-6 regulates M1 phenotype of macrophages through regulating the TLR4/MyD88/NF-κB signaling pathway. As shown in Fig. 4A, our data suggested that ESAT-6 increased the expression of M1-associated genes (IL-6 and iNOS) at 12 h, which were all abrogated by silencing TLR4. In consistent with the above findings, it was shown that ESAT-6 suppressed the enrichment of M1-polarized macrophages by repressing the expression levels of IL-6 and iNOS at 36 h, while overexpressing of TLR4 observably rescued the effect of ESAT-6 (Fig. 4B). Coherently, immunofluorescence staining indicated that the promotion of the M1 macrophages marker CD86 by ESAT-6 treatment within 12 h could be neutralized by the downregulation of TLR4 (Fig. 4C, D). Likewise, the inhibitory effect of ESAT-6 treatment for 36 h on CD86 also could be rescued by overexpression of TLR4 (Fig. 4E, F). Collectively, these data suggested that ESAT-6 regulated M1 polarization though the TLR4/MyD88/NF-κB pathway in a time-dependent manner.
ESAT-6 protein regulated M1 polarization of the THP-1 macrophages via regulating the TLR4/MyD88/NF-κB pathway. (A): The effect of knockdown of TLR4 on the expression of IL-6 and iNOS in THP-1 cells induced by ESAT-6 for 12 h. (B): The effect of overexpression of TLR4 on the expression of IL-6 and iNOS in THP-1 cells induced by ESAT-6 for 36 h. (C): Immunofluorescence detection of the effect of TLR4 on M1 macrophages (CD86) by ESAT-6 treatment for 12 h. (D): Quantification analyses of M1 macrophages in THP-1 cells in Figure C. (E): Immunofluorescence detection of the effect of TLR4 on M1 macrophages (CD86) by ESAT-6 treatment for 36 h. (F): Quantification analyses of M1 macrophages in THP-1 cells in Figure E. *P < 0.05; **P < 0.01; ***P < 0.001
Targeting the TLR4/MyD88/NF-κB pathway restrains ESAT-6-induced cell apoptosis in M1 macrophages
We finally explored whether ESAT-6 regulates apoptotic cell death in M1 macrophages through the TLR4/MyD88/NF-κB signaling pathway. Since ESAT-6 treatment did not alter apoptosis within 24 h, we chose ESAT-6 treatment for 36 h to detect this biological function. The apoptotic levels of M1 macrophages were analyzed by flow cytometry, and the results were shown in Fig. 5A and B, ESAT-6 treated with 36 h significantly boosted cell apoptosis in M1 macrophages, while overexpression of TLR4 reversed the promotion of cell apoptosis of ESAT-6. Similarly, the apoptosis-associated protein was detected by Western blot analysis, and the data uncovered that ESAT-6 promoted the expression of cleaved Caspase-3 and Bax, and inhibited the Bcl-2 expression, but the effect of ESAT-6 on the apoptosis-associated protein could be rescued by upregulation of TLR4 (Fig. 5C, D). Taken together, these findings indicated that ESAT-6 regulated M1 macrophages apoptosis through the TLR4/MyD88/NF-κB pathway.
Discussion
TB, infected by Mtb, is one of the oldest known chronic inflammatory diseases (Lam et al. 2017). Mtb infection provokes an immune response in the body, and the ultimate healing of the body depends on the virulence of Mtb and the function of immune cells (Bade et al. 2021; de Martino et al. 2019). Macrophages, as a type of immune cell, play nonnegligible roles in the initiation and progression of TB (Weiss and Schaible 2015). Macrophages could differentiate into the pro-inflammatory M1 macrophages and the anti-inflammatory M2 macrophages (Huang et al. 2018). The pro-inflammatory cytokines including TNF-α, IL-6, IL-1β and iNOS, while the anti-inflammatory cytokines IL-4, IL-10, TGF-β and Arg-1 (Zhan et al. 2022). Furthermore, ESAT-6 has been shown to be involved in macrophages polarization. For example, Refai et al. disclosed that ESAT-6 driven macrophage differentiation to M1 phenotype and subsequent conversion to M2 phenotype (Refai et al. 2018). Buka et al. suggested that ESAT-6 effected the pro-inflammatory responses (Samten et al. 2011). The results in our studies figured out that ESAT-6 firstly promoted the differentiation of macrophages to M1 phenotype, then switching from the M1 phenotype to the M2 phenotype resulted in a decreased in the M1 phenotype and an increased in the M2 phenotype. This change may be due to the fact that at the beginning of the infection M1-type macrophages achieve pathogen killing by promoting an inflammatory response, and when the infection is finished the macrophages transform into M2-type or undergo apoptosis to protect the organism from excessive damage. Our study may provide a theoretical basis for the pathogenesis of tuberculosis.
It is reported that the secreted and immunogenic proteins of Mtb play an irreplaceable role in the pathogenesis of Mtb, including PPE44, HSPX, CFP-10 and ESAT-6 (Liu et al. 2021; Omar et al. 2021; Poulakis et al. 2016; Valizadeh et al. 2022). The cooperation of ESAT-6 and HSPX improved the effectiveness of BCG vaccination by inducing T-lymphocyte and NK-cell-mediated immune responses (Marongiu et al. 2013). Besides, it has been demonstrated that ESAT-6 promoted macrophage apoptosis by targeting downstream signaling pathways (Grover and Izzo 2012; Yang et al. 2015). And Shivraj et al. uncovered that ESAT-6 elevated expression of mitochondrial SOD and repressed autophagy (Yabaji et al. 2020). In our research, ESAT-6 initially induced macrophage conversion to the pro-inflammatory M1 phenotype but did not alter apoptosis, and subsequently promoted apoptosis in M1 macrophages, and increased the proportion of macrophages of the anti-inflammatory M2 phenotype, favoring Mtb immune escape and leading to chronic TB development. Therefore, ESAT-6 may be an effective therapeutic target for TB against Mtb infection, but the exact mechanism remains unclear.
Increasing evidence indicated that the TLR4/MyD88/NF-κB signaling pathway participates in many biological activities. For example, inhibition of the TLR4/MyD88/NF-κB signaling pathway alleviated ISE-induced cornea inflammation (Wu et al. 2021). Silencing TLR4/MyD88/NF-κB signaling pathway reduced the inflammatory response of the myocardium and improved cardiac function after CME (Su et al. 2018). Blocking the TLR4/MyD88/NF-κB signaling pathway ameliorated asthma pathology processes by hampering peribronchial and perivascular inflammation (Ma et al. 2021). Furthermore, TLRs-mediated signaling also plays an essential role in Mtb-induced macrophage (Sánchez et al. 2010). Li et al. indicated that inhibition of the TLR4/MyD88/NF-κB pathway depleted the progression of atherosclerosis by regulating lipid metabolism and macrophage-mediated inflammation (Li et al. 2021b). And Wang et al. showed that omentin-1 alleviated LPS-induced macrophage activation by blocking TLR4/MyD88/NF-κB pathway (Wang et al. 2020b). TLR2 was involved in the immune response of ESAT-6-stimulated cell in TB (Mandala et al. 2020). ESAT6 interacts with TLR2 to inhibit TLR signaling in TB (Pathak et al. 2007). Here, TLR4 mRNA levels and the protein expression levels of TLR4, MyD88 and NF-κB were remarkably improved by ESAT-6 treatment within 18 h, but its expression levels were visibly downregulated after 18 h. In addition, the effects of ESAT-6 on macrophage polarization and apoptosis could be regulated by the TLR4/MyD88/NF-κB pathway. However, whether TLR2 is involved in ESAT-6-mediated macrophage polarization and apoptosis remains to be further investigated. The present study revealed the different directions of polarization performed by macrophages in response to different periods of Mtb infection to participate in the immune response.
In conclusion, our data proposed that ESAT-6 primarily promoted M1 polarization in macrophages and then M1 transition to M2 or M1 apoptosis in macrophages via the TLR4/MyD88/NF-κB signaling pathway. Blocking the TLR4/MyD88/NF-κB pathway in early stages or activating it in late stages to inhibit apoptosis of M1 macrophages and the ratio of M2 macrophages facilitates the clearance of Mtb, inhibits the progression of TB chronicity, and improves disease prognosis.
Data Availability
All available data supporting the results of this study are included in the article.
References
Araujo Z, Fernández de Larrea C, López D, Isern-Kebschull J, de Waard JH, Hagel I, Camargo M, Vanegas M, Patarroyo MA (2021) ESAT-6 and Ag85A Synthetic Peptides as Candidates for an Immunodiagnostic Test in Children with a Clinical Suspicion of Tuberculosis. Dis Markers 2021:6673250
Bade P, Simonetti F, Sans S, Laboudie P, Kissane K, Chappat N, Lagrange S, Apparailly F, Roubert C, Duroux-Richard I (2021) Integrative Analysis of Human Macrophage Inflammatory Response Related to Mycobacterium tuberculosis Virulence. Front Immunol 12:668060
Bussi C, Gutierrez MG (2019) Mycobacterium tuberculosis Infection of host cells in space and time. FEMS Microbiol Rev 43:341–361
C ÓM, Cox DJ, Phelan JJ, Mitermite M, Murphy DM, Leisching G, Thong L, O’Leary SM, Gogan KM, McQuaid K et al (2021) Lactate alters metabolism in human macrophages and improves their ability to kill Mycobacterium tuberculosis. Front Immunol 12:663695
Chai Q, Wang L, Liu CH, Ge B (2020) New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol 17:901–913
Chen SN, Tan Y, Xiao XC, Li Q, Wu Q, Peng YY, Ren J, Dong ML (2021) Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol Sin 42:1610–1619
Clemmensen HS, Knudsen NPH, Billeskov R, Rosenkrands I, Jungersen G, Aagaard C, Andersen P, Mortensen R (2020) Rescuing ESAT-6 specific CD4 T cells from terminal differentiation is critical for Long-Term Control of Murine Mtb Infection. Front Immunol 11:585359
Coppola M, Arroyo L, van Meijgaarden KE, Franken KL, Geluk A, Barrera LF, Ottenhoff THM (2017) Differences in IgG responses against Infection phase related Mycobacterium tuberculosis (Mtb) specific antigens in individuals exposed or not to Mtb correlate with control of TB Infection and progression. Tuberculosis (Edinb) 106:25–32
de Martino M, Lodi L, Galli L, Chiappini E (2019) Immune Response to Mycobacterium tuberculosis: a narrative review. Front Pediatr 7:350
Faridgohar M, Nikoueinejad H (2017) New findings of toll-like receptors involved in Mycobacterium tuberculosis Infection. Pathog Glob Health 111:256–264
Gopalaswamy R, Shanmugam S, Mondal R, Subbian S (2020) Of Tuberculosis and non-tuberculous mycobacterial Infections - a comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci 27:74
Grover A, Izzo AA (2012) BAT3 regulates Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis of macrophages. PLoS ONE 7:e40836
Hsieh WY, Zhou QD, York AG, Williams KJ, Scumpia PO, Kronenberger EB, Hoi XP, Su B, Chi X, Bui VL et al (2020) Toll-like receptors induce Signal-Specific reprogramming of the macrophage lipidome. Cell Metab 32:128–143e125
Huang X, Li Y, Fu M, Xin HB (2018) Polarizing macrophages in Vitro. Methods Mol Biol 1784:119–126
Jha V, Pal R, Kumar D, Mukhopadhyay S (2020) ESAT-6 protein of Mycobacterium tuberculosis increases holotransferrin-mediated Iron Uptake in macrophages by Downregulating Surface hemochromatosis protein HFE. J Immunol 205:3095–3106
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu D, Cang J, Luo Z (2018) MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle 17:2001–2018
Khanna M, Rady H, Dai G, Ramsay AJ (2021) Intranasal boosting with MVA encoding secreted mycobacterial proteins Ag85A and ESAT-6 generates strong pulmonary immune responses and protection against M. Tuberculosis in mice given BCG as neonates. Vaccine 39:1780–1787
Korbecki J, Bajdak-Rusinek K (2019) The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res 68:915–932
Lam A, Prabhu R, Gross CM, Riesenberg LA, Singh V, Aggarwal S (2017) Role of apoptosis and autophagy in Tuberculosis. Am J Physiol Lung Cell Mol Physiol 313:L218–l229
Li C, Yang S, Ma H, Ruan M, Fang L, Cheng J (2021a) Influence of icariin on inflammation, apoptosis, invasion, and Tumor immunity in Cervical cancer by reducing the TLR4/MyD88/NF-κB and Wnt/β-catenin pathways. Cancer Cell Int 21:206
Li Y, Zhang L, Ren P, Yang Y, Li S, Qin X, Zhang M, Zhou M, Liu W (2021b) Qing-Xue-Xiao-Zhi formula attenuates Atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine 93:153812
Liu S, Wu M, Wu AE, Geng S, Li S, Li Z, Li M, Pang L, Kang Y W et al (2021) Factors associated with differential T cell responses to antigens ESAT-6 and CFP-10 in pulmonary Tuberculosis patients. Med (Baltim) 100:e24615
Ma B, Athari SS, Mehrabi Nasab E, Zhao L (2021) PI3K/AKT/mTOR and TLR4/MyD88/NF-κB signaling inhibitors attenuate pathological mechanisms of allergic Asthma. Inflammation 44:1895–1907
Mandala JP, Ahmad S, Pullagurla A, Thada S, Joshi L, Ansari MSS, Valluri VL, Gaddam SL (2020) Toll-like receptor 2 polymorphisms and their effect on the immune response to ESAT-6, Pam3CSK4 TLR2 agonist in pulmonary Tuberculosis patients and household contacts. Cytokine 126:154897
Marongiu L, Donini M, Toffali L, Zenaro E, Dusi S (2013) ESAT-6 and HspX improve the effectiveness of BCG to induce human dendritic cells-dependent Th1 and NK cells activation. PLoS ONE 8:e75684
Mily A, Kalsum S, Loreti MG, Rekha RS, Muvva JR, Lourda M, Brighenti S (2020) Polarization of M1 and M2 human monocyte-derived cells and analysis with Flow Cytometry upon Mycobacterium tuberculosis Infection. J Vis Exp
Mir SA, Sharma S (2022) Immunotherapeutic potential of n-terminally formylated ESAT-6 protein in murine Tuberculosis. Int J Mycobacteriol 11:108–112
Omar RA, Verma N, Arora PK (2021) Development of ESAT-6 based Immunosensor for the detection of Mycobacterium tuberculosis. Front Immunol 12:653853
Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J (2007) Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8:610–618
Poulakis N, Gritzapis AD, Ploussi M, Leventopoulos M, Papageorgiou CV, Anastasopoulos A, Constantoulakis P, Karabela S, Vogiatzakis E, Tsilivakos V (2016) Intracellular ESAT-6: a new biomarker for Mycobacterium tuberculosis Infection. Cytometry B Clin Cytom 90:312–314
Powers M, Sanchez TR, Welty TK, Cole SA, Oelsner EC, Yeh F, Turner J, O’Leary M, Brown RH, O’Donnell M et al (2020) Lung function and respiratory symptoms after Tuberculosis in an American Indian Population. The strong heart study. Ann Am Thorac Soc 17:38–48
Refai A, Gritli S, Barbouche MR, Essafi M (2018) Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front Cell Infect Microbiol 8:327
Saba K, Sameeullah M, Asghar A, Gottschamel J, Latif S, Lössl AG, Mirza B, Mirza O, Waheed MT (2020) Expression of ESAT-6 antigen from Mycobacterium tuberculosis in broccoli: an edible plant. Biotechnol Appl Biochem 67:148–157
Samten B, Wang X, Barnes PF (2011) Immune regulatory activities of early secreted antigenic target of 6-kD protein of Mycobacterium tuberculosis and implications for Tuberculosis vaccine design. Tuberculosis (Edinb) 91(Suppl 1):S114–118
Sánchez D, Rojas M, Hernández I, Radzioch D, García LF, Barrera LF (2010) Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis-induced macrophage death. Cell Immunol 260:128–136
Satoh T, Akira S (2016) Toll-like receptor signaling and its inducible proteins. Microbiol Spectr 4
Sawai M, Miyauchi Y, Ishida T, Takechi S (2022) Dihydropyrazine suppresses TLR4-dependent inflammatory responses by blocking MAPK signaling in human hepatoma HepG2 cells. J Toxicol Sci 47:381–387
Shneerson JM (2004) Respiratory Failure in Tuberculosis: a modern perspective. Clin Med (Lond) 4:72–76
Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, Mukhopadhyay S (2014) The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLoS Pathog 10:e1004446
Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J (2018) Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in Coronary Microembolization-Induced Myocardial Injury. Cell Physiol Biochem 47:1497–1508
Swanson L, Katkar GD, Tam J, Pranadinata RF, Chareddy Y, Coates J, Anandachar MS, Castillo V, Olson J, Nizet V et al (2020) TLR4 signaling and macrophage inflammatory responses are dampened by GIV/Girdin. Proc Natl Acad Sci U S A 117:26895–26906
Valizadeh A, Imani Fooladi AA, Sedighian H, Mahboobi M, Gholami Parizad E, Behzadi E, Khosravi A (2022) Evaluating the performance of PPE44, HSPX, ESAT-6 and CFP-10 factors in Tuberculosis Subunit vaccines. Curr Microbiol 79:260
Wang Y, Chen H, Chen Q, Jiao FZ, Zhang WB, Gong ZJ (2018) The Protective Mechanism of CAY10683 on Intestinal Mucosal Barrier in Acute Liver Failure through LPS/TLR4/MyD88 Pathway. Mediators Inflamm 2018:7859601
Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, Cao Y, Fisher M, Liu CZ (2020a) Acupuncture attenuates inflammation in Microglia of vascular Dementia rats by inhibiting miR-93-Mediated TLR4/MyD88/NF-κB signaling pathway. Oxid Med Cell Longev 2020:8253904
Wang J, Gao Y, Lin F, Han K, Wang X (2020b) Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem Biophys 679:108187
Wang P, Xiao T, Li J, Wang D, Sun J, Cheng C, Ma H, Xue J, Li Y, Zhang A et al (2021) miR-21 in EVs from pulmonary epithelial cells promotes myofibroblast differentiation via glycolysis in arsenic-induced pulmonary fibrosis. Environ Pollut 286:117259
Weiss G, Schaible UE (2015) Macrophage defense mechanisms against intracellular bacteria. Immunol Rev 264:182–203
Welin A, Eklund D, Stendahl O, Lerm M (2011) Human macrophages infected with a high burden of ESAT-6-expressing M. Tuberculosis undergo caspase-1- and cathepsin B-independent necrosis. PLoS ONE 6:e20302
Wu L, Du L, Ju Q, Chen Z, Ma Y, Bai T, Ji G, Wu Y, Liu Z, Shao Y et al (2021) Silencing TLR4/MyD88/NF-κB signaling pathway alleviated inflammation of corneal epithelial cells infected by ISE. Inflammation 44:633–644
Xie X, Lv H, Liu C, Su X, Yu Z, Song S, Bian H, Tian M, Qin C, Qi J et al (2021) HBeAg mediates inflammatory functions of macrophages by TLR2 contributing to hepatic fibrosis. BMC Med 19:247
Yabaji SM, Dhamija E, Mishra AK, Srivastava KK (2020) ESAT-6 regulates autophagous response through SOD-2 and as a result induces intracellular survival of Mycobacterium bovis BCG. Biochim Biophys Acta Proteins Proteom 1868:140470
Yang S, Li F, Jia S, Zhang K, Jiang W, Shang Y, Chang K, Deng S, Chen M (2015) Early secreted antigen ESAT-6 of Mycobacterium Tuberculosis promotes apoptosis of macrophages via targeting the microRNA155-SOCS1 interaction. Cell Physiol Biochem 35:1276–1288
Zhan L, Liu H, Zheng J, Meng J, Fu D, Pang L, Ji C (2022) Electroacupuncture at Zusanli Alleviates Sepsis by Regulating the TLR4-MyD88-NF-Kappa B Pathway and Diversity of Intestinal Flora. Evid Based Complement Alternat Med 2022:6706622
Zhang L, Zhang H, Zhao Y, Mao F, Wu J, Bai B, Xu Z, Jiang Y, Shi C (2012) Effects of Mycobacterium tuberculosis ESAT-6/CFP-10 fusion protein on the autophagy function of mouse macrophages. DNA Cell Biol 31:171–179
Zhang L, Jiang X, Pfau D, Ling Y, Nathan CF (2021) Type I interferon signaling mediates Mycobacterium tuberculosis-induced macrophage death. J Exp Med 218
Zhao R, Luo T, Ma P, Ge L, Chen Z, Wang X, Liao W, Bao L (2021) Improvement of the immunogenicity of ESAT-6 via fusion with the dodecameric protein dodecin of Mycobacterium tuberculosis. Microb Pathog 155:104890
Zhu L, Yang T, Li L, Sun L, Hou Y, Hu X, Zhang L, Tian H, Zhao Q, Peng J et al (2014) TSC1 controls macrophage polarization to prevent inflammatory Disease. Nat Commun 5:4696
Funding
This study was supported by the State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia Fund (SKL-HIDCA-2021-JH2) and the Youth Fund of Natural Science Foundation of Xinjiang Uygur Autonomous Region (2018D01C207).
Author information
Authors and Affiliations
Contributions
Feng Sun: conception, study design and wrote the manuscript. Jiangbo Li: performed the experiments and statistical analysis. Cunzi Yan and Ling Cao: collected, analyzed and interpretated the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to participate
All authors approved and were directly involved in the planning, execution, and analysis of the data presented here.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, F., Li, J., Cao, L. et al. Mycobacterium tuberculosis virulence protein ESAT-6 influences M1/M2 polarization and macrophage apoptosis to regulate tuberculosis progression. Genes Genom 46, 37–47 (2024). https://doi.org/10.1007/s13258-023-01469-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-023-01469-4