Abstract
Mutations in PARK2 are considered a common cause of Parkinson’s disease (PD). To assess the frequency of PARK2 mutations in the Korean population, we screened the PARK2 gene in 83 Korean PD patients: two young onset (YO, ≤ 49), 32 middle onset (MO, 50–69) and 49 late onset (LO, ≥ 70). Detection of the point mutations was performed by direct sequencing of the PARK2 exons, and exonic rearrangements were analyzed using multiplex ligation-dependent probe amplification. Five known PARK2 variants were identified in 53 (63.9 %) of the Korean PD patients: two missense mutations (Y267H and M458L) and three polymorphisms (S167N, L272I and V380L). We also found an increased frequency of PARK2 variants in PD patients and a lowered PD age at onset (AAO) in those having two variants, suggesting that the genetic variation in PARK2 gene might be a genetic risk factor of PD in Korean population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A PARK2 mutation was first described in a Japanese family with an autosomal recessive juvenile Parkinson’s disease (PD) (Kitada et al. 1998) and 213 different mutations have since been identified throughout PARK2, including large deletions or amplifications, small deletions or insertions, as well as missense mutations (The Parkinson Disease Mutation Database, accessed July 2015). Studies have shown PARK2 mutations in about 40-50 % of early onset FPD, and about 1.3–20 % of SPD patients (Choi et al. 2008; Hedrich et al. 2004; Kann et al. 2002; Mellick et al. 2009; Oczkowska et al. 2013; Sironi et al. 2008), suggesting that PARK2 dysfunction by mutations might be involved in the pathogenesis of both FPD and SPD.
PARK2, an E3 ubiquitin ligase regulates a variety of processes, including receptor trafficking and mitochondrial quality control, via mono- or poly-ubiquitinations of PARK2 substrates (Dawson and Dawson 2014). Several distinct pathomechanisms by PARK2 mutations have been reported. Many missense mutations reduce the enzymatic activity of PARK2, leading to the abnormal accumulation of toxic proteins and neurodegeneration (Corti et al. 2011; Dawson and Dawson 2010; Sul et al. 2013). The mutations in PARK2 gene also change the general physical characteristics of the protein such as stability and/or solubility, promoting aggregate formation and impairing mitochondrial maintenance, collectively resulting in increased cellular toxicity (Gaweda-Walerych and Zekanowski 2013; Hampe et al. 2006; Oczkowska et al. 2013; Sriram et al. 2005). These results suggest that PARK2 mutants might influence the risk of developing PD.
The frequency of PARK2 mutation has been reported in the 5.5–15.8 % of the Korean population with early onset PD (EOPD) (Choi et al. 2008; Chu et al. 2014; Kim et al. 2012). However, the frequency of PARK2 mutation has not been examined in Korean PD patients in other age at onset (AAO) groups. Therefore, this is the first analysis of frequency of PARK2 variants in Korean PD patients with AAO ≥ 50 years.
Materials and methods
Subjects
A total of 83 PD patients were included in the study. To analyze the relationship between AAO and variants, we divided patients into three groups based on their AAO of the disease (Mehanna et al. 2014): those younger than 49 years (young onset PD, YOPD), those between 50 and 69 years (middle onset PD, MOPD), and those older than 70 years (late onset PD, LOPD).
Mutation analysis
Genomic DNA was extracted from peripheral blood by standard protocol. The exons 1–12 of the PARK2 gene were amplified from genomic DNA using intronic primers (Table 1). The reaction mixture for PCR contained 50 ng of genomic DNA and 10 pmol of each primer. The PCR reactions were denatured at 94 °C for 5 min followed by 30 cycles of 94 °C for 45 s, 50–60 °C for 45 s, and 72 °C for 45 s with a final extension of 7 min at 72 °C. The PCR products were purified and sequenced directly using the ABI 3730XL DNA analyzer (Applied Biosystems).
Gene dosage analysis
Exon rearrangements were analyzed with a multiplex ligation-dependent probe amplification (MLPA) assay using commercially available probes (SALSA P051 Parkinson MLPA kits, MRC Holland). Sequence-specific probes enclosed in this kit are against all exons of PARK2 and PINK1, and specific exons of SNCA (exons1, 3–6); PARK7 (DJ-1, exons 1, 3, 5, 7); TNFRSF9 (exon 3); ATP13A2 (exon 2, 9); LPA (6q26) and two-point mutations (A30P in SNCA and G2019S in LRRK2). Experimental procedures were conducted for all 83 samples according to the manufacturer protocol. MLPA peaks analysis, normalization, and calculation of dosage ratio were performed with the GeneMarker software (SoftGenetics LLC) with standard parameters of analysis.
Statistical evaluation
The paired t test was used to compare mean AAOs among PD patients with no variant, with one variant, and with ≥2 variants. Values were expressed as mean ± SD and P < 0.05 was considered statistically significant.
Results
We sequenced all of the 12 exons of the PARK2 gene from 15 patients selected randomly from our PD samples in order to see a preliminary profile of the PARK2 gene of our PD patients. Sequence analysis of 15 patients disclosed three different missense mutations: S167N (exon 4), V380L (exon 10), and M458L (exon 12). We did not detect novel mutations other than the reported variations in exon 4, 10, and 12. Thereafter, we screened sequence variations in these three exons (exon 4, 10 and 12) of other patient samples, based on the above data. In addition, we also screened exon 2 encoding the ubiquitin-like (Ubl) domain, and exon 6 and 7 encoding RING1 domain, because mutations in these domains tend to affect the enzymatic function of PARK2 (Caulfield et al. 2014).
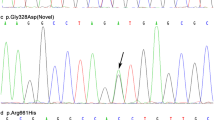
The demographic characteristics of the 83 patients are presented in Table 2 (YOPD, N = 2; MOPD, N = 32; LOPD, N = 49). Five variants identified in this study are S167N (c.500G > A), Y267H (c.799T > C), L272I (c.814C > A), V380L (c.1138G > C), and M458L (c.1372A > C) (Table 3). S167N polymorphism in exon 4 was the commonest variant (allele frequency of 40.4 %) when compared to the other polymorphisms. L272I and V380L alleles were found relatively infrequently (0.6 and 4.2 % of the allele frequencies in the PD patients, respectively).
In order to detect exon dosage changes caused by genomic rearrangements of the known PD genes, we used the SALSA P051-B1 kit. The kit consists of probes for specific exons of SNCA, PARK7, TNFRSF9, ATP13A2, and LPA, as well as point mutations of SNCA A30P and LRRK2 G2019S. Three out of 83 patients had genomic duplications in the PARK2 and PINK1 loci (Fig. 1; Supplementary Table 1) and other major PD gene rearrangements were not detected: two patients had duplication of the PARK2 gene and one patient had duplication of the PINK1 gene.
When point mutations and exonic rearrangements were taken together, PARK2 variants were found in 54 (65 %) of 83 PD patients (Table 4): 23 patients carried compound heterozygous or homozygous variants, and 31 patients carried a single heterozygous variant. One patient with PINK1 mutation did not have PARK2 variant.
Discussion
PARK2 is a well-known risk factor for juvenile and early onset occurrence of PD (Kitada et al. 1998). Moreover, the importance of PARK2 mutations in sporadic PD have also been reported (Foroud et al. 2003; Oczkowska et al. 2013). In the Korean population, PARK2 mutations were reported in about 5.5–18.5 % of EOPD and FPD (Choi et al. 2008; Chu et al. 2014; Kim et al. 2011, 2012). However, genetic study of late onset PD has not yet been reported. Therefore, this study assessed the frequency of PARK2 mutation in Korean PD patients, with AAO after 50 years.
We found that S167N polymorphism was the commonest with high frequency (40.4 %) in our study. Studies regarding the allele frequency of S167N vary greatly among populations with different ethnic origins ((Li et al. 2005; Martinez et al. 2010; Mellick et al. 2001; Sakai et al. 2010; Sinha et al. 2005). In the Asian SPD populations (Japanese, Chinese, and Taiwanese), the frequency of S167N polymorphism was 38.6–46.6 % (Supplementary Table 2) and that of Korean SPD in this study also fits in this range. On the other hand, S167N allele was found in only 0.2–2.5 % of a Caucasian population. The frequency of S167N polymorphism was also higher in the Asian EOPD population (around 40 %) than in the Caucasian EOPD population (2.6–12.9 %) (Supplementary Table 3). Taken together, S167N polymorphism may be a susceptibility factor to PD in Asian populations.
The study by Ghione et al.(Ghione et al. 2007) reported that the combination of PARK2 polymorphisms including S167N (14 % of cases, heterozygous) and environmental factors including pesticides, organic solvents, and rural living, strongly affects lowering the AAO of PD. S167N is located in exon 4 encoding the RING0/unique Parkin domain (UPD), a PINK1 interacting domain. PINK1 kinase activates PARK2 via phosphorylation of PARK2 on residue Ser65 (Iguchi et al. 2013; Kondapalli et al. 2012; Okatsu et al. 2012; Seirafi et al. 2015). It would be interesting to know whether S167N polymorphism interferes with the binding of PARK2 to PINK1 when an environmental risk factor is added. It is also probable that S167N polymorphism may promote aggregation or impair mitochondrial homeostasis by environmental and/or other genetic factors. Further functional studies of PARK2 polymorphisms would be useful for diagnostic and prognostic processes related to PD.
In the Korean LOPD patients, we found PARK2 exon duplication in only two patients: one had exon 5 duplication and the other had multiple duplications (exon 2, 3, 5–11) (Fig. 1 and Supplementary Table 1). On the other hand, a larger case study showed duplication of PARK2 on exons 2–7 in 2,091 PD patients (mean AAO 58.3 ± 12.1 years), and duplication of exon 1, 2, and 3 in 1,686 controls (mean age 66.1 ± 13.1 years) (Kay et al. 2010). Interestingly, only duplication of exon 4 was reported in American, Polish, and French EOPD patients (Ambroziak et al. 2015; Hedrich et al. 2001; Kay et al. 2010; Periquet et al. 2003). Therefore, it seems worthy to further investigate the role of exon 4 of PARK2 in the pathomechanism of EOPD.
To assess the relevance between variants and AAO, we performed a subgroup analysis (Mehanna et al. 2014): YOPD, MOPD and LOPD (Table 2). In MOPD, the average AAO was lower in patients with ≥2 variants than in patients with one variant, or without variant of statistical significance. We also observed lowered average AAO in LOPD patients with ≥2 variants; however, this was without statistical significance.
In this study, two variants (Y267H and L272I) in exon 7 encoding the RING1 domain were found in LOPD, while two variants (V380L and M458L) in exon 10 and 12 encoding c-terminus, were observed in MOPD (Table 3). RING1 recruits the E2 enzyme, but loss of the RING1 domain does not result in a loss of ligase activity (Chew et al. 2011; Matsuda et al. 2006; Spratt et al. 2013). This suggests that PARK2 is an E2-independent ubiquitin ligase, a RING between RING (RBR) E3 ligase. RING2 in the c-terminus of PARK2 is the true catalytic domain, and structural study of RING2 has shown that it is required for E2 recruitment, catalysis, and trans autoubiquitination. Consistent with this, AAOs of patients with c-terminal variant (V380L and M458L) were lowered when compared to those of patients with RING1 variant in our study (Table 3). Our data also suggest that the c-terminus of PARK2 might play a more important role than the RING1 region, in PD pathogenesis.
In conclusion, we have for the first time, presented a genetic analysis of the PARK2 gene in relation to Korean MOPD and LOPD. A high frequency of PARK2 variants in PD patients was found, suggesting that PARK2 variants might be a genetic risk factor for PD in Korean populations.
References
Ambroziak W, Koziorowski D, Duszyc K, Gorka-Skoczylas P, Potulska-Chromik A, Slawek J, Hoffman-Zacharska D (2015) Genomic instability in the PARK2 locus is associated with Parkinson’s disease. J Appl Genet. doi:10.1007/s13353-015-0282-9
Caulfield TR, Fiesel FC, Moussaud-Lamodiere EL, Dourado DF, Flores SC, Springer W (2014) Phosphorylation by PINK1 releases the UBL domain and initializes the conformational opening of the E3 ubiquitin ligase Parkin. PLoS Comput Biol 10:e1003935
Chew KC, Matsuda N, Saisho K, Lim GG, Chai C, Tan HM, Tanaka K, Lim KL (2011) Parkin mediates apparent E2-independent monoubiquitination in vitro and contains an intrinsic activity that catalyzes polyubiquitination. PLoS ONE 6:e19720
Choi JM, Woo MS, Ma HI, Kang SY, Sung YH, Yong SW, Chung SJ, Kim JS, Shin HW, Lyoo CH et al (2008) Analysis of PARK genes in a Korean cohort of early-onset Parkinson disease. Neurogenetics 9:263–269
Chu MK, Kim WC, Choi JM, Hong JH, Kang SY, Ma HI, Kim YJ (2014) Analysis of dosage mutation in PARK2 among Korean patients with early-onset or familial Parkinson’s disease. J Clin Neurol 10:244–248
Corti O, Lesage S, Brice A (2011) What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev 91:1161–1218
Dawson TM, Dawson VL (2010) The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord 25(Suppl 1):S32–S39
Dawson TM, Dawson VL (2014) Parkin plays a role in sporadic Parkinson’s disease. Neurodegener Dis 13:69–71
Foroud T, Uniacke SK, Liu L, Pankratz N, Rudolph A, Halter C, Shults C, Marder K, Conneally PM, Nichols WC et al (2003) Heterozygosity for a mutation in the parkin gene leads to later onset Parkinson disease. Neurology 60:796–801
Gaweda-Walerych K, Zekanowski C (2013) Integrated pathways of parkin control over mitochondrial maintenance—relevance to Parkinson’s disease pathogenesis. Acta Neurobiol Exp (Wars) 73:199–224
Ghione I, Di Fonzo A, Saladino F, Del Bo R, Bresolin N, Comi GP, Rango M (2007) Parkin polymorphisms and environmental exposure: decrease in age at onset of Parkinson’s disease. Neurotoxicology 28:698–701
Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O (2006) Biochemical analysis of Parkinson’s disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet 15:2059–2075
Hedrich K, Kann M, Lanthaler AJ, Dalski A, Eskelson C, Landt O, Schwinger E, Vieregge P, Lang AE, Breakefield XO et al (2001) The importance of gene dosage studies: mutational analysis of the parkin gene in early-onset parkinsonism. Hum Mol Genet 10:1649–1656
Hedrich K, Eskelson C, Wilmot B, Marder K, Harris J, Garrels J, Meija-Santana H, Vieregge P, Jacobs H, Bressman SB et al (2004) Distribution, type, and origin of Parkin mutations: review and case studies. Mov Disord 19:1146–1157
Iguchi M, Kujuro Y, Okatsu K, Koyano F, Kosako H, Kimura M, Suzuki N, Uchiyama S, Tanaka K, Matsuda N (2013) Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation. J Biol Chem 288:22019–22032
Kann M, Jacobs H, Mohrmann K, Schumacher K, Hedrich K, Garrels J, Wiegers K, Schwinger E, Pramstaller PP, Breakefield XO et al (2002) Role of parkin mutations in 111 community-based patients with early-onset parkinsonism. Ann Neurol 51:621–625
Kay DM, Stevens CF, Hamza TH, Montimurro JS, Zabetian CP, Factor SA, Samii A, Griffith A, Roberts JW, Molho ES et al (2010) A comprehensive analysis of deletions, multiplications, and copy number variations in PARK2. Neurology 75:1189–1194
Kim HJ, Kim HJ, Lee JY, Yun JY, Kim SY, Park SS, Jeon BS (2011) Phenotype analysis in patients with early onset Parkinson’s disease with and without parkin mutations. J Neurol 258:2260–2267
Kim SY, Seong MW, Jeon BS, Kim SY, Ko HS, Kim JY, Park SS (2012) Phase analysis identifies compound heterozygous deletions of the PARK2 gene in patients with early-onset Parkinson disease. Clin Genet 82:77–82
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608
Kondapalli C, Kazlauskaite A, Zhang N, Woodroof HI, Campbell DG, Gourlay R, Burchell L, Walden H, Macartney TJ, Deak M et al (2012) PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol 2:120080
Li X, Kitami T, Wang M, Mizuno Y, Hattori N (2005) Geographic and ethnic differences in frequencies of two polymorphisms (D/N394 and L/I272) of the parkin gene in sporadic Parkinson’s disease. Parkinsonism Relat Disord 11:485–491
Martinez HR, Gonzalez-Gonzalez H, Cantu-Martinez L, Rangel-Guerra R, Hernandez-Castillo CD, Vergara-Saavedra JJ, Ramos-Gonzalez MR, Cerda-Flores RM, Morales-Garza MA, Guerrero-Munoz MJ et al (2010) PARKIN-coding polymorphisms are not associated with Parkinson’s disease in a population from northeastern Mexico. Neurosci Lett 468:264–266
Matsuda N, Kitami T, Suzuki T, Mizuno Y, Hattori N, Tanaka K (2006) Diverse effects of pathogenic mutations of Parkin that catalyze multiple monoubiquitylation in vitro. J Biol Chem 281:3204–3209
Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC (2014) Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism Relat Disord 20:530–534
Mellick GD, Buchanan DD, Hattori N, Brookes AJ, Mizuno Y, Le Couteur DG, Silburn PA (2001) The parkin gene S/N167 polymorphism in Australian Parkinson’s disease patients and controls. Parkinsonism Relat Disord 7:89–91
Mellick GD, Siebert GA, Funayama M, Buchanan DD, Li Y, Imamichi Y, Yoshino H, Silburn PA, Hattori N (2009) Screening PARK genes for mutations in early-onset Parkinson’s disease patients from Queensland, Australia. Parkinsonism Relat Disord 15:105–109
Oczkowska A, Kozubski W, Lianeri M, Dorszewska J (2013) Mutations in PRKN and SNCA genes important for the progress of Parkinson’s disease. Curr Genomics 14:502–517
Okatsu K, Oka T, Iguchi M, Imamura K, Kosako H, Tani N, Kimura M, Go E, Koyano F, Funayama M et al (2012) PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun 3:1016
Periquet M, Latouche M, Lohmann E, Rawal N, De Michele G, Ricard S, Teive H, Fraix V, Vidailhet M, Nicholl D et al (2003) Parkin mutations are frequent in patients with isolated early-onset parkinsonism. Brain: J Neurol 126:1271–1278
Sakai M, Tsujino A, Eguchi H, Sato K, Shirabe S, Tateishi Y, Sato A, Tsujihata M, Yoshimura T, Eguchi K (2010) A single-nucleotide polymorphism of PARK2 affects the phenotype in sporadic Parkinson disease. Acta Med Nagasaki 54:67–71
Seirafi M, Kozlov G, Gehring K (2015) Parkin structure and function. FEBS J 282:2076–2088
Sinha R, Racette B, Perlmutter JS, Parsian A (2005) Prevalence of parkin gene mutations and variations in idiopathic Parkinson’s disease. Parkinsonism Relat Disord 11:341–347
Sironi F, Primignani P, Zini M, Tunesi S, Ruffmann C, Ricca S, Brambilla T, Antonini A, Tesei S, Canesi M et al (2008) Parkin analysis in early onset Parkinson’s disease. Parkinsonism Relat Disord 14:326–333
Spratt DE, Martinez-Torres RJ, Noh YJ, Mercier P, Manczyk N, Barber KR, Aguirre JD, Burchell L, Purkiss A, Walden H et al (2013) A molecular explanation for the recessive nature of parkin-linked Parkinson’s disease. Nat Commun 4:1983
Sriram SR, Li X, Ko HS, Chung KK, Wong E, Lim KL, Dawson VL, Dawson TM (2005) Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet 14:2571–2586
Sul JW, Park MY, Shin J, Kim YR, Yoo SE, Kong YY, Kwon KS, Lee YH, Kim E (2013) Accumulation of the parkin substrate, FAF1, plays a key role in the dopaminergic neurodegeneration. Hum Mol Genet 22:1558–1573
Acknowledgments
This study was supported by an internal grant (#2014-0810-01) of Chungnam National University, Daejeon, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no financial conflicts of interest.
Compliances of bioethical regulation
Written informed consent was approved by the Institutional Review Board of Eulji University Hospital. The experimental procedures followed the standard regulation of the Review Board.
Additional information
Min-young Park and In won Park have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, My., Park, I.w., Ihm, C.h. et al. PARK2 gene variants in Korean patients with Parkinson’s disease. Genes Genom 38, 163–169 (2016). https://doi.org/10.1007/s13258-015-0351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-015-0351-9