Abstract
Electrocardiogram (ECG) and photoplethysmograph (PPG) are non-invasive techniques that provide electrical and hemodynamic information of the heart, respectively. This information is advantageous in the diagnosis of various cardiac abnormalities. Arrhythmia is the most common cardiovascular disease, manifested as single or multiple irregular heartbeats. However, due to the continuous manual observation, it becomes troublesome for experts sometimes to identify the paroxysmal nature of arrhythmia correctly. Moreover, due to advancements in technology, there is an inclination towards wearable sensors which monitor such patients continuously. Thus, there is a need for automatic detection techniques for the identification of arrhythmia. In the presented work, ECG and PPG-based state-of-the-art methods have been described, including preprocessing, feature extraction, and classification techniques for the detection of various arrhythmias. Additionally, this review exhibits various wearable sensors used in the literature and public databases available for the evaluation of results. The study also highlights the limitations of the current techniques and pragmatic solutions to improvise the ongoing effort.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the 2017 world health organization (WHO) report, cardiovascular disease is one of the most prevalent causes of death, with an estimated 17.3 million deaths [1]. Arrhythmia is a commonly diagnosed cardiovascular disease caused due to an irregular heartbeat. It is caused when the electrical impulses that coordinate with the heartbeats do not work rightly, resulting in the heart beating either too fast, too slow, or skipping a beat, showing hardly any insignia of heart disease [2]. The irregularity of the heartbeat caused due to arrhythmias can be classified into two categories: (a) arrhythmias due to impulse production and (b) arrhythmias due to impulse conduction [3]. Arrhythmias due to impulse production can be further classified into four categories: premature beats, non-sinus rhythm, fibrillation, tachycardias/Bradycardias, and flutter. Premature beats are the ecotopic beats that arise outside the sinus node and occurs before the following sinus rhythm [4], where ventricular ectopic beat (VEB) occurs in the heart's lower chambers. It is caused when the heart is unable to fill with an adequate amount of blood, resulting in inadequate pumping of blood from the heart to other parts of the body [5]. Premature ventricular contraction (PVC) is a commonly occurred VEB arrhythmia caused due to the early contraction of the ventricles [5]. Atrial fibrillation (AF) and ventricular fibrillation (VF) are the types of fibrillation that arise in the heart [5], where AF occurs when the upper chambers of the heart sense unrestricted electric signals that result in the bombardment of electric signals between the atria and ventricles. On the contrary, VF occurs when the heart is unable to pump blood throughout the body due to the variation in left and right atrium contraction [5]. Tachycardia occurs when the impulse travels rapidly around the heart, resulting in the heart beating swiftly, whereas bradycardia occurs when the heart beats slower than normal [5]. Arrhythmias due to impulse conduction can be classified into atrioventricular (AV) block, bundle branch block, Wolff Parkinson White (WPW) syndrome, and escape beats [3]. AV block is an arrhythmia occurring at the atrioventricular node. In this, the atria and ventricles of the heart become impaired due to the anatomical heart’s conduction system. Thus impulses are delayed or disturbed in transmitting an impulse from the atria to the ventricles [6]. In bundle branch block (BBB), there is a blockage in pathways of the heart, caused due to damaged heart muscle, and in WPW syndrome, extra electrical pathways occur between the atria and ventricular chambers of the heart resulting into rapid heart rate [7].
These irregular heartbeats can be monitored using different ECG or PPG modalities [8], as shown in Fig. 1, where ECG monitors the electric activity of the heart, and PPG monitors the volumetric change of blood in the microvascular bed of the tissue. In ECG, the electrical signals generated in the atrium of the heart are recorded by placing electrodes on the chest and calculating the potential difference between the electrodes at different angles [9]. 12-lead ECG is a standard method to diagnose various cardiovascular diseases. However, due to its fewer limitations, i.e., mobility, uncomfortable, high cost, limited to hospital settings etc., various single-lead, multiple-lead portable ECG sensors are being developed for the measurement of a heartbeat in out-of-hospital settings. The waveform obtained using an ECG sensor is shown in Fig. 2. The waveform consists of P-wave, QRS-wave, and T-wave, where P-wave represents depolarization of atria, QRS-wave represents depolarization contraction of ventricles, and T-wave represents repolarization of ventricles [2].
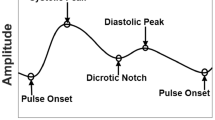
On the other hand, in PPG, the light emitted by diodes reflects/transmits from the skin and measures blood flow. The change in blood flow is thereby used in the detection of arrhythmia. Since the wavelength of green light used in the PPG sensor is strongly absorbed by blood compared to other neighbouring tissues, therefore is preferably used in the detection of change in the blood flow from the microvascular tissue bed [10]. The working principle of the PPG sensors is shown in Fig. 3, where Fig. 3i shows, incident light travels from the sensor. Through a microvascular bed of tissues, the light either gets reflected or transmitted. The amount of light reflected through the epidermis layer of the skin is thus used in the detection of abnormality. Figure 3ii represents the PPG pulse obtained on signals reflection, where a represents depolarization of atria (systolic phase), b repolarization of ventricles (diastolic phase), and c is the pulse width of the heart. The change observed in these pulsatile waveforms is used to identify various cardiac abnormalities. PPG sensors can be mounted at different locations of the body viz ear (transmitting sensor) [11], finger (transmitting sensor) [12], wrist (reflective sensor) [13], or forehead (reflective sensor) [12].
i Working principle of PPG, where dashed line indicates the incident light being transmitted, reflected from the epidermis layer, and absorbed in the dermis layer of the skin. The reflected light is obtained in the form of PPG signal, and ii Representation of PPG waveform, where a represents the depolarization of atria, b represents the repolarization of ventricles, and c represents the pulse width
Due to the paroxysmal nature of arrhythmia, it becomes troublesome for experts to monitor every heartbeat continuously. Thus, there is an unmet need for automated detection of arrhythmia. State-of-the-art shows various review articles for arrhythmia detection using machine learning and deep learning methods on ECG signals [14,15,16,17,18,19]. Studies also reported several PPG-based vital signs monitoring, i.e., heart rate and arrhythmia monitoring review articles [12, 20, 21]. However, a collective review on ECG and PPG-based detection techniques for arrhythmia detection has not been reported so far.
The amount of research articles published on arrhythmia detection using ECG and PPG modalities is shown in Fig. 4. The data utilized in the graph has been obtained from the web of science database published since 2010. Furthermore, the number of research articles on single/multiple-lead ECG, reflected/transmissive PPG sensors has been obtained by observing the type of sensor used. It can be observed from the graph that 1209 research articles were published on ECG-based arrhythmia detection, where 812 articles are on single-lead ECG sensors and 142 on multiple-lead ECG sensors. The majority of the literature on single-lead ECG data for arrhythmia detection has used lead-II ECG for identification. On the other hand, PPG reported 109 published research articles on arrhythmia detection, where 62 articles are on the use of reflective PPG sensors and 34 on transmissive PPG sensors. The review presents a comprehensive study on selected articles every year on ECG and PPG-based arrhythmia detection and classification methods carried out since the last decade. Studies related to various feature extraction approaches, that is, wavelets, machine learning, morphological, statistical, etc., on ECG data have been included in the review. Various noise removal and classification techniques advantageous in arrhythmia detection are also included in the study, and merits/demerits of the methods have also been discussed. On the contrary, the review has included an exploration of the use of PPG signals for noise and motion artifacts removal, heartbeat detection, and arrhythmia detection as an alternate technique to ECG for in-and-out-of-hospital settings. The use of ECG and PPG methods for detecting arrhythmia in combination with other abnormalities, other CV diseases, and comparison of ECG and PPG methods for heart rate measurements during other abnormalities are excluded from the study. The publically available databases that could be used in the research are also discussed in this review.
The graph shows the number of research articles on arrhythmia detection using ECG and PPG modalities. The first column in each modality depicts all arrhythmia detection methods, second column represents arrhythmia detection methods using single-lead ECG/reflected PPG sensor, third column represents multiple-lead ECG/transmissive PPG sensors, and forth represents the number of review articles for each modality. The data has been acquired from Web of science database published since 2010
The paper is organized as follows: Sect. “Databases” discusses publically available databases that can be used for experimentation. Sect. “Review” discusses the various preprocessing, feature extraction, and classification methods that can be used for arrhythmia detection. Finally, Sects. “Discussion” and “Conclusion” discuss the limitations, challenges, and areas of arrhythmia classification research with particular reference to ECG and PPG signals are highlighted.
Databases
Various databases are publically available containing patients’ cardiac records. These records could be used for the evaluation of several cardiovascular diseases. The American National Standards Institute (ANSI) and AAMI approved the national consensus records and practices, giving technical information reports for medical devices. Following are the publically available databases recommended and widely used for the evaluation of several cardiovascular diseases [19]:
-
PhysioNet database: It is a research resource for physiological signals initiated by the Massachusetts Institute of Technology—Beth Israel Hospital Arrhythmia Database (MIT-BIH), providing free access to the recorded biomedical and physiologic signals since 1999. The database also provides ECG and PPG arrhythmia data, where ECG arrhythmia data can be found in the MIT-BIH arrhythmia database [22]. The database contains 48 records obtained from 47 subjects. The sampling frequency of the signals is 360 Hz. The majority of the records were collected from lead II, the remaining using V2, V4, or V5 leads. PhysioNet database also provides PPG data which can be found namely in MIMIC-II [23] and Challenge 2015 [24] along with ECG signals, namely lead II, where MIMIC-II contains 25,328 records of clinical and waveform data from the medical, surgical intensive care unit (ICU), cardiac care unit, and coronary care unit sampled at 125 Hz. However, annotations of the signals are not provided in the PPG database. Thus signals need to be annotated before usage. Challenge 2015 database contains signals having false alarm signals, thus encouraging the development of an algorithm to reduce these false alarms. It includes 1250 train test records sampled at 250 Hz.
-
AHA: The American Heart Association provides arrhythmia and Normal signal 12-lead ECG data from more than 6300 hospitals and 400 healthcare systems [25]. It contains eight categories of arrhythmia from 154 records [26]. The ECG signals acquired are sampled at 250 Hz. However, the data is not public and does not contain PPG signals.
-
European ST-T Database: It is a European database set up by European Community in 1985. The database contains 90 annotated ECG records obtained from 79 subjects having 372 ST and 423 T changes. Each record is of 2 h duration sampled at 250 Hz. The database is public and was acquired using 2-channel ECG [27]. The limitation of the record is that it does not contain a PPG database.
The databases discussed above provide annotations containing fiducial points with the type of heartbeat for ECG recordings. However, the MIT-BIH arrhythmia database presented the majority of the published article for ECG records. In contrast, the PhysioNet MIMIC-II and PhysioNet Challenge 2015 databases showed most of the published articles for PPG records.
Review
The functional state of the heart is limited by the shape of the ECG and PPG waveform. It may comprise important pointers that suggest the nature of diseases troubling the heart. In this section, several ECG and PPG-based arrhythmia detection methods are discussed in detail.
ECG based arrhythmia detection
Electrical signals of the heart obtained using ECG can be used for the detection of various cardiovascular diseases. However, the signals directly received from the sensor contain noise and motion artifacts that complicate the accurate analysis of the disease. Hence, at first, there is a need to preprocess the signals to remove noise and motion artifacts. Thereafter, pivotal points are extracted for the detection of arrhythmic beats. Finally, identification of the type of arrhythmia is carried out using various classification approaches. The following sub-sections discuss various preprocessing, feature extraction, and classification techniques used for the ECG signal analysis.
Preprocessing
ECG signals possess the following categories of noise: powerline interference, electrode–skin interference, radio frequency surgical noise, and baseline drift due to respiration [28]. Various noise removal techniques reported in the literature are bandpass filtering [29,30,31,32,33,34,35,36], average and median filter [37,38,39,40,41], wavelet transform [40, 42,43,44]. Table 1 shows the list of commonly used ECG-based preprocessing methods found in the literature. Bayasi et al.[36]. implemented bandpass filtering for noise and artifacts removal, with cuts off delay of 5 Hz. The filtering technique has been able to remove noise effectively. However, the bandpass filtering approach has few limitations, i.e., it cannot track time-varying ECG characteristics that change quasi-periodically. Moreover, the cut-off frequency is fixed in this filtering technique, resulting in significant distortion of the ST segment and the QRS segment. Thus, authors in [40, 42,43,44] implemented higher-scale wavelet coefficients to de-noise ECG signals. In wavelets, coefficients obtained can be eliminated using a predefined threshold, resulting in additive noise removal. The study reported by Lee et al.[45] presents a novel first-order intrinsic mode function for motion and noise artifacts detection.
The preprocessing method used also depends on the quality of the signal received from the database or sensor. The majority of the studies reported in the literature implemented the filtering techniques on the MIT-BIH database. The commonly used filtering methods on the database are bandpass and wavelet-based filtering methods. Once a preprocessed signal is obtained, the next is to extract features that are useful in arrhythmia detection. In the following subsection, various ECG-based feature extraction methods have been discussed.
ECG based feature extraction
Feature extraction is an essential step in arrhythmia detection, where features represent the characteristics of a signal that may vary from the other part or signal. These features can be obtained from ECG morphology in frequency as well as in the time domain. Various approaches have been proposed in the literature for feature extraction of the ECG signals, respectively. Table 2 shows some significant studies reported in the literature for feature extraction of ECG signals.
Time-domain features are the prominent feature extraction approach in ECG arrhythmia detection. It is anticipated that the variation in the difference between RR intervals is correlated with the chance of having an arrhythmia. Mert et al. [49] extracted nine time-domain features, including R wave detection, PR, ST, QRS, and slopes, RR interval, etc. Later, bagged decision trees (BDT) and a single decision tree (DT) were used for learning. The study was carried out on 22 ECG signals containing 56,569 heartbeats. The results show that the BDT classifier gives improved results than the DT method. Salam et al. [2] detected R wave, QRS segment, and ST-segment by analyzing the inflection point of the signal using an adaptive filter. These inflection points are recognized by the change in the slope of the respective segments. The authors also analyzed non-stationary signals using discrete wavelet transform in this research work [2]. The detection accuracy of peaks obtained using these features is 98.65%. Deep learning methods have also been used for the extraction of appropriate features [50]. Isin et al. [50] extracted QRS wave features using a deep learning approach. Zeraatkar et al. [51] segment T-wave features into five different levels using the Daubechies wavelet. The accuracy obtained in detecting T-wave is satisfactory. Sayadi et al. [52] proposed a model-based beat segmentation approach, where every heartbeat is used as a combination of Gaussian kernels. Later, the kernels are compared to the peak points and each kernel is represented as a PQRST waveform.
The study reported by Ozbay et al. [53] demonstrates a complex wavelet transform for feature extraction using complex random numbers. The results show that the time taken to train a model is reduced without affecting the model's accuracy [53]. Ye et al. [31] proposed a method to extract independent components using the JADE algorithm. In this authors, calculated independent components using fixed-point algorithms. Later, these independent components are ordered using the JADE algorithm, and independent components are evaluated using various preprocessing, feature extraction, and classification methods [31]. In [54], the authors proposed an approach where ICA and wavelet transform is used in extracting morphological features for each heartbeat. Later, its corresponding coefficients are dimensionally reduced using PCA. However, to attain higher accuracy and sensitivity, several independent components would be required, resulting in an increase in the complexity of the algorithm. Fira and Goras [55] presented adaptive hysteretic filtering, extreme local extraction, and Lempel–Ziv-Welch based signal compression technique. In this skeleton, samples are discarded. A few samples are added using hysteretic filtering. Later, a signal reconstruction is carried out using the Lempel–Ziv-Welch method. A study by Lee et al. [56] reported five steps for ECG signal compression. First, ECG signals are downsampled by ½ and represented as 1 byte. Second, R-peaks are detected using a downslope trace window algorithm and are classified from the previous peak. Third, a discrete cosine transform of the data is obtained. Fourth, a window filter is used to filter out the transformed data. Lastly, the Huffman coding algorithm is used for data compression. The reported algorithm shows its robustness on all MIT-BIH arrhythmia databases. Ozbay et al. [57] proposed a clustering algorithm that reduces ECG samples from 106 to 67 clusters. The proposed approach is also used to inflate the number of features. However, the result shows an insignificant difference from the previous literature. Thereby, Korurek et al. [58] reduced the sample size from 100 to 20 clusters. The authors cluster the QRS segment during the learning phase using the Ant Colony Optimization (ACO) technique. The study reports an overall sensitivity of 94.40%. Yaghouby et al. [59] proposed a hybrid approach for the detection of atrial fibrillation using genetic programming orthogonal least squares and simulated annealing methods.
In [40, 41, 43, 46, 60,61,62] and [33], authors implemented a DWT approach to extract ECG signal features. Llamedo et al. [62] considered the features from ECG samples containing RR series and scales of the wavelet transform. Thereafter, the floating feature selection algorithm is used to select eight features. The results show that the generalization capability of the method outperforms other DWT-based state-of-the-art methods. Rai et al. [40] separated 64 features into two DWT based and morphological based classes, where DWT contain 48 features and morphological based class contain 16 features. These features are fed as an input to the classifier. The results show that the specificity obtained using MLP and BPN is 100% [40]. The use of the Daubechies wavelet approach for the extraction of features is most appropriate and obtains preeminent accuracy. In [60], the ECG signal is distributed into eight levels, and basic features such as minimum and maximum of the signal are obtained along with the time features. Thereafter, these time features and discrete wavelet features are feed-forward for further classification. Kutlu et al. [41] proposed automatic heartbeat recognition using the high order statistics (HOS) of the wavelet packet decomposition (WPD) [41]. In this approach, the authors first calculated coefficients for each varied ECG beat. Then HOS of these coefficients is derived, and finally, the obtained features are fed into the classifier. Thomas et al. [33] extract time-domain features and other features like kurtosis, skewness, and timing information using the dual-tree CWT approach. The results obtained using wavelet-based approaches for feature extraction are significant in arrhythmia detection. However, implementing the wavelet transform for feature extraction also has fewer limitations. DWT can resolve only three spatial domain orientations, resulting in poor selectivity of diagonal features. Moreover, DWT is shift sensitive, thus it is not able to distinguish between signal shifts. Therefore it becomes important to appropriately preprocess the input ECG signal to avoid variations in obtained transformation coefficients. Faezipour et al. [63] presented a profiling scheme where wavelets are used for beat detection and classification [63]. In this, authors considered ECG waveform as a data packet and implemented packet processing techniques. Plawiak et al. [64] used power spectral density based on Welsh and Fourier transform method for feature extraction. To calculate power spectral density, the authors used hamming windows of 128, 256, 512, and 1024 samples.
Hong et al. [65] and Zhang et al. [66] proposed a neural network features extraction approach where authors in [65] combined deep neural networks and engineered features for the detection of arrhythmia. The engineered features used are medical knowledge-based hand-crafted. Thus authors in [66] proposed Spatio-temporal mechanism incorporation into convolutional neural networks for the extraction of informative features. Asl et al. [68] proposed feature extraction using generalized discriminant analysis (GDA) [68]. GDA is a generalization of LDA that maps input data into an expedient high dimensional feature space with LDA properties. Thus, GDA can be used for dimensionality reduction and for discriminating features as well.
In addition to feature extraction, compression techniques are used to preserve useful diagnostic information. Most of the data extracted from the heart's electrical signals contain useless information; nearly 10% of the data contain valuable signals. Therefore, various compression techniques like Huffman coding [37], delta coding [37], and skeleton coding have been used to extract this useful data. Whereas, for dimensionality reduction, principal component analysis (PCA) [43, 53, 69, 70] and independent component analysis (ICA) [31, 43, 54] methods can be implemented. Table 3 shows some significant signal compression techniques reported by various studies for ECG signals. PCA is a statistical procedure that transforms a large set of correlated variables into fewer ones, i.e., it is used for data compression and for evaluating de-correlation between noisy and useful ECG signals. ICA is used for searching the statistically independent components, i.e., nonlinear features. Castells et al. [69] proposed a combined PCA and Karhumen-Loeve transform for data compression. Though these techniques provide useful diagnostic information, there are some limitations to these approaches, i.e., when T waves are irregular, resulting compression of the features is less efficient. The resampling of the ST segment can improve this limitation. Chen et al. implemented PCA to decrease the complexity of sparse matrix computing architecture and SVM classifiers. Elhaj et al.[43] first investigated linear and nonlinear features independently, thereafter proposed a combined set of linear and nonlinear features. The proposed approach leads to an increase in the classification accuracy of ECG signals [43]. According to Elhaj et al. [43], the proposed combination of PCA and ICA method for compressed feature extraction. This approach of combining features has been able to obtain maximum accuracy for five types of arrhythmia detection of 98.90%.
Although different methods have been used in feature extraction, it is difficult to determine which feature extraction method would be more advantageous than others, owing to the physiological difference in patient heartbeats. However, features obtained using the neural networks approach have detected multiple arrhythmias with higher accuracy on varied patients' heartbeats. After feature extraction and arrhythmia detection, there is a need to classify the arrhythmia type in the human body. The following section discusses various ECG-based arrhythmia classification methods.
ECG based arrhythmia classification
Classification plays a critical role in the identification of the particular class of arrhythmia. State-of-the-art proposed various machine learning algorithms for multiple types of arrhythmia classification using ECG sensors. The most commonly used machine learning classifiers are support vector machine (SVM) [2, 31, 61, 68, 70, 71], neural network [29, 31, 40, 41, 51, 72,73,74], and fuzzy classifier [75, 76]. Table 4 shows a list of some significant ECG-based arrhythmia classification approaches.
SVM is a widely used supervised learning technique for data analyses and pattern recognition. The purpose of this approach is to minimize the confidence interval or margin between hyper-plane and data points. Later, these data points are plotted into clusters, representing a different class [2]. Salam et al. [2] plots feature vectors in different clusters for normal and abnormal ECG classification using an SVM classifier and obtain an accuracy of 98.65% in peak detection. Yu et al. [54] implemented SVM and probabilistic neural networks (PNN) independently as a multi-class problem to identify eight different types of ECG beats. The authors claimed that results obtained from SVM and probabilistic neural networks are similar. Asl et al. [68] cascade SVM with generalized discriminant analysis (GDA) to obtain mapped input features. The features obtained are mapped on a radial basis to function non-linearly. Chen et al. [70] proposed a hybrid classifier architecture approach for feature classification using nonlinear SVM and weak linear classifier (WLC). A hybrid approach is implemented to avoid negative behavior of SVM for imbalanced classes. The results concludes that the proposed method is energy efficient in ECG processing and obtains negligible performance loss.
Neural network (NN)-based arrhythmia classification is a popular model for classification. A fuzzy state in a neural network is responsible for distributing data into clusters providing different membership values. Later, classification is carried out using these membership values [57]. Oresko et al. [75] proposed an adaptive artificial neural network (ANN) based technique for precise and faster training of data. In [40], Rae et al. compared different neural network techniques for the classification of arrhythmia based on ECG records. Ozbay et al. [76] implemented a fuzzy c-means clustering algorithm (type 2) along with the wavelet transform. The fuzzy layer is responsible for performing a pre-classification task, and the wavelet is used to eliminate trivial features. Jiang et al. [80] implemented a block-based neural network method that optimized weights using evolutionary operators and local gradient search. The method obtains detection accuracy of 98.1% and 96.6% for VEB and SEB, respectively.
Llamedo et al. [81] proposed the integration of a clustering algorithm with a linear discriminant classifier (LDC) for the classification of arrhythmia, where clustering is liable to patient-specific data ordering, and LDC is for automatic cluster labeling. According to the authors, the beat being grouped in a cluster belongs to one class, and the remaining belongs to the other classes. In [77], the authors proposed a one-versus-one SVM classifier for arrhythmia classification. In this approach, time-based, morphological, and wavelet coefficient features have been used to classify four classes of arrhythmia. Average classification accuracy of 87.88% has been achieved.
Others classifiers that can be used for the classification of arrhythmia are non-singleton fuzzy logic classifier [82], modular neural network [83] and [42], multi-layered perceptron [40], fuzzy clustering NN [57], naive Bayes classifier [36], neuro SVM-KNN fusion classifier [47], convolutional neural network [29, 84, 85] linear discriminant classifier [81, 86], particle swarm optimization classifier [32, 87] and conditional random fields classifier [39].
Although twelve lead ECG is a standard method for the detection of various cardiovascular diseases, however, this approach has fewer limitations, such as discomfort, limited freedom of movement for patients, limited to clinical practices only, etc. Moreover, due to continuous manual observation, it becomes troublesome for experts sometimes to identify the paroxysmal nature of arrhythmia correctly. To overcome these limitations, various research work has been carried for the automated detection of arrhythmia using fewer ECG leads. The review discusses various methods for the automated detection of arrhythmia using fewer ECG leads. On the contrary, various research also focuses on the detection of arrhythmia using PPG signals due to its low cost and convenience to used advantages. Thus, the following section discusses various PPG-based methods used for the detection of arrhythmias.
PPG based arrhythmia detection
In recent years, data acquisition from PPG signals has gained significant importance in biomedical signal processing due to its low cost, ease of use, and non-invasive benefits. In ECG, electrodes need to be deployed onto the patient’s body which discomforts the patient. Moreover, when deployed for a long duration, feeling of unhealthiness, limited freedom of movement, etc. Results in for the patient. Although 12-lead ECG is a gold standard for the measurement of heart activity, it cannot be used as a portable device. Therefore, since the last decade, with the advancement in technology development, portable devices have been in great demand to monitor cardiac activity for a longer duration. Castaneda et al. [20] discuss current PPG-based developments and their applications in the real world. It explained various PPG sensors and discussed analysis methods. Moreover, various medical diagnosis tools stating how PPG sensors can be used as personalized health tools for advancement in health care and e-health are also presented. The detection process of the arrhythmia is mainly categorized into preprocessing of the PPG signal, followed by feature extraction and classification to identify the type of arrhythmia abnormality. Various preprocessing, feature extraction, and classification techniques found in the literature have been reviewed in the following sub-sections.
Preprocessing
Before the detection of an arrhythmia, artifact removal from the signal are of utmost importance. Artifacts obtained in the PPG signals are noisier compared to ECG signals due to: (a) technical artifacts or experimental artifacts, (b) environmental artifacts, and (c) biological artifacts or physiological artifacts. Technical artifacts that occur due to the body movements might result in the displacement of the sensor from its original location. Environmental artifacts occur due to the presence of ambient light that may intervene with the sensor, resulting in the corruption of the captured signals. Biological artifacts occur due to the varied optical response of the blood with a person’s hemodynamic parameters. The amplitude of the artifacts is increased when the data collection is transferred to a patient’s home by the hospital [8]. However, the most common artifacts leading to false alarms are motion and noise artifacts. Solosenko et al. [88] implemented low and high-pass finite impulse response filters to minimize noise and remove baseline wandering with cut-off frequencies of 5 and 0.4 Hz, respectively. Paradkar et al. [89] demonstrated a pulse quality index for noise removal by selecting only the highest quality pulses for estimating heart rate. Similarly, authors in [90,91,92,93] used bandpass filters for preprocessing, whereas the study reported in [90] implemented a second-order Butterworth bandpass filter based on brute force search. Later, the second derivative has been, implemented to extract the acceleration plethysmography (APG) signal from a filtered PPG signal. Poh et al. [91] used a moving average filter with a bandpass filter to refine blood volume pulse peak fiducial points. However, due to the presence of ambient light, the filtered signals obtained after averaging are weak. To limit this problem, Solosenko et al. [93] used a second-order butter-worth bandpass filter to remove baseline wandering and non-overlapping noises with a cut-off frequency of 0.5 and 3.5 Hz. The use of a bandpass filter was able to reduce only non-overlapping noise from the signal. Poh et al. [94] used Widrow’s ANC technique to minimize motion artifacts. Later, these motion artifacts were subtracted from the corrupted output to obtain a physiological signal. In this approach, the authors assume that the obtained physiological signal and the motion-induced signal are additive. To limit this noise, accelerometers could be used for adaptive noise cancellation [97, 98]. Yousefi et al. [97] proposed an artificial noise generation approach using an accelerometer. In this singular value decomposition, ICA and FFT generate three references that are later used to quantify the randomness of each signal. Fukushima et al. [98] calculated the difference in the spectrum between PPG and acceleration. Reliability and heart rate frequency are later detected, and artifacts are rejected. Lee et al. [99] use a fixed interval-based Kalman filter for noise cancellation. The results show that this filter can remove noise effectively compared to the other adaptive filtering techniques. However, it has been found that the obtained smoothening filter is computationally expensive compared to the other algorithms. Sayadi et al. [4] proposed a joint dynamical state-space model along with the Kalman filter. This technique provides a synchronized estimation of multiple cardiovascular signals such as PPG, ECG, blood pressure, venous pressure.
Patterson et al. [95] proposed a radiometric comparison based noise cancellation method. In this, a comparison was made between the characteristics of infrared and red light. The absorption characteristics were used to cancel out multiplicative noise, whereas amplitude modulation is used to remove additive noise. However, the method compensates when ambient light levels are varied. To overwhelmed the limitation, another approach by Bhowmik et al. [96] was proposed for noise removal. In this, the authors implemented Daubechies wavelet transform where signals are passed through multiple filter banks, and the baseline of the signal is formed. After approximating the coefficient value, any distinction in the baseline of the signal is removed. This method was able to remove artifacts to a large extent however, due to its complexity and resolution trade-off between time and frequency domain, the approach cannot be used for real-world application effectively. Table 5 shows some studies carried out in the literature for noise and artifact removal in PPG signals. PPG signals being more prone to motion artifacts, accurate preprocessing of the signal is required for efficient detection of arrhythmia. In the following subsection, various feature extraction techniques for arrhythmia detection have been discussed.
Arrhythmia detection and feature extraction
After preprocessing the PPG signal, signal features need to be extracted to detect the arrhythmia. This section provides brief literature on various arrhythmia detection techniques. PPG signals can be captured from the different body parts such as a finger, wrist, ear, forehead, ankle [12]. Table 6 shows the various feature extraction method found in the literature for the detection of arrhythmia. Elgendi et al. [100] presented a review on various features such as pulse width, pulse area, peak-to-peak interval, etc. that can be used to process the PPG signal further. In [101,102,103], authors developed a smartphone-based algorithm for arrhythmia detection, where the user is required to place the index finger over the camera lens for signal acquisition. Chong et al. [101] proposed a root mean square of successive differences (RMSSD) and Shannon entropy (ShE) based feature extraction method. These features identify AF, PVC, normal sinus rhythm (NSR), and premature atrial contraction (PAC) based on these features. The limitation of this method is that threshold values have been used for the detection of a normal and abnormal pulse. Due to these fixed values, the robustness of the algorithm decreases. Lee et al. [102] used statistical methods such as RMSSD, ShE to decide for AF. Although the method has been able to detect AF, however, due to the asymptomatic nature of the AF, all AF’s are not detected accurately. Scully et al. [103] used a green band from the RGB-based video recordings for the detection of AF with the same features as in [101]. Other methods described in [96, 99, 104,105,106] are also finger-based arrhythmia detection algorithms. Poh et al. [104] extracted the pulse rate using a webcam and finger-based blood volume pulse (BVP) sensor to observe the rate. The author has used Bland–Altman and the correlation approach for pulse rate estimation. The advantage of the approach is that it is tolerant of motion artifacts and is capable of examining multiple people at a time. Though this method is based on automatic face tracking, the artifacts that occur during face tracking have not been addressed in the paper, hence, limits its use as a robust method. Elgendi et al. [90] proposed a novel approach for the detection of atrial PPG signal components by implementing a second derivative to a filtered PPG signal. The second-order of the PPG signal can be used to detect cardiovascular diseases like atrial stiffness and atherosclerosis. The results obtained has a 99.82% positive predictivity. Bhowmik et al. [96] developed an on-board feature detection algorithm using wavelet transform. Solosenko et al. [107] proposed a method for generating simulated PPG signals from the ECG RR intervals series. In this PPG, the pulse is modeled from the ECG signal as a combination of one logarithm waveform and two Gaussian functions. This method signifies the change in the PPG signal during atrial fibrillation.
ICA has been used [91, 108, 109] for extracting physiological parameters using a webcam by separating color channels obtained during video recordings. These features are used to extract blood volume pulse and thereby measure heart rate variability (HRV). The methods proposed by these authors are based on the detection of blood volume pulse from the face of the subject. Poh et al. [91] require the subject to be stationary; however, Lee et al. [108] and Lam et al. [109] proposed the HRV detection approach while the subject is in motion. Balakrishnan et al. [110] proposed a PCA approach to track volumetric features on a person’s head and find a periodic signal of the pulse accordingly. Whereas Tulyakov et al. [111] estimate HRV using matrix completion theory by processing skin pixels in the facial regions. This matrix completion-based framework is used for finding the preeminent areas of the face for the correct prediction of HRV. The methods mentioned above for arrhythmia detection for video recordings have been able to detect HRV. However, the use of ICA and PCA increases the processing time of the algorithm. Moreover, artifacts that occur during face tracking have not been addressed in the existing literature. In [92, 112, 113], the author used the wrist-based sensor to extract features and detect arrhythmia. This sensor is considered the most preferred sensing device due to its convenience in wearing the device. Authors in [113], proposed a TROIKA framework for signal decomposition and spectral peak tracking using a wristwatch PPG probe. But this framework has few limitations, such as peak spectral and acceleration spectrum in the PPG spectrum that does not appear at the same frequency bin. Besides, this method is limited to the monitoring of the heart rate. In contrast to the TROIKA, The Joint sparse spectra model [112] is used for the spectrum estimation of the PPG signals. The result shows that this method has great potential for PPG-based HRV detection during motion. Moreover, it does not require signal decomposition like in TROIKA. However, when the heart rate is not located in the frequency grid, then accurate detection of heart rate is not achieved.
Zhang et al. [92] used a sparse signal recovery algorithm for the heart rate estimation. It calculates high-resolution power spectra for PPG signals. This method works well when the movement of the wrist is stable. Fukushima et al. [98] use an accelerometer for motion artifacts removal and heart rate estimation. In this approach, frequency analysis of PPG signals is analyzed by obtaining the difference of spectrum between PPG to acceleration. Parak et al.[117] used the heart timing signal algorithm for arrhythmia detection in PPG, where R-peaks are verified manually in the reference signal and later resampled to the 10 Hz sampling frequency.
Among the discussed PPG sensors, ear-based sensors are less affected by motion artifacts. Moreover, an earlobe has no cartilage resulting in ample arterial supply [94]. Poh et al.[94] used magnetic earring (clips) based sensors for feature extraction. In this method, the least mean square algorithm is used to minimize the signal's power to estimate filter coefficients. According to them, the proposed design approach is more tolerant to motion artifacts during physical activities. Other methods such as Paradkar et al.[89], expressed each pulse as a mixture of two Gaussian curves, and accordingly, Gaussian fit parameters are extracted. The other features, such as peak intervals and pulse amplitude features, are also used for false arrhythmia detection. These features are later normalized over the PPG window containing 30 pulses. Fischer et al.[8] implemented a pulse wave analysis of the signal for detecting clipping values of the raw signals. Later, these clipped signals are filtered, and annotations are compared to the raw input signals. Then, rise time, pulse rate, pulse wave duration features are extracted for the detection of arrhythmia. Solosenko et.al [88], used a sliding window approach for the extraction of normalized peak-to-peak interval and power ratio features. The main emphasis of the PPG signal is on heart rate variability detection such that the device could be used as a portable device. However, limited research explores the use of PPG signals for arrhythmia detection. Authors extracted various time, area, and statistical features for the detection of arrhythmia, and later classification is implemented for identifying the type of arrhythmias’. In the next section, various PPG-based arrhythmia classification methods will be discussed.
Arrhythmia classification
After feature extraction, there is a need for the identification of the type of PPG pulse. To undertake these, several automatic classification methods have been presented in the literature. Various algorithms such as neural networks [88], naive Bayes classifier [93], and linear discriminant classifier [118] are proposed in the literature for arrhythmia classification. Solosenko et al.[88] proposed an ANN approach for linear and nonlinear feature classification. The backpropagation method has been used to train the model, and overfitting has been overwhelmed to some extent by adding random noise to the data.
Gil et al.[118] proposed a linear discriminant analysis (LDA) based method for ventricular premature beats (VPB) classification. In this, features are normalized with their mean values. The normalization is done before classification. Later, the author’s employed LDA, where features are added for improving performance accuracy at each step. Solosenko et al.[93] implemented the Naive Bayes classification for the different types of arrhythmia detection. This method calculates the value of each feature, assuming they are conditionally independent. This approach is preferred in embedded systems due to its ease in the implementation, increased efficiency, and high sensitivity for trained data. It has been observed that the state-of-the-art methods have reported very few works for the detection of the single or multiple types of arrhythmias using a machine learning approach. These approaches may provide more prominent results compared to conventional methods. Table 7 shows various classification methods used in the literature for arrhythmia detection from PPG signals.
Other classifiers that can be used for the classification of arrhythmia are a non-singleton fuzzy logic classifier, multi-layered perceptron, fuzzy clustering NN [8], SVM- KNN fusion classifier, convolutional neural network, etc. The limitation of the current methods is that the classification methods used are only applicable to binary class classification. The detection of all types of life-threatening arrhythmia has not been addressed using a classification strategy. Hence, there is a need to improve classification methods that can detect to classify all types of arrhythmia using learning-based approaches.
Discussion
Automated arrhythmia detection is a stimulating issue in the area of biomedical engineering. This paper presents a comprehensive review of ECG and PPG signals based on automated arrhythmia detection and classification techniques. Firstly, the review highlights various preprocessing techniques proposed in the literature for noise and motion artifacts removal, along with their limitations. Thereafter, feature extraction methods for extracting pivotal points from the ECG and PPG signals are summarized to detect arrhythmia automatically. Finally, the classification approaches presented in the literature for the automated classification of the type of arrhythmia have been discussed. Tables 1, 2, 3, 4, 5, 6 and 7 included in the paper shows a brief comparative analysis of the methods, the database used, data description, and types of arrhythmias identified. Also, the number of channels used for ECG data collection and the type of sensor used for PPG in the literature are discussed in the review.
ECG and PPG modalities are used for arrhythmia detection; however, as the review highlighted following challenges needs to be addressed. Automated ECG-based arrhythmia detection has been carried out mostly on a single channel or two-channel publically available dataset, where annotated ECG data are available. On the contrary, annotated PPG dataset is not available publically. Thus, PPG-based arrhythmia detection has been carried out mainly on self-generated datasets using various transmitting and reflective sensors.
In ECG-based arrhythmia detection methods, bandpass, wavelet-based, and median filter are some preprocessing approaches that have been used in the literature. Among these, wavelet filtering has been widely used in the literature for noise and artifacts removal due to the reconstruction properties of wavelets with linear and higher-order polynomial shapes. Although wavelet-based noise removal technique shows a promising future in ECG signal preprocessing. However, the use of wavelets leads to an increase in computational time and complexity of the algorithm. Moreover, selecting an approximate sub-band is also complicated on using wavelets. Contrary to wavelet filtering for ECG preprocessing, an increase in signal-to-noise ratio caused due to motion artifacts and sensor dislocation in PPG signals are discarded in the bandpass filter effectively. Thus, the bandpass filter is preferred in most of the available methods for noise and motion artifact removal in PPG signal preprocessing.
After preprocessing, the foremost emphasis of the ECG and PPG signal is to extract fiducial points for heart rate variability and arrhythmia detection. Several morphological, statistical, Fourier transform, feature engineered, wavelet, and deep learning-based feature extraction methods have been discussed in detail in the ECG-based arrhythmia detection approach. These features can detect many different classes of arrhythmias with significant accuracy. Among these feature extraction techniques, wavelets and deep learning-based approaches have been able to detect almost all types of arrhythmias with significant accuracy. However, the deep learning model requires a large dataset for training, resulting in an expensive data model. Moreover, deep learning methods are black-box models resulting in difficulty in the interpretability of the model. Thus, it becomes challenging for the expert to diagnose based on the results of these models without giving a clinical explanation. In the PPG-based arrhythmia detection approach, literature reported various feature extraction techniques to extract pivotal points for heart rate variability and arrhythmia detection. Several time-based, frequency, statistical, and entropy-based feature extraction methods have been widely used in the literature. However, limited research work has been carried in the area of arrhythmia detection using PPG signals. Furthermore, most of the work reported AF arrhythmia detection, where other commonly occurring arrhythmias are also required. Subsequently, to feature extraction, feature selection also plays a pivotal role in obtaining significant accuracy. However, the use of current PCA and LDA feature selection techniques have few limitations. For instance, LDA is not preferred for nonlinear datasets, and PCA may sometimes lead to information loss. To overcome these limitations, the usage of generalized discriminant analysis (GDA) and ant colony optimization (ACO) in ECG-based applications for feature selection and feature dimensionality reduction can be studied. GDA is a generalization of LDA that maps input data into an expedient high dimensional feature space using LDA properties. This generalized mapping leads to the feature dimensionality reduction of nonlinear datasets as well. Thus, usage of GDA would be able to reduce dimensionality for both linear and nonlinear features. Moreover, the ACO-based ECG feature selection method can also be studied. It uses local feature importance and overall performance of feature set to search the feature space to obtain optimal feature set. Further research may provide efficient solutions to feature reduction and selection problems.
Various classification approaches are discussed in detail for both ECG and PPG techniques to identify the type of arrhythmia. The widely used classification techniques used in the literature are Bayes classification, SVM, regression, neural network, etc. However, in PPG-based arrhythmia detection, the results that have been obtained are on a small dataset. Thus there is a need to study these methods on a larger dataset for their real-time applicability. Moreover, further research is needed for new feature extraction techniques for multiple types of arrhythmias detection and classification. Table 8 shows a comparison of some technological and arrhythmia detection aspects between ECG and PPG signals.
Twelve-lead ECG is a gold-standard method for CV detection. However, it has fewer limitations, such as limited mobility, discomfort, and clinical expertise required. The recent trends show that the research is being focused on the automatic detection of arrhythmia using single-channel ECG or PPG techniques for in-and-out-of-hospital settings to overcome these limitations. The presented paper shows that the literature has been able to detect multiple types of arrhythmias using single-channel ECG with significant accuracy. However, these techniques have fewer limitations: first, the single-channel ECG data used has been acquired from 12-lead ECG settings. Thus, detecting multiple types of arrhythmia using a single-channel standalone ECG device is still a research gap. Second, other CV diseases cannot be effectively identified using single-channel ECG. Thus data from other channels would be required for accurate estimation of the abnormality, and adding more leads to the device would increase the complexity of the device, resulting in difficulty to set up. At last, tall T-wave obtained in ECG due to other CV diseases could be sometimes misclassified with the R-wave in ECG signals, resulting in incorrect abnormality detection. Contrary to single-channel ECG, PPG sensors also prove to be an alternate solution to 2-lead ECG. PPG signals are captured from a single spot of the body. PPG signals and their derivatives could be useful for the detection of arrhythmia and other CV diseases such as coronary artery diseases without adding complexity to the device. However, these techniques have fewer limitations: first, PPG signals are more prone to noise and motion artifacts caused due to sensor dislocation, body movement, muscular movement, etc. Second, skin color, ambient light, and cold body temperature will also affect signal acquisition. Thus, further research is needed to overcome these limitations and provide an optimum solution for the efficient detection of arrhythmia and other CV diseases.
Conclusion
Arrhythmia detection using user-friendly, portable devices is one of the promising areas in the field of biomedical applications. This paper presents a comprehensive review of various methods used for the detection and classification of arrhythmias using single-channel or multiple-channel ECG and transitive or reflective PPG sensors. The limitations of the current techniques for noise and artifacts removal, feature extraction, and classification of ECG/PPG-based arrhythmia detection have been discussed, and rational solutions are provided. The review also exhibits a list of public databases that can be further used for the evaluation of the results. Typically, ECG-based methods have been validated on the MIT-BIH database, and PPG-based methods have been validated on the self-generated or PhysioNet database. A list of ECG and PPG sensors is also reported in the paper for further analysis. The review presented a detailed overview of the literature on automated arrhythmia detection using ECG and PPG modalities. The review highlights various state-of-the-art methods, challenges, gaps, and promising areas for further research in arrhythmia detections.
References
Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, Stevens G et al (2019) World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 7(10):e1332–e1345
Salam KA, Srilakshmi G (eds.) (2015) An algorithm for ECG analysis of arrhythmia detection. In: 2015 IEEE International Conference on Electrical, Computer and Communication Technologies (ICECCT). IEEE
Kléber AG, Rudy Y (2004) Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84(2):431–488
Sayadi O, Shamsollahi MB (2011) Life-threatening arrhythmia verification in ICU patients using the joint cardiovascular dynamical model and a Bayesian filter. IEEE Trans Biomed Eng 58(10):2748
Richards KJ, Cohen AT (2006) Types of arrhythmia. Anaesth Intensive Care Med 8(7):289–293
Lim Y, Singh D, Poh KK (2018) High-grade atrioventricular block. Singap Med J 59(7):346
Jazayeri M-A, Jazayeri MM-R (2018) Conduction disturbances: sinus node disease/sick sinus syndrome, AV conduction disturbances, AV blocks, bundle branch blocks, and fascicular blocks. ECG masters’ collection: favorite ECGs from master teachers around the world, vol 2. Cardiotext Pubblishing, Minneapolis
Fischer C, Dömer B, Wibmer T, Penzel T (2017) An algorithm for real-time pulse waveform segmentation and artifact detection in photoplethysmograms. IEEE J Biomed Health Inform 21(2):372–381
Biel L, Pettersson O, Philipson L, Wide P (2001) ECG analysis: a new approach in human identification. IEEE Trans Instrum Meas 50(3):808–812
Kanawade R, Klämpfl F, Riemann M, Knipfer C, Tangermann-Gerk K, Schmidt M et al (2014) Novel method for early signs of clinical shock detection by monitoring blood capillary/vessel spatial pattern. J Biophoton 7(10):841–849
Wang L, Lo BP, Yang G-Z (2007) Multichannel reflective PPG earpiece sensor with passive motion cancellation. IEEE Trans Biomed Circuits Syst 1(4):235–241
Tamura T, Maeda Y, Sekine M, Yoshida M (2014) Wearable photoplethysmographic sensors—past and present. Electronics 3(2):282–302
Suzuki T, Kameyama K-i, Tamura T (eds.) (2009) Development of the irregular pulse detection method in daily life using wearable photoplethysmographic sensor. In: 2009 EMBC 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE
Xie L, Li Z, Zhou Y, He Y, Zhu J (2020) Computational diagnostic techniques for electrocardiogram signal analysis. Sensors 20(21):6318
Sevakula RK, Au-Yeung WTM, Singh JP, Heist EK, Isselbacher EM, Armoundas AA (2020) State-of-the-art machine learning techniques aiming to improve patient outcomes pertaining to the cardiovascular system. J Am Heart Assoc 9(4):e013924
Murat F, Yildirim O, Talo M, Baloglu UB, Demir Y, Acharya UR (2020) Application of deep learning techniques for heartbeats detection using ECG signals-analysis and review. Comput Biol Med 120:103726
Kooman JP, Wieringa FP, Han M, Chaudhuri S, van der Sande FM, Usvyat LA et al (2020) Wearable health devices and personal area networks: can they improve outcomes in haemodialysis patients? Nephrol Dial Transplant 35(Supplement_2):ii43–ii50
Hong S, Zhou Y, Shang J, Xiao C, Sun J (2020) Opportunities and challenges of deep learning methods for electrocardiogram data: a systematic review. Comput Biol Med 2020:103801
Luz EJDS, Schwartz WR, Cámara-Chávez G, Menotti D (2016) ECG-based heartbeat classification for arrhythmia detection: a survey. Comput Methods Programs Biomed 127:144–164
Castaneda D, Esparza A, Ghamari M, Soltanpur C, Nazeran H (2018) A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelectron 4(4):195
El-Hajj C, Kyriacou PA (2020) A review of machine learning techniques in photoplethysmography for the non-invasive cuff-less measurement of blood pressure. Biomed Signal Process Control 58:101870
Moody GB, Mark RG (2001) The impact of the MIT-BIH arrhythmia database. IEEE Eng Med Biol Mag 20(3):45–50
Saeed M, Villarroel M, Reisner AT, Clifford G, Lehman L-W, Moody G et al (2011) Multiparameter intelligent monitoring in intensive care II (MIMIC-II): a public-access intensive care unit database. Crit Care Med 39(5):952
Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG et al (2000) PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101(23):e215–e220
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA et al (2006) ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 8(9):651–745
Zheng J, Zhang J, Danioko S, Yao H, Guo H, Rakovski C (2020) A 12-lead electrocardiogram database for arrhythmia research covering more than 10,000 patients. Sci Data 7(1):1–8
Taddei A, Distante G, Emdin M, Pisani P, Moody G, Zeelenberg C et al (1992) The European ST-T database: standard for evaluating systems for the analysis of ST-T changes in ambulatory electrocardiography. Eur Heart J 13(9):1164–1172
Sun Y, Chan KL, Krishnan SM (2002) ECG signal conditioning by morphological filtering. Comput Biol Med 32(6):465–479
Kiranyaz S, Ince T, Gabbouj M (2016) Real-time patient-specific ECG classification by 1-D convolutional neural networks. IEEE Trans Biomed Eng 63(3):664–675
De Chazal P (ed.) (2014) Heartbeat classification system using adaptive learning from selected beats. In: Computing in Cardiology Conference (CinC). IEEE
Ye C, Kumar BV, Coimbra MT (2012) Heartbeat classification using morphological and dynamic features of ECG signals. IEEE Trans Biomed Eng 59(10):2930–2941
Daamouche A, Hamami L, Alajlan N, Melgani F (2012) A wavelet optimization approach for ECG signal classification. Biomed Signal Process Control 7(4):342–349
Thomas M, Das MK, Ari S (2015) Automatic ECG arrhythmia classification using dual tree complex wavelet based features. AEU Int J Electron Commun 69(4):715–721
Lu J, Jia H, Verma N, Jha NK (2018) Genetic programming for energy-efficient and energy-scalable approximate feature computation in embedded inference systems. IEEE Trans Comput 67(2):222–236
Saini I, Singh D, Khosla A (2013) QRS detection using K-Nearest Neighbor algorithm (KNN) and evaluation on standard ECG databases. J Adv Res 4(4):331–344
Bayasi N, Tekeste T, Saleh H, Mohammad B, Khandoker A, Ismail M (2016) Low-power ECG-based processor for predicting ventricular arrhythmia. IEEE TransVery Large Scale Integr Syst 24(5):1962–1974
Kim H, Yazicioglu RF, Merken P, Van Hoof C, Yoo H-J (2010) ECG signal compression and classification algorithm with quad level vector for ECG holter system. IEEE Trans Inf Technol Biomed 14(1):93–100
Chen H, Cheng B-C, Liao G-T, Kuo T-C (2014) Hybrid classification engine for cardiac arrhythmia cloud service in elderly healthcare management. J Vis Lang Comput 25(6):745–753
De Lannoy G, François D, Delbeke J, Verleysen M (2012) Weighted conditional random fields for supervised interpatient heartbeat classification. IEEE Trans Biomed Eng 59(1):241–247
Rai HM, Trivedi A, Shukla S (2013) ECG signal processing for abnormalities detection using multi-resolution wavelet transform and artificial neural network classifier. Measurement 46(9):3238–3246
Kutlu Y, Kuntalp D (2012) Feature extraction for ECG heartbeats using higher order statistics of WPD coefficients. Comput Methods Programs Biomed 105(3):257–267
Javadi M, Arani SAAA, Sajedin A, Ebrahimpour R (2013) Classification of ECG arrhythmia by a modular neural network based on mixture of experts and negatively correlated learning. Biomed Signal Process Control 8(3):289–296
Elhaj FA, Salim N, Harris AR, Swee TT, Ahmed T (2016) Arrhythmia recognition and classification using combined linear and nonlinear features of ECG signals. Comput Methods Programs Biomed 127:52–63
Elgendi M, Fletcher R, Liang Y, Howard N, Lovell NH, Abbott D et al (2019) The use of photoplethysmography for assessing hypertension. NPJ Digit Med 2(1):1–11
Lee J, McManus DD, Merchant S, Chon KH (2012) Automatic motion and noise artifact detection in holter ECG data using empirical mode decomposition and statistical approaches. IEEE Trans Biomed Eng 59(6):1499–1506
Vijaya V, Rao KK, Rama V (2011) Arrhythmia detection through ECG feature extraction using wavelet analysis. Eur J Sci Res 66(3):441–448
Homaeinezhad MR, Atyabi S, Tavakkoli E, Toosi HN, Ghaffari A, Ebrahimpour R (2012) ECG arrhythmia recognition via a neuro-SVM–KNN hybrid classifier with virtual QRS image-based geometrical features. Expert Syst Appl 39(2):2047–2058
Tuncer T, Dogan S, Pławiak P, Acharya UR (2019) Automated arrhythmia detection using novel hexadecimal local pattern and multilevel wavelet transform with ECG signals. Knowl Based Syst 186:104923
Mert A, Kılıç N, Akan A (2014) Evaluation of bagging ensemble method with time-domain feature extraction for diagnosing of arrhythmia beats. Neural Comput Appl 24(2):317–326
Isin A, Ozdalili S (2017) Cardiac arrhythmia detection using deep learning. Procedia Comput Sci 120:268–275
Zeraatkar E, Kermani S, Mehridehnavi A, Aminzadeh A, Zeraatkar E, Sanei H (2011) Arrhythmia detection based on morphological and time-frequency features of t-wave in electrocardiogram. J Med Signals Sens 1(2):99
Sayadi O, Shamsollahi MB (2009) A model-based Bayesian framework for ECG beat segmentation. Physiol Meas 30(3):335
Özbay Y (2009) A new approach to detection of ECG arrhythmias: complex discrete wavelet transform based complex valued artificial neural network. J Med Syst 33(6):435
Yu S-N, Chou K-T (2009) Selection of significant independent components for ECG beat classification. Expert Syst Appl 36(2):2088–2096
Fira CM, Goras L (2008) An ECG signals compression method and its validation using NNs. IEEE Trans Biomed Eng 55(4):1319–1326
Lee S, Kim J, Lee M (2011) A real-time ECG data compression and transmission algorithm for an e-health device. IEEE Trans Biomed Eng 58(9):2448–2455
Özbay Y, Ceylan R, Karlik B (2006) A fuzzy clustering neural network architecture for classification of ECG arrhythmias. Comput Biol Med 36(4):376–388
Korürek M, Nizam A (2008) A new arrhythmia clustering technique based on ant colony optimization. J Biomed Inform 41(6):874–881
Yaghouby F, Ayatollahi A, Bahramali R, Yaghouby M, Alavi AH (2010) Towards automatic detection of atrial fibrillation: a hybrid computational approach. Comput Biol Med 40(11–12):919–930
Sahab A, Gilmalek YM (2011) An automatic diagnostic machine for ECG arrhythmias classification based on wavelet transformation and neural networks. Int J Circuits Syst Signal Process 5(3):255–262
Shen C-P, Kao W-C, Yang Y-Y, Hsu M-C, Wu Y-T, Lai F (2012) Detection of cardiac arrhythmia in electrocardiograms using adaptive feature extraction and modified support vector machines. Expert Syst Appl 39(9):7845–7852
Llamedo M, Martínez JP (2011) Heartbeat classification using feature selection driven by database generalization criteria. IEEE Trans Biomed Eng 58(3):616–625
Faezipour M, Saeed A, Bulusu SC, Nourani M, Minn H, Tamil L (2010) A patient-adaptive profiling scheme for ECG beat classification. IEEE Trans Inf Technol Biomed 14(5):1153–1165
Pławiak P (2018) Novel methodology of cardiac health recognition based on ECG signals and evolutionary-neural system. Expert Syst Appl 92:334–349
Hong S, Zhou Y, Wu M, Shang J, Wang Q, Li H et al (2019) Combining deep neural networks and engineered features for cardiac arrhythmia detection from ECG recordings. Physiol Meas 40(5):054009
Zhang J, Liu A, Gao M, Chen X, Zhang X, Chen X (2020) ECG-based multi-class arrhythmia detection using spatio-temporal attention-based convolutional recurrent neural network. Artif Intell Med 106:101856
Liu C-M, Chang S-L, Yeh Y-H, Chung F-P, Hu Y-F, Chou C-C et al (2021) Enhanced detection of cardiac arrhythmias utilizing 14-day continuous ECG patch monitoring. Int J Cardiol 332:78–84
Asl BM, Setarehdan SK, Mohebbi M (2008) Support vector machine-based arrhythmia classification using reduced features of heart rate variability signal. Artif Intell Med 44(1):51–64
Castells F, Laguna P, Sörnmo L, Bollmann A, Roig JM (2007) Principal component analysis in ECG signal processing. EURASIP J Adv Signal Proces 2007(1):074580
Chen Z, Luo J, Lin K, Wu J, Zhu T, Xiang X et al (2018) An energy-efficient ecg processor with weak-strong hybrid classifier for arrhythmia detection. IEEE Trans Circuits Syst II Express Briefs 65(7):948–952
De Lannoy G, François D, Delbeke J, Verleysen M (2010) Weighted SVMs and feature relevance assessment in supervised heart beat classification. International joint conference on biomedical engineering systems and technologies. Springer, Heidelberg
Al Rahhal MM, Bazi Y, AlHichri H, Alajlan N, Melgani F, Yager RR (2016) Deep learning approach for active classification of electrocardiogram signals. Inf Sci 345:340–354
Oresko JJ, Jin Z, Cheng J, Huang S, Sun Y, Duschl H et al (2010) A wearable smartphone-based platform for real-time cardiovascular disease detection via electrocardiogram processing. IEEE Trans Inf Technol Biomed 14(3):734–740
Zhang X, Li J, Cai Z, Zhang L, Chen Z, Liu C (2021) Over-fitting suppression training strategies for deep learning-based atrial fibrillation detection. Med Biol Eng Comput 59:1–9
Vafaie M, Ataei M, Koofigar HR (2014) Heart diseases prediction based on ECG signals’ classification using a genetic-fuzzy system and dynamical model of ECG signals. Biomed Signal Process Control 14:291–296
Özbay Y, Ceylan R, Karlik B (2011) Integration of type-2 fuzzy clustering and wavelet transform in a neural network based ECG classifier. Expert Syst Appl 38(1):1004–1010
Zhang Z, Luo X (2014) Heartbeat classification using decision level fusion. Biomed Eng Lett 4(4):388–395
Alfaras M, Soriano MC, Ortín S (2019) A fast machine learning model for ECG-based heartbeat classification and arrhythmia detection. Front Phys 7:103
Ullah A, Tu S, Mehmood RM, Ehatisham-ul-haq M (2021) A hybrid deep CNN model for abnormal arrhythmia detection based on cardiac ECG signal. Sensors 21(3):951
Jiang W, Kong SG (2007) Block-based neural networks for personalized ECG signal classification. IEEE Trans Neural Netw 18(6):1750–1761
Llamedo M, Martínez JP (2012) An automatic patient-adapted ECG heartbeat classifier allowing expert assistance. IEEE Trans Biomed Eng 59(8):2312–2320
Chua TW, Tan WW (2011) Non-singleton genetic fuzzy logic system for arrhythmias classification. Eng Appl Artif Intell 24(2):251–259
Jadhav SM, Nalbalwar SL, Ghatol AA (2011) Modular neural network based arrhythmia classification system using ECG signal data. Int J Inform Technol Knowl Manage 4(1):205–209
Krizhevsky A, Sutskever I, Hinton GE (2012) Imagenet classification with deep convolutional neural networks. Adv Neural Inform Process Syst 25:1097
Acharya UR, Fujita H, Lih OS, Hagiwara Y, Tan JH, Adam M (2017) Automated detection of arrhythmias using different intervals of tachycardia ECG segments with convolutional neural network. Inf Sci 405:81–90
Mar T, Zaunseder S, Martínez JP, Llamedo M, Poll R (2011) Optimization of ECG classification by means of feature selection. IEEE Trans Biomed Eng 58(8):2168–2177
Melgani F, Bazi Y (2008) Classification of electrocardiogram signals with support vector machines and particle swarm optimization. IEEE Trans Inf Technol Biomed 12(5):667–677
Sološenko A, Petrėnas A, Marozas V (2015) Photoplethysmography-based method for automatic detection of premature ventricular contractions. IEEE Trans Biomed Circuits Syst 9(5):662–669
Paradkar N, Chowdhury SR (eds.) (2017) Cardiac arrhythmia detection using photoplethysmography. In: 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE
Elgendi M (2014) Detection of c, d, and e waves in the acceleration photoplethysmogram. Comput Methods Programs Biomed 117(2):125–136
Poh M-Z, McDuff DJ, Picard RW (2011) Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans Biomed Eng 58(1):7–11
Zhang Z (ed.) (2014) Heart rate monitoring from wrist-type photoplethysmographic (PPG) signals during intensive physical exercise., In: 2014 IEEE Global Conference on Signal and Information Processing (GlobalSIP). IEEE
Solosenko A, Marozas V (2014) Automatic extrasystole detection using photoplethysmographic signals. XIII mediterranean conference on medical and biological engineering and computing 2013. Springer, Cham
Poh M-Z, Swenson NC, Picard RW (2010) Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans Inf Technol Biomed 14:786
Patterson JA, Yang G-Z (2011) Ratiometric artifact reduction in low power reflective photoplethysmography. IEEE Trans Biomed Circuits Syst 5(4):330–338
Bhowmik T, Dey J, Tiwari VN (eds.) (2017) A novel method for accurate estimation of HRV from smartwatch PPG signals. In: 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE
Yousefi R, Nourani M, Ostadabbas S, Panahi I (2014) A motion-tolerant adaptive algorithm for wearable photoplethysmographic biosensors. IEEE J Biomed Health Inform 18(2):670–681
Fukushima H, Kawanaka H, Bhuiyan MS, Oguri K (eds.) (2012) Estimating heart rate using wrist-type photoplethysmography and acceleration sensor while running. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE
Lee B, Han J, Baek HJ, Shin JH, Park KS, Yi WJ (2010) Improved elimination of motion artifacts from a photoplethysmographic signal using a Kalman smoother with simultaneous accelerometry. Physiol Meas 31(12):1585
Elgendi M (2012) On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 8(1):14–25
Chong JW, Esa N, McManus DD, Chon KH (2015) Arrhythmia discrimination using a smart phone. IEEE J Biomed Health Inform 19(3):815–824
Lee J, Reyes BA, McManus DD, Maitas O, Chon KH (2013) Atrial fibrillation detection using an iPhone 4S. IEEE Trans Biomed Eng 60(1):203–206
Scully CG, Lee J, Meyer J, Gorbach AM, Granquist-Fraser D, Mendelson Y et al (2012) Physiological parameter monitoring from optical recordings with a mobile phone. IEEE Trans Biomed Eng 59(2):303–306
Poh M-Z, McDuff DJ, Picard RW (2010) Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt Express 18(10):10762–10774
Shin HS, Lee C, Lee M (2009) Adaptive threshold method for the peak detection of photoplethysmographic waveform. Comput Biol Med 39(12):1145–1152
Doostdar H, Khalilzadeh M (2014) Quantification the effect of ageing on characteristics of the photoplethysmogram using an optimized windkessel model. J Biomed Phys Eng 4(3):103
Sološenko A, Petrėnas A, Marozas V, Sörnmo L (2017) Modeling of the photoplethysmogram during atrial fibrillation. Comput Biol Med 81:130–138
Li X, Chen J, Zhao G, Pietikainen M (eds.) (2014) Remote heart rate measurement from face videos under realistic situations. In: Proceedings of the IEEE conference on computer vision and pattern recognition
Lam A, Kuno Y (eds.) (2015) Robust heart rate measurement from video using select random patches. In: Proceedings of the IEEE International Conference on Computer Vision
Balakrishnan G, Durand F, Guttag J (eds.) (2013) Detecting pulse from head motions in video. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition
Tulyakov S, Alameda-Pineda X, Ricci E, Yin L, Cohn JF, Sebe N (eds.) (2016) Self-adaptive matrix completion for heart rate estimation from face videos under realistic conditions. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition
Zhang Z (2015) Photoplethysmography-based heart rate monitoring in physical activities via joint sparse spectrum reconstruction. IEEE Trans Biomed Eng 62(8):1902–1910
Zhang Z, Pi Z, Liu B (2015) TROIKA: a general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans Biomed Eng 62(2):522–531
Bashar SK, Han D, Hajeb-Mohammadalipour S, Ding E, Whitcomb C, McManus DD et al (2019) Atrial fibrillation detection from wrist photoplethysmography signals using smartwatches. Sci Rep 9(1):1–10
Eerikäinen LM, Bonomi AG, Schipper F, Dekker L, de Morree HM, Vullings R et al (2019) Detecting atrial fibrillation and atrial flutter in daily life using photoplethysmography data. IEEE J Biomed Health Inform 24:1610
Väliaho E-S, Kuoppa P, Lipponen JA, Hartikainen JE, Jäntti H, Rissanen TT et al (2021) Wrist band photoplethysmography autocorrelation analysis enables detection of atrial fibrillation without pulse detection. Front Physiol. https://doi.org/10.3389/fphys.2021.654555
Parak J, Korhonen I (eds.) (2014) Evaluation of wearable consumer heart rate monitors based on photopletysmography. In: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE
Gil E, Laguna P, Martínez JP, Barquero-Pérez O, García-Alberola A, Sörnmo L (2013) Heart rate turbulence analysis based on photoplethysmography. IEEE Trans Biomed Eng 60(11):3149–3155
Aschbacher K, Yilmaz D, Kerem Y, Crawford S, Benaron D, Liu J et al (2020) Atrial fibrillation detection from raw photoplethysmography waveforms: a deep learning application. Heart Rhythm O2 1(1):3–9
Gothwal H, Kedawat S, Kumar R (2011) Cardiac arrhythmias detection in an ECG beat signal using fast fourier transform and artificial neural network. J Biomed Sci Eng 4(04):289
Pławiak P, Acharya UR (2020) Novel deep genetic ensemble of classifiers for arrhythmia detection using ECG signals. Neural Comput Appl 32(15):11137–11161
Mahri N, Gan KB, Meswari R, Jaafar MH, Mohd Ali MA (2017) Utilization of second derivative photoplethysmographic features for myocardial infarction classification. J Med Eng Technol 41(4):298–308
Chakraborty A, Sadhukhan D, Pal S, Mitra M (2020) Automated myocardial infarction identification based on interbeat variability analysis of the photoplethysmographic data. Biomed Signal Process Control 57:101747
Pilt K, Ferenets R, Meigas K, Lindberg L-G, Temitski K, Viigimaa M (2013) New photoplethysmographic signal analysis algorithm for arterial stiffness estimation. Sci World J 2013:1
Singh T, Bing R, Dweck MR, van Beek EJ, Mills NL, Williams MC et al (2020) Exercise electrocardiography and computed tomography coronary angiography for patients with suspected stable angina pectoris: a post hoc analysis of the randomized SCOT-HEART trial. JAMA Cardiol 5(8):920–928
Acknowledgements
The research work has been funded by CSIR HQ under Intelligent Systems Mission HCP0013 WP 1.3 at CSIR-CSIO, Chandigarh. Neha is thankful to the Council of Scientific and Industrial Research—Senior Research Fellow (CSIR-SRF), Grant No. 31/31/(0053)/2k19-EMR-I.
Funding
The research work has been funded by the Council of Scientific and Industrial Research—Senior Research Fellow (CSIR-SRF), Grant No. 31/31/(0053)/2k19-EMR-1.
Author information
Authors and Affiliations
Contributions
Neha: Conceptualization, review analysis, written original draft. HKS: Supervision, review analysis, manuscript editing. RK: Supervision, review analysis, manuscript editing. ST: Review analysis, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neha, Sardana, H.K., Kanwade, R. et al. Arrhythmia detection and classification using ECG and PPG techniques: a review. Phys Eng Sci Med 44, 1027–1048 (2021). https://doi.org/10.1007/s13246-021-01072-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-021-01072-5