Abstract
In clinical rhythmology, intracardiac bipolar electrograms (EGMs) play a critical role in investigating the triggers and substrates inducing and perpetuating atrial fibrillation (AF). However, the interpretation of bipolar EGMs is ambiguous due to several aspects of electrodes, mapping algorithms and wave propagation dynamics, so it requires several variables to describe the effects of these uncertainties on EGM analysis. In this narrative review, we critically evaluate the potential impact of such uncertainties on the design of cardiac mapping tools on AF-related substrate characterization. Literature suggest uncertainties are due to several variables, including the wave propagation vector, the wave’s incidence angle, inter-electrode spacing, electrode size and shape, and tissue contact. The preprocessing of the EGM signals and mapping density will impact the electro-anatomical representation and the features extracted from the local electrical activities. The superposition of multiple waves further complicates EGM interpretation. The inclusion of these uncertainties is a nontrivial problem but their consideration will yield a better interpretation of the intra-atrial dynamics in local activation patterns. From a translational perspective, this review provides a concise but complete overview of the critical variables for developing more precise cardiac mapping tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is a global health burden that affects an estimated 60 million people worldwide [1, 2]. It is the most common arrhythmia in humans, which occurs in the presence of irregular and disorganized electrical activities in the atrial conduction system (ACS) [3,4,5,6,7,8,9]. An electrical signal passes rhythmically through specific (cardiac) conduction pathways and maintains the heart’s pumping activity in sinus rhythm (SR) [10, 11]. However, structural remodeling of the arrhythmogenic substrates disrupts regular cardiac conduction dynamics in ACS, leading to AF.
Catheter-based isolation of the pulmonary veins represents the cornerstone of interventional AF treatment. Electro-anatomical mapping of local intracardiac bipolar electrograms (EGMs) is an established way to characterize the atrial substrate and identify potential ablation targets [12,13,14]. Bipolar EGMs are constructed from unipolar EGMs to quantify the arrhythmogenic substrates because bipolar EGMs are less likely to be contaminated with far-field potentials, i.e., ventricular artefacts [13,14,15,16,17,18]. However, multiple variables, including wave propagation direction relative to the bipolar electrode orientation, electrode size, inter-electrode spacing, electrode-tissue contact, filtering, mapping density, and mapping resolution, impact bipolar EGM [19,20,21,22,23]. This review aims to evaluate the effects of the variables on bipolar EGM-based AF mapping.
Cardiac Conduction Dynamics and Atrial Fibrillation
There are four fundamental characteristics of the cardiomyocyte cells, i.e., contraction, autorhythmicity, intercellular conduction and electromechanical coupling [10, 11, 24,25,26,27,28,29,30]. The sinoatrial (SA) node is the natural pacemaker located in the upper region of the right atrium, which initiates each heartbeat. Electrical impulses from the SA node propagate through junctional fibers to the atrioventricular (AV) node in the right atrium and via the inter-atrial Bachmann’s bundle in the left atrium. With a delay in the AV node, impulses are then passed into the bundle of His where they bifurcate into right and left branches. Finally, the impulses proceed down to the Purkinje fibers throughout the ventricular walls. Figure 1 illustrates the components and conduction pathways of the cardiac conduction system.
Mapping Intracardiac Ablation Target
The current intervention, intracardiac catheter ablation, is based on various hypotheses regarding AF initiation and perpetuation over time. Haissaguerre et al. have shown that ectopic electrical impulses coming from pulmonary veins could cause AF. Electrically isolating pulmonary veins may restore SR and has become a standard intervention for AF [31, 32]. A hypothesis of substrate-based AF characterization refers to ectopic source identification. The sources produce disruptive signals in the atrial anatomy and disturb regular cardiac conduction dynamics. Autonomic nervous system plays role in AF initiation and maintenance, and recent studies have identified ganglionated plexuses as ablation targets [33,34,35]. Other hypotheses include multiple wavelets, primary rotors and multiple functional re-entry circuits [3,4,5,6, 8, 36,37,38] (Fig. 2).
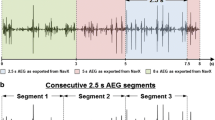
Current ablation strategies depend on accurately identifying AF-related substrates (ablation targets) based on EGM characteristics. Structural changes associated with an electrical scar or diseased myocardium may introduce re-entry circuits within the ACS. Low voltage areas (bipolar peak-to-peak voltage \(< 0.5\) mV) are assumed to be an indication of scar or structural defects [39,40,41,42,43]. Fractionated EGMs are sometimes the ablation targets [44, 45]. According to Nademanee et al., fractionated EGMs follow two criteria [46]. Atrial EGMs have two and more deflections and a variable baseline with complex atrial activation patterns, showing transient activities (i.e., cycle length \(\le 120\) ms) over a prolonged 10 s period. However, inconsistent definitions of EGM fractionation can impede success in clinical ablation [45, 47]. Local activation time and conduction velocity-based AF source mapping are potential alternatives in the clinical setting [48,49,50]. Recent studies have proposed recurrent wave cycle length and their morphological similarity-based ablation target detection [51,52,53]. Additional bipolar EGM-derived features such as dominant frequency and Shannon entropy have been clinically used to investigate the propagation of predominant waves and to identify the pivot of a rotor, respectively, which may guide substrate-based ablation [54,55,56].
Genesis of Focal Ectopic Source and Reentry Circuit
AF mechanisms manifest in ectopic firing, and various re-entry circuits in the ACS are assumed to occur due to atrial fibrotic substrates. Pathological fibrosis results from malfunctioning ion channels, unusual \(Ca^{2+}\) handling, autonomic neural regulation dysfunction, or structural remodeling. Extracellular matrix (ECM) triggers its regulatory protein cells to adjust for any changes during fibrosis to maintain cardiac homeostasis [57,58,59,60,61,62,63,64]. ECM provides structural support to cardiomyocytes, facilitates intra- and inter-cardiomyocyte cellular crosstalk, and transduces critical signals to vascular and interstitial cells [61]. The essential components of ECM include transcriptionally active fibroblasts and endothelial cells [65]. When ECM starts synthesizing its proteins for fibrogenesis as a response to microenvironmental inflammatory and pro-fibrotic changes, increased fibroblasts and myofibroblasts may interfere with intra- and inter-cardiomyocyte electrical conduction. Fibroblasts and cardiomyocytes scarcely share similar intra- and inter-cellular conduction dynamics, and their complex interplay might cause disturbances in physiologic ACS [59,60,61]. Recent studies implicated that asynchrony between endocardial and epicardial conduction causes AF initiation and maintenance [57, 66,67,68,69,70].
Complex cardiomyocyte-fibroblast interactions alter the electrophysiological properties of atrial cardiomyocytes [59,60,61,62,63, 63, 64, 71]. For example, the shortening of action potential duration and effective refractory period due to ion channel dysfunction could cause the fibrotic substrates to develop spontaneous re-entry circuits. \(Ca^{2+}\) handling abnormalities and errors in autonomic neural regulation sometimes result in focal ectopic firing, which is a potential manifestation of AF-related substrates. Focal ectopic sources further trigger re-entry circuits. \(Ca^{2+}\) handling abnormalities can also contribute to structural remodeling leading to fibrotic or scar tissues and conduction blocks that induce re-entry circuits. Figure 3 describes the complex interactions between the cardiomyocytes and ECM protein cells and the induction of AF. Takahashi et al. have studied that fibrosis, growth intercellular space, myofibrillar loss and reduced nuclear density are histological correlates of structural remodelling causing AF [72].
Pathogenesis of atrial conduction block, focal ectopic source and re-entry circuit from a complex interplay between fibroblasts and cardiomyocytes. [Created with https://www.BioRender.com]
Uncertainties Introduced by The Catheter
Unipolar Versus Bipolar Signal Acquisition
For unipolar EGMs, an electrode is connected to the anodal (positive) input of the recording amplifier and the cathodal (negative) input is connected to a remote amplifier (reference electrode) [16, 73, 74]. A bipolar EGM can then be constructed by subtracting two unipolar EGMs [16, 73, 75]. Figure 4 shows unipolar and bipolar recording configurations from the myocardial surface. Both unipolar and bipolar EGM have specific advantages and disadvantages for AF-related substrate characterization.
In unipolar mapping, only one electrode within the heart is used, with the second electrode located outside the heart. The anode can be Wilson’s central terminal, which uses the extremity electrodes, an electrode located within the inferior vena, or an internal close unipolar reference electrode [76]. Unipolar mapping has a critical role, particularly during ablation; the signal of interest is obtained from the tip electrode rather than a combined signal from a distal and proximal electrode.
Because the reference electrode is placed at a distance from the heart, unipolar EGM is contaminated by far-field potentials, e.g., ventricular artefacts [13,14,15,16,17, 77]. Ventricular artefacts, i.e., undesired signals originating from ventricles, are the dominant sources that can be distinguished during sinus rhythm. However, AF is characterized by chaotic and disorganized electrical activities within atria that may potentially corrupt local EGM morphology by the superposition of waves [15, 78]. Constructing a bipolar EGM from a pair of unipolar electrodes may inherently reduce far-field artefacts [18]. However, bipolar EGM is affected by several measurement uncertainties due to bipolar vector orientation, inter-electrode spacing, and tissue contact [20, 22, 75, 79].
Electrode Size and Inter-electrode Spacing
Electrode material, size, shape, thickness and inter-electrode spacing are catheter design specifications and impact EGM-derived features for substrate characterization, for example, when differentiating between healthy and scar tissues [79,80,81]. An electrode’s size, shape and thickness define the coverage area for signal acquisition and influence both unipolar and bipolar EGM [16, 79, 80, 82]. Takigawa et al. have demonstrated that larger electrodes increase the amplitude and duration of unipolar and bipolar EGMs [80]. Thus, scar detection using a low-voltage threshold may be impacted. Inter-electrode spacing also affects substrate characterization. Closer inter-electrode spacing intrinsically reduces the contribution of far-field signals and better identifies the boundary between scar and healthy tissues [81].
Relation Between the Angle of Incidence and Bipolar EGM Vector
Bipolar EGMs are less prone to far-field artefacts and predominantly capture changes in local electrical activities; however, they depend highly on bipolar vector orientation. From a signal processing perspective, bipolar vector orientation could be exploited as a mapping tool for accurate identification of AF sources [18, 83,84,85,86]. Figure 4 demonstrates the directional placement of electrodes (bipolar vector orientation) and wave propagation vector. Assuming a plannar wave propagating through a two-dimensional medium, a bipolar EGM measures either the maximum amplitude of a signal if the angle of incidence is \(0^\circ\) or the minimum of 0 mV if the angle of incidence is \(90^\circ\). Although variability in EGM measurement using diverse bipolar vector orientations is obvious, the extent of uncertainty during AF-related substrate characterization requires rigorous clinical validation [18, 75, 82,83,84,85,86,87]. AF is a pathological condition delineating very complex and chaotic electrical wave propagation dynamics in a three-dimensional anatomical network of the atria. Thus, interpreting the impact of bipolar vector orientation on AF-related substrate characterization should not be straightforward.
Variable Tissue Contact
Identification of AF-related sources depends on the precise reconstruction of the atrial electro-anatomy and the spatiotemporal distribution of myocardial electrical potentials and associated features [13, 14]. Continuous mechanical contraction of the complex atrial anatomy makes the positioning of catheter electrodes onerous [21, 23]. Sometimes the electrodes are not in full contact with the surface, which may impact the EGM characteristics leading to some recording points being excluded from the analysis. If the distance between electrode and recording surface is too large to maintain acceptable signal quality, the corresponding EGMs are excluded. Figure 5 illustrates variable electrode-tissue contact. Nonetheless, the earlier cardiac mapping tools allow us to verify anatomical landmarks and atrial geometry with computed tomography or magnetic resonance scans, intracardiac echocardiographic and positron emission tomography imaging for guiding the ablation procedure [13, 14, 23, 88]. Registering the recording points with the corresponding atrial anatomy is key to successful AF mapping. Notably, recent cardiac mapping systems (e.g., CARTO\(^{\text{TM}}\), EnSite Precision\(^{\text{TM}}\) and Rhythmia HDx\(^{\text{TM}}\)) use non-fluoroscopic, i.e., magnetic- or impedance-based localization of electrodes [13]. Inaccurate localization of the electrode postion (i.e., EGM recording point) may mislead the electrophysiological interpretation of AF [19].
Sources of Mapping Uncertainties
Preprocessing of EGM Signals
Constructing a bipolar EGM may reduce noise contents’ dominance over local atrial signals intrinsically. However, further processing is essential to eliminate noise and artefacts while preserving local EGM morphology [89]. Zero-phase band-pass filtering is typically applied to eliminate baseline shifts and high-frequency noise [73]. The cut-off frequencies clinically used are 30 Hz and 300 Hz [18, 90]. Botteron and Smith proposed band-pass filtering with cut-off frequencies of 40 Hz and 250 Hz, followed by rectification and low-pass filtering to discern the spatial organization of AF dynamics [91]. This filtering technique has also been utilized to extract dominant frequency mapping [73, 92]. Ciaccio et al. demonstrated the frequency range \(3-12\) Hz associated with main atrial components. Band-pass filtering might distort the local EGM morphology by eliminating potential low-frequency signal of interest [93]. Thus, there is a trade-off between noise or artefacts elimination and preserving local EGM morphology. Comprehensive retrospective clinical studies correlating substrate characterization with ablation outcomes can evaluate the efficacy of any signal processing method.
Electroanatomic Mapping System: Density and Resolution
Multimodal electroanatomic cardiac mapping systems such as CARTO\(^{\text{TM}}\), EnSite Precision\(^{\text{TM}}\) and Rhythmia HDx\(^{\text{TM}}\) detect the electrode positions during EGM recording, which is represented by a three-dimensional point cloud after matching each point with the corresponding atrial anatomy [13, 94]. The electrode position information is typically as accurate as 1 mm [88], though the accuracy in electrode localization strongly depends on the mapping system in use. Mapping density refers to the tightness of point cloud (i.e., recording sites) distribution. It depends on the inter-electrode spacing and sequential navigation of the catheter electrodes inside the atrial anatomy. Densely collected points may offer a better resolution of reconstructed atrial anatomy.
Once the three-dimensional point cloud is approximated from the cardiac mapping system, the next step is to reconstruct the atrial anatomical surface with representations of spatio-temporal distributions of EGMs [95]. Using methods derived from computational geometry (e.g., Delaunay triangulation), first, the surface is reconstructed so that anatomically relevant information is preserved. Then, the surface is represented in colored maps (rendering by EGM-derived features) to investigate AF-related sources [13]. Due to irregularly spaced points, the resolution of the reconstructed atrial anatomy is nonhomogeneous. Figure 6 shows an irregularly spaced two-dimensional point cloud to illustrate surface reconstruction and color rendering.
Consequences of Uncertainties for Data Interpretation
Electro-anatomical mapping of AF requires a comprehensive clinical environment with multimodal electro-magnetic devices and medical instruments [13, 88]. Cardiologists’ skillful maneuvering of mapping catheters in the presence of mechanical contraction of the heart and continuous blood circulation further affects the quality of EGM signals. Innovation in the catheter design enables the navigation of electrodes inside the complex atrial anatomy and acquiring high-fidelity local EGM signals, potentially leading to a successful AF source localization. From an engineering perspective, bipolar vector orientation, electrode size, inter-electrode spacing, electrode-tissue contact, filtering, mapping density and mapping resolution are essential variables to be included while constructing 3-dimensional electro-anatomical maps [13, 18, 21, 73, 75, 79,80,81,82,83,84,85,86, 88, 89, 93, 94]. The extent of the impact of the variables above requires further clinical validation. Kim et al. have rigorously discussed the potential pitfalls of state-of-the-art cardiac mapping systems and highlighted the technological advances [19].
Although ablation targets (AF sources) identification is sometimes onerous, various clinically accepted AF initiation and perpetuation hypotheses exist. Analyzing EGM signals to define clinically relevant features associated with the hypotheses for identifying AF sources is often ambiguous. Besides pulmonary vein isolation [31, 32], a widely accepted precursor procedure, other substrate-based ablation strategies depend upon characterizing ectopic sources, re-entry, and rotational circuits. Fibrotic or scar tissues are supposedly responsible for re-entry and rotational circuits while obstructing common cardiac conduction pathways [3,4,5,6, 8, 36]. Complex AF source identification in atrial anatomy is demanding, and uncertainties in EGM interpretation pose additional challenges. However, recent innovations in signal processing algorithms, including mutual information or artificial intelligence-based analysis of multi-electrode arrays, may offer complementary information to clinical decision-making [96, 97].
Voltage mapping enables defining low-voltage areas (peak-to-peak voltage \(<0.5\) mV) associated with the scar of fibrotic tissues [39,40,41,42,43]. Studies have implicated that bipolar vector orientation, inter-electrode spacing, and electrode-tissue contact directly impact the amplitude of EGM signals and thus voltage mapping [18, 80, 81, 85, 86]. Studies have demonstrated in silico that the diagnostic catheter shapes influence the EGM-derived markers for AF [98,99,100,101]. These variables also impact other EGM-derived features such as local activation time, conduction velocity, fractionated EGM, dominant frequency and Shannon entropy [49, 50, 54,55,56].
The State-of-The-Art Diagnostic Catheters
Multi-electrode array with innovative catheter design is at the forefront of cardiac mapping tool development, advancing personalized diagnostic and therapeutic interventions. Table 1 lists some state-of-the-art diagnostic catheters with their specifications and design strengths [18, 40, 75, 76, 79,80,81, 83,84,85,86, 102,103,104,105,106,107,108,109]. Recently introduced OctaRay\(^{\text{TM}}\) mapping catheter attributes 48 electrodes, and their simultaneous recordings enable faster mapping of the whole atrium than its predecessor PentaRay\(^{\text{TM}}\) mapping catheter [76, 103,104,105]. OctaRay multi-electrode catheter with CARTO\(^{\text{TM}}\) signal processing unit renders the atrial anatomy based on locally collected EGM features and assists clinicians in detecting ablation targets more accurately. HD Grid\(^{\text{TM}}\) catheter has been another sought-after mapping tool in the clinical community because of its orthogonally arranged electrodes [18, 40, 75, 79,80,81, 83,84,85,86,87, 106, 107, 110,111,112,113,114,115,116,117,118,119]. IntellaMap Orion\(^{\text{TM}}\) and Constellation\(^{\text{TM}}\) catheters are basket catheters having 64 electrodes and can cover a large area simultaneously [120,121,122]. Notably, the constellation catheter is not on the market anymore, but the design showcases innovation in the cardiac mapping industry. The multi-electrode catheter technology has evolved significantly from single spline Decapolar\(^{\text{TM}}\) (flexible) or Lasso\(^{\text{TM}}\) (circular) catheters to multi-spline HD Grid\(^{\text{TM}}\), OctaRay\(^{\text{TM}}\) or Optrell\(^{\text{TM}}\). Unlike grid-type catheters, Octaray\(^{\text{TM}}\) and Pentaray\(^{\text{TM}}\) are multi-spline catheters featuring flower shape open branch design. Simultaneous EGM from the electrode array can integrate advanced signal processing and artificial intelligence-based algorithms for more accurate ablation target selection and, thus, superior intervention outcome [73, 97, 98].
The Principle of Wavefront Direction-Aware EGM
Innovation in multi-electrode mapping catheters, especially grid-type HD Grid\(^{\text{TM}}\) and Optrell\(^{\text{TM}}\) catheters, enable integrating multiple bipolar electrode orientations into a wavefront direction-aware omnipolar technology [85, 87, 110,111,112,113,114,115,116,117,118,119]. Deno et al. proposed the construction of omnipolar EGM from multiple bipolar EGM considering specific alignments of the electrodes, called clique [85, 110]. Assuming four unipolar electrodes arranged in a square area and \(\overline{W}\) is a propagating wavefront passing through locally (Fig. 7), omnipolar technology exploits the wavefront characteristics such as propagation direction. Then, bipolar EGMs from orthogonally placed bipolar electrode pairs can be calculated without maneuvering the catheter. If \(V_{x}\) and \(V_{y}\) are voltages captured by two mutually orthogonal bipolar orientations using a triangular clique, omnipolar technology features the maximum measurable voltages using a particular mapping catheter. Notably, the type of clique formation can vary using different sets of bipolar orientations [123]. For a more detailed calculation of the omnipolar voltage, please refer to [85, 110]. Omnipolar EGM explicitly reduces the impact of the directionality of bipolar orientations [85, 87, 110,111,112,113,114,115,116,117,118,119].
A schematic illustration of wavefront direction-aware EGM approximation concepts. [Created with https://www.BioRender.com]
Saha et al. proposed a beamforming-inspired spatial filtering technique for minimizing the impact of directionality while utilizing the diversity gain from multiple bipolar electrode orientations [18]. Current grid-type mapping catheters, such as HD Grid\(^{\text{TM}}\) and Optrell\(^{\text{TM}}\), offer special arrangements of the electrodes, allowing the simultaneous processing of various EGMs. Array signal processing is a popular topic in the wireless or cellular communication industry [124, 125], and the advancements may contribute to current cardiac mapping tools after adequate clinical validation. Figure 7 schematically compares the concepts of omnipolar and beamforming EGM constructions for reducing the effect of directionality.
Future Perspectives and Conclusion
There are still scopes for improvement while considering the uncertainties of bipolar EGM-based cardiac mapping tools, as discussed in the review. Three factors could be vital for the next frontier of AF mapping technologies, i.e., the user-centric design of catheter electrodes, the precision of electrode localization, and the integration of artificial intelligence (AI) [13, 14, 18, 19, 23, 40, 75, 76, 79,80,81, 83,84,85,86, 88, 102,103,104,105,106,107, 126,127,128]. The intrinsic nonlinearity in the AI-based algorithm may capture the time-variant and nonstationary EGM dynamics for AF source mapping utilizing multi-electrode catheters. The in silico simulation is a precursor for validating novel algorithms while quantifying their differences in AF source selection [98,99,100,101].
Cardiologists’ feedback on the existing state-of-the-art cardiac mapping tools concerning maneuverability, ease-of-use and accuracy in ablation target identification can revise the problem statement for engineers, prompting them to develop catheter electrodes fulfilling user needs. Then, increasing the accuracy of electrode localization (registering electrodes in the atrial anatomy) tools could play an essential role in AF target selection. Finally, how effectively we could integrate artificial intelligence in the mapping system, from surface reconstruction to AF-related source localization, is crucial.
Comprehensive studies from engineering perspectives are critical for demonstrating the effects of the variables reviewed in this paper and the clinical implications of detecting unsuitable ablation targets (AF-related sources). Recent advances in multielectrode catheter design provide high-density acquisition of spatially distributed atrial electrical activities. Retrospective investigation of the collected data and clinically annotated AF-related targets could be a precursor to exploiting the power of advanced signal processing and artificial intelligence to identify the AF sources accurately.
Data Availability
All data are included in this manuscript.
Code Availability
Not applicable.
References
Elliott, A. D., M. E. Middeldorp, I. C. Van Gelder, C. M. Albert, and P. Sanders. Epidemiology and modifiable risk factors for atrial fibrillation. Nat. Rev. Cardiol. 20(6):404–417, 2023.
Essien, U. R., J. Kornej, A. E. Johnson, L. B. Schulson, E. J. Benjamin, and J. W. Magnani. Social determinants of atrial fibrillation. Nat. Rev. Cardiol. 18(11):763–773, 2021.
Schotten, U., S. Lee, S. Zeemering, and A. L. Waldo. Paradigm shifts in electrophysiological mechanisms of atrial fibrillation. EP Europace. 23(Suppl 2):9–13, 2021.
Quah, J. X., D. Dharmaprani, K. Tiver, A. Lahiri, T. Hecker, R. Perry, J. B. Selvanayagam, M. X. Joseph, A. McGavigan, and A. Ganesan. Atrial fibrosis and substrate based characterization in atrial fibrillation: time to move forwards. J. Cardiovasc. Electrophysiol. 32(4):1147–1160, 2021.
Roney, C. H., A. L. Wit, and N. S. Peters. Challenges associated with interpreting mechanisms of AF. Arrhythm. Electrophysiol. Rev. 8(4):273, 2019.
Wijesurendra, R. S., and B. Casadei. Mechanisms of atrial fibrillation. Heart. 105(24):1860–1867, 2019.
Cheniti, G., K. Vlachos, T. Pambrun, D. Hooks, A. Frontera, M. Takigawa, F. Bourier, T. Kitamura, A. Lam, C. Martin, et al. Atrial fibrillation mechanisms and implications for catheter ablation. Front. Physiol. 9:1458, 2018.
Nattel, S., and D. Dobrev. Controversies about atrial fibrillation mechanisms: aiming for order in chaos and whether it matters. Circ. Res. 120(9):1396–1398, 2017.
Schotten, U., S. Verheule, P. Kirchhof, and A. Goette. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol. Rev. 91(1):265–325, 2011.
Karki, R., A. Raina, F. M. Ezzeddine, M. C. Bois, and S. J. Asirvatham. Anatomy and pathology of the cardiac conduction system. Cardiac Electrophysiol. Clin. 13(4):569–584, 2021.
Padala, S. K., J.-A. Cabrera, and K. A. Ellenbogen. Anatomy of the cardiac conduction system. Pacing Clin. Electrophysiol. 44(1):15–25, 2021.
Garcia, J. V., and D. K. Wan, The kit: access, catheter placement, transeptal puncture, ablation technology, 3d mapping. Decoding Cardiac Electrophysiology: understanding the Techniques and Defining the Jargon, pp. 21–39, 2020.
Ladas, T. P., A. Sugrue, J. Nan, V. R. Vaidya, D. Padmanabhan, K. Venkatachalam, and S. J. Asirvatham. Fundamentals of cardiac mapping. Cardiac Electrophysiol. Clin. 11(3):433–48, 2019.
Issa, Z. F., J. M. Miller, and D. P. Zipes. Chapter 3—mapping and navigation modalities. In: Clinical Arrhythmology and Electrophysiology, edited by Z. F. Issa, J. M. Miller, and D. P. Zipes. Philadelphia: W.B. Saunders, 2009, pp. 57–99. https://doi.org/10.1016/B978-1-4160-5998-1.00006-9.
Ragot, D., S. Nayyar, S. Z. Massin, A. C. Ha, S. M. Singh, C. Labos, A. Suszko, R. Dalvi, and V. S. Chauhan. Unipolar electrogram-based voltage mapping with far-field cancellation to improve detection of abnormal atrial substrate during atrial fibrillation. J. Cardiovasc. Electrophysiol. 32(6):1572–1583, 2021.
de Groot, N., D. Shah, P. M. Boyle, E. Anter, G. D. Clifford, I. Deisenhofer, T. Deneke, P. van Dessel, O. Doessel, P. Dilaveris, et al. Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the european heart rhythm association and european society of cardiology working group on ecardiology in collaboration with the heart rhythm society, Asia pacific heart rhythm society, latin American heart rhythm society and computing in cardiology. EP Europace, 2021.
Frisch, D., T. G. Oesterlein, L. A. Unger, G. Lenis, R. Wakili, C. Schmitt, A. Luik, O. Dössel, and A. Loewe. Mapping and removing the ventricular far field component in unipolar atrial electrograms. IEEE Trans. Biomed. Eng. 67(10):2905–2915, 2020.
Saha, S., D. Linz, P. Sanders, and M. Baumert, Beamforming-inspired spatial filtering technique for intracardiac electrograms. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2019, pp. 4254–4257. IEEE.
Kim, Y.-H., S.-A. Chen, S. Ernst, C. E. Guzman, S. Han, Z. Kalarus, C. Labadet, Y.-J. Lin, L.-W. Lo, A. Nogami, et al. 2019 aphrs expert consensus statement on three-dimensional mapping systems for tachycardia developed in collaboration with hrs, ehra, and lahrs. J. Arrhythm. 36(2):215, 2020.
Abdi, B., M. S. van Schie, N. M. de Groot, and R. C. Hendriks. Analyzing the effect of electrode size on electrogram and activation map properties. Comput. Biol. Med.134:104467, 2021.
Lydiard, S., B. Pontré, B. S. Lowe, H. Ball, G. Sasso, and P. Keall. Cardiac radioablation for atrial fibrillation: target motion characterization and treatment delivery considerations. Med. Phys. 48(3):931–941, 2021.
Anter, E., and M. E. Josephson. Bipolar voltage amplitude: what does it really mean? Heart Rhythm. 13(1):326–327, 2016.
Klemm, H. U., D. Steven, C. Johnsen, R. Ventura, T. Rostock, B. Lutomsky, T. Risius, T. Meinertz, and S. Willems. Catheter motion during atrial ablation due to the beating heart and respiration: impact on accuracy and spatial referencing in three-dimensional mapping. Heart Rhythm. 4(5):587–592, 2007.
Del Corso, G., R. Verzicco, and F. Viola. A fast computational model for the electrophysiology of the whole human heart. J. Comput. Phys.457:111084, 2022.
Arshad, A., and A. J. Atkinson. A 21st century view of the anaotmy of the cardiac conduction system. Transl. Res. Anatomy.28:100204, 2022.
Anderson, R. H., D. Sánchez-Quintana, D. E. Spicer, J. Farré, and E. B. Sternick. How does the cardiac impulse pass from the sinus to the atrioventricular node? Heart Rhythm. 19(10):1738–1746, 2022.
Prabhu, S., and A. Sohaib, The basic language of cardiac electrophysiology—an introduction to intracardiac electrograms and electrophysiology studies. Decoding Cardiac Electrophysiology: Understanding the Techniques and Defining the Jargon, pp. 3–19 (2020)
Zhang, Y., L. Sun, L. Xuan, Z. Pan, X. Hu, H. Liu, Y. Bai, L. Jiao, Z. Li, L. Cui, et al. Long non-coding RNA CCRR controls cardiac conduction via regulating intercellular coupling. Nat. Commun. 9(1):1–14, 2018.
Pfeiffer, E. R., J. R. Tangney, J. H. Omens, and A. D. McCulloch. Biomechanics of cardiac electromechanical coupling and mechanoelectric feedback. J. Biomech. Eng.136(2):021007, 2014.
Anderson, R. H., J. Yanni, M. R. Boyett, N. J. Chandler, and H. Dobrzynski. The anatomy of the cardiac conduction system. Clin. Anatomy. 22(1):99–113, 2009.
Ramirez, F. D., V. Y. Reddy, R. Viswanathan, M. Hocini, and P. Jaïs. Emerging technologies for pulmonary vein isolation. Circ. Res. 127(1):170–183, 2020.
Haissaguerre, M., P. Jaïs, D. C. Shah, A. Takahashi, M. Hocini, G. Quiniou, S. Garrigue, A. Le Mouroux, P. Le Métayer, and J. Clémenty. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339(10):659–666, 1998.
Aksu, T., J. R. Skeete, and H. H. Huang. Ganglionic plexus ablation: a step-by-step guide for electrophysiologists and review of modalities for neuromodulation for the management of atrial fibrillation. Arrhythm. Electrophysiol. Rev.12:e02, 2023.
Chen, P.-S., L. S. Chen, M. C. Fishbein, S.-F. Lin, and S. Nattel. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 114(9):1500–1515, 2014.
Coyle, C., S. Koutsoftidis, M.-Y. Kim, B. Porter, D. Keene, V. Luther, B. Handa, J. Kay, E. Lim, L. Malcolme-Lawes, et al. Feasibility of mapping and ablating ectopy-triggering ganglionated plexus reproducibly in persistent atrial fibrillation. J. Interventional Cardiac Electrophysiol., 1–8, 2023.
Narayan, S. M., D. E. Krummen, K. Shivkumar, P. Clopton, W.-J. Rappel, and J. M. Miller. Treatment of atrial fibrillation by the ablation of localized sources: confirm (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J. Am. Coll. Cardiol. 60(7):628–636, 2012.
Narayan, S. M., D. E. Krummen, and W.-J. Rappel. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J. Cardiovasc. Electrophysiol. 23(5):447–454, 2012.
Nattel, S. New ideas about atrial fibrillation 50 years on. Nature. 415(6868):219–226, 2002.
Nairn, D., C. Nagel, B. Mueller-Edenborn, H. Lehrmann, A. Jadidi, and A. Loewe. Spatial and quantitative assessment of the correlation between sinus rhythm and atrial fibrillation voltage mapping to identify low voltage substrate in persistent atrial fibrillation. EP Europace. 23(Suppl 3):116–163, 2021.
Linz, D., S. Saha, R. Kutieleh, K. Kadhim, D. Lau, M. Baumert, and P. Sanders. Impact of bipolar vector orientation and inter-electrode spacing on electrograms during human atrial fibrillation. Eur. Heart J. 40(Suppl 1):748–1153, 2019.
Yamaguchi, T., T. Tsuchiya, S. Nakahara, A. Fukui, Y. Nagamoto, K. Murotani, K. Eshima, and N. Takahashi. Efficacy of left atrial voltage-based catheter ablation of persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 27(9):1055–1063, 2016.
Harrison, J. L., H. K. Jensen, S. A. Peel, A. Chiribiri, A. K. Grøndal, L. Ø. Bloch, S. F. Pedersen, J. F. Bentzon, C. Kolbitsch, R. Karim, et al. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur. Heart J. 35(22):1486–1495, 2014.
Badger, T. J., M. Daccarett, N. W. Akoum, Y. A. Adjei-Poku, N. S. Burgon, T. S. Haslam, S. Kalvaitis, S. Kuppahally, G. Vergara, L. McMullen, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circulation. 3(3):249–259, 2010.
Baher, A., B. Buck, M. Fanarjian, J. Paul Mounsey, A. Gehi, E. Chung, F. G. Akar, C. L. Webber Jr., J. G. Akar, and J. P. Hummel. Recurrence quantification analysis of complex-fractionated electrograms differentiates active and passive sites during atrial fibrillation. J. Cardiovasc. Electrophysiol. 30(11):2229–2238, 2019.
van der Does, L. J., and N. M. de Groot. Inhomogeneity and complexity in defining fractionated electrograms. Heart Rhythm. 14(4):616–624, 2017.
Nademanee, K., J. McKenzie, E. Kosar, M. Schwab, B. Sunsaneewitayakul, T. Vasavakul, C. Khunnawat, and T. Ngarmukos. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 43(11):2044–2053, 2004.
Almeida, T. P., D. C. Soriano, M. Mase, F. Ravelli, A. S. Bezerra, X. Li, G. S. Chu, J. Salinet, P. J. Stafford, G. A. Ng, et al. Unsupervised classification of atrial electrograms for electroanatomic mapping of human persistent atrial fibrillation. IEEE Trans. Biomed. Eng. 68(4):1131–1141, 2020.
Roney, C. H., J. Whitaker, I. Sim, L. O’Neill, R. K. Mukherjee, O. Razeghi, E. J. Vigmond, M. Wright, M. D. O’Neill, S. E. Williams, et al. A technique for measuring anisotropy in atrial conduction to estimate conduction velocity and atrial fibre direction. Comput. Biol. Med. 104:278–290, 2019.
Coveney, S., C. Corrado, C. H. Roney, D. O’Hare, S. E. Williams, M. D. O’Neill, S. A. Niederer, R. H. Clayton, J. E. Oakley, and R. D. Wilkinson. Gaussian process manifold interpolation for probabilistic atrial activation maps and uncertain conduction velocity. Philos. Trans. R. Soc. A. 378(2173):20190345, 2020.
Wong, K. C., P. P. Sadarmin, J. De Bono, N. Qureshi, M. Jones, K. Rajappan, Y. Bashir, and T. R. Betts. Local activation times at the high posterior wall of the left atrium during left atrial appendage pacing predict roof line block with high specificity and sensitivity. Europace. 13(9):1243–1249, 2011.
Zaatari, G., R. Mitrani, J. Bohorquez, J. Ng, J. Ng, H. Rivner, A. Velasquez, L. Lambrakos, R. Arora, and J. J. Goldberger. Electrogram morphology recurrence for mapping persistent atrial fibrillation: initial vs redo catheter ablation. Clin. Electrophysiol. 9(4):526–540, 2023.
Ravelli, F., M. Mase, A. Cristoforetti, M. Marini, and M. Disertori. The logical operator map identifies novel candidate markers for critical sites in patients with atrial fibrillation. Prog. Biophys. Mol. Biol. 115(2–3):186–197, 2014.
Honarbakhsh, S., R. J. Schilling, R. Providencia, E. Keating, A. Chow, S. Sporton, M. Lowe, M. J. Earley, P. D. Lambiase, and R. J. Hunter. Characterization of drivers maintaining atrial fibrillation: correlation with markers of rapidity and organization on spectral analysis. Heart Rhythm. 15(9):1296–1303, 2018.
Li, X., G. S. Chu, T. P. Almeida, F. J. Vanheusden, J. Salinet, N. Dastagir, A. R. Mistry, Z. Vali, B. Sidhu, P. J. Stafford, et al. Automatic extraction of recurrent patterns of high dominant frequency mapping during human persistent atrial fibrillation. Front. Physiol.12:649486, 2021.
Hwang, M., J.-S. Song, Y.-S. Lee, C. Li, E. B. Shim, and H.-N. Pak. Electrophysiological rotor ablation in in-silico modeling of atrial fibrillation: comparisons with dominant frequency, shannon entropy, and phase singularity. PLoS ONE. 11(2):0149695, 2016.
Ganesan, A. N., P. Kuklik, D. H. Lau, A. G. Brooks, M. Baumert, W. W. Lim, S. Thanigaimani, S. Nayyar, R. Mahajan, J. M. Kalman, et al. Bipolar electrogram shannon entropy at sites of rotational activation: implications for ablation of atrial fibrillation. Circulation. 6(1):48–57, 2013.
Bianca J. J. M. Brundel, M.T.H.M.F.K.G.Y.H.L. Xun Ai, N.M.S. de Groot, Atrial fibrillation. Nat. Rev. Dis. Primers 8(21):2056–676, 2022.
Andersen, J. H., L. Andreasen, and M. S. Olesen. Atrial fibrillation-a complex polygenetic disease. Eur. J. Hum. Genet. 29(7):1051–1060, 2021.
Andrade, J., P. Khairy, D. Dobrev, and S. Nattel. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114(9):1453–1468, 2014.
Frangogiannis, N. G. The extracellular matrix in ischemic and nonischemic heart failure. Circ. Res. 125(1):117–146, 2019.
Reese-Petersen, A. L., M. S. Olesen, M. A. Karsdal, J. H. Svendsen, and F. Genovese. Atrial fibrillation and cardiac fibrosis: a review on the potential of extracellular matrix proteins as biomarkers. Matrix Biol. 91:188–203, 2020.
Nguyen, T. P., Z. Qu, and J. N. Weiss. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J. Mol. Cell. Cardiol. 70:83–91, 2014.
Pellman, J., R. C. Lyon, and F. Sheikh. Extracellular matrix remodeling in atrial fibrosis: mechanisms and implications in atrial fibrillation. J. Mol. Cell. Cardiol. 48(3):461–467, 2010.
Burstein, B., and S. Nattel. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 51(8):802–809, 2008.
Pelouch, V., I. Dixon, L. Golfman, R. E. Beamish, and N. S. Dhalla. Role of extracellular matrix proteins in heart function. Mol. Cell. Biochem. 129(2):101–120, 1993.
van der Does, L., C. Kik, M. Allessie, and N. de Groot. Endo-epicardial dissociation in conduction. Eur. Heart J. 38(22):1775–1775, 2017.
de Groot, N., L. Van Der Does, A. Yaksh, E. Lanters, C. Teuwen, P. Knops, P. van de Woestijne, J. Bekkers, C. Kik, A. Bogers, et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circulation.9(5):003648, 2016.
Verheule, S., J. Eckstein, D. Linz, B. Maesen, E. Bidar, A. Gharaviri, and U. Schotten. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog. Biophys. Mol. Biol. 115(2–3):173–185, 2014.
de Groot, N. M., R. P. Houben, J. L. Smeets, E. Boersma, U. Schotten, M. J. Schalij, H. Crijns, and M. A. Allessie. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 122(17):1674–1682, 2010.
Ravelli, F., M. Masè, A. Cristoforetti, L. Avogaro, E. D’Amato, F. Tessarolo, F. Piccoli, and A. Graffigna. Quantitative assessment of transmural fibrosis profile in the human atrium: evidence for a three-dimensional arrhythmic substrate by slice-to-slice histology. Europace. 25(2):739–747, 2023.
Pagani, S., L. Dede’, A. Frontera, M. Salvador, L. Limite, A. Manzoni, F. Lipartiti, G. Tsitsinakis, A. Hadjis, P. Della Bella, et al. A computational study of the electrophysiological substrate in patients suffering from atrial fibrillation. Front. Physiol.12:673612, 2021.
Takahashi, Y., T. Yamaguchi, T. Otsubo, K. Nakashima, K. Shinzato, R. Osako, S. Shichida, Y. Kawano, A. Fukui, A. Kawaguchi, et al. Histological validation of atrial structural remodelling in patients with atrial fibrillation. Eur. Heart J. 396, 2023.
Baumert, M., P. Sanders, and A. Ganesan. Quantitative-electrogram-based methods for guiding catheter ablation in atrial fibrillation. Proc. IEEE. 104(2):416–431, 2016.
Tedrow, U. B., and W. G. Stevenson. Recording and interpreting unipolar electrograms to guide catheter ablation. Heart Rhythm. 8(5):791–796, 2011.
Gaeta, S., T. D. Bahnson, and C. Henriquez. Mechanism and magnitude of bipolar electrogram directional sensitivity: characterizing underlying determinants of bipolar amplitude. Heart Rhythm. 17(5):777–785, 2020.
Sroubek, J., M. Rottmann, M. Barkagan, E. Leshem, A. Shapira-Daniels, E. Brem, C. Fuentes-Ortega, J. Malinaric, S. Basu, M. Bar-Tal, et al. A novel octaray multielectrode catheter for high-resolution atrial mapping: electrogram characterization and utility for mapping ablation gaps. J. Cardiovasc. Electrophysiol. 30(5):749–757, 2019.
Saha, S., S. Hartmann, D. Linz, P. Sanders, and M. Baumert, A ventricular far-field artefact filtering technique for atrial electrograms. In: 2019 Computing in Cardiology (CinC), p. 1, 2019. IEEE.
Dalvi, R., S. Nayyar, A. Suszko, and V. S. Chauhan, A least squares approach to estimation of far-field voltage in unipolar electrograms in atrial fibrillation. In: 2018 52nd Asilomar Conference on Signals, Systems, and Computers, pp. 1230–1233, 2018. IEEE.
Stinnett-Donnelly, J. M., N. Thompson, N. Habel, V. Petrov-Kondratov, D. D. C. de Sa, J. H. Bates, and P. S. Spector. Effects of electrode size and spacing on the resolution of intracardiac electrograms. Coron. Artery Dis. 23(2):126–132, 2012.
Takigawa, M., T. Kitamura, S. Basu, M. Bartal, C. A. Martin, R. Martin, G. Cheniti, K. Vlachos, X. Pillois, A. Frontera, et al. Effect of electrode size and spacing on electrograms: optimized electrode configuration for near-field electrogram characterization. Heart Rhythm. 19(1):102–112, 2021.
Takigawa, M., J. Relan, R. Martin, S. Kim, T. Kitamura, G. Cheniti, K. Vlachos, X. Pillois, A. Frontera, G. Massoullié, et al. Detailed analysis of the relation between bipolar electrode spacing and far-and near-field electrograms. Clin. Electrophysiol. 5(1):66–77, 2019.
Beheshti, M., K. Magtibay, S. Massé, A. Porta-Sanchez, S. Haldar, A. Bhaskaran, S. Nayyar, B. Glover, D. C. Deno, E. J. Vigmond, et al. Determinants of atrial bipolar voltage: Inter electrode distance and wavefront angle. Comput. Biol. Med. 102:449–457, 2018.
Kuo, M.-J., L.-W. Lo, Y.-J. Lin, S.-L. Chang, Y.-F. Hu, F.-P. Chung, T.-C. Tuan, T.-F. Chao, J.-N. Liao, T.-Y. Chang, et al. Low voltage zones detected by omnipolar vmax map accurately identifies the potential atrial substrate and predicts the af ablation outcome after pv isolation. Int. J. Cardiol. 351:42–47, 2022.
Yavin, H. D., J. Sroubek, J. Yarnitsky, Z. P. Bubar, K. Higuchi, I. Zilberman, S. Basu, and E. Anter. Direction-aware mapping algorithms have minimal impact on bipolar voltage maps created using high-resolution multielectrode catheters. J. Cardiovasc. Electrophysiol. 33(1):73–80, 2022.
Deno, D. C., R. Balachandran, D. Morgan, F. Ahmad, S. Massé, and K. Nanthakumar. Orientation-independent catheter-based characterization of myocardial activation. IEEE Trans. Biomed. Eng. 64(5):1067–1077, 2016.
Massé, S., K. Magtibay, N. Jackson, J. Asta, M. Kusha, B. Zhang, R. Balachandran, M. Radisic, D. C. Deno, and K. Nanthakumar. Resolving myocardial activation with novel omnipolar electrograms. Circulation.9(7):004107, 2016.
Castells, F., S. Ruipérez-Campillo, I. Segarra, R. Cervigón, R. Casado-Arroyo, J. L. Merino, and J. Millet. Performance assessment of electrode configurations for the estimation of omnipolar electrograms from high density arrays. Comput. Biol. Med.154:106604, 2023.
Shenasa, M., S.-M. Razavi, H. Shenasa, and A. Al-Ahmad. The ideal cardiac mapping system. Cardiac Electrophysiol. Clin. 11(4):739–748, 2019.
Martinez-Iniesta, M., J. Ródenas, R. Alcaraz, and J. J. Rieta. Waveform integrity in atrial fibrillation: the forgotten issue of cardiac electrophysiology. Ann. Biomed. Eng. 45(8):1890–1907, 2017.
Unger, L. A., T. G. Oesterlein, A. Loewe, and O. Dössel, Noise quantification and noise reduction for unipolar and bipolar electrograms. In: 2019 Computing in Cardiology (CinC), p. 1, 2019. IEEE.
Botteron, G. W., and J. M. Smith. A technique for measurement of the extent of spatial organization of atrial activation during atrial fibrillation in the intact human heart. IEEE Trans. Biomed. Eng. 42(6):579–586, 1995.
Berenfeld, O., R. Mandapati, S. Dixit, A. C. Skanes, J. Chen, M. Mansour, and J. Jalife. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the langendorff-perfused sheep heart. J. Cardiovasc. Electrophysiol. 11(8):869–879, 2000.
Ciaccio, E. J., A. B. Biviano, and H. Garan. Computational method for high resolution spectral analysis of fractionated atrial electrograms. Comput. Biol. Med. 43(10):1573–1582, 2013.
Borlich, M., and P. Sommer. Cardiac mapping systems: rhythmia, topera, ensite precision, and carto. Cardiac Electrophysiol. Clin. 11(3):449–458, 2019.
Xiong, Z., M. K. Stiles, Y. Yao, R. Shi, A. Nalar, J. Hawson, G. Lee, and J. Zhao. Automatic 3d surface reconstruction of the left atrium from clinically mapped point clouds using convolutional neural networks. Front. Physiol.13:880260, 2022.
Sha, Q., L. Elliott, X. Zhang, T. Levy, T. Sharma, and A. Abdelaal. Atrial fibrillation driver identification through regional mutual information networks: a modeling perspective. J. Interv. Card. Electrophysiol. 64(3):649–660, 2022.
Rodrigo, M., M. I. Alhusseini, A. J. Rogers, C. Krittanawong, S. Thakur, R. Feng, P. Ganesan, and S. M. Narayan. Atrial fibrillation signatures on intracardiac electrograms identified by deep learning. Comput. Biol. Med.145:105451, 2022.
Sánchez, J., G. Luongo, M. Nothstein, L. A. Unger, J. Saiz, B. Trenor, A. Luik, O. Dössel, and A. Loewe, Using machine learning to characterize atrial fibrotic substrate from intracardiac signals with a hybrid in silico and in vivo dataset. Front. Physiol. 1000, 2021.
Bartolucci, C., C. Fabbri, C. Tomasi, P. Sabbatani, S. Severi, and C. Corsi. Computational analysis of mapping catheter geometry and contact quality effects on rotor detection in atrial fibrillation. Front. Physiol.12:732161, 2021.
Heijman, J., H. Sutanto, H. J. Crijns, S. Nattel, and N. A. Trayanova. Computational models of atrial fibrillation: achievements, challenges, and perspectives for improving clinical care. Cardiovasc. Res. 117(7):1682–1699, 2021.
Aronis, K. N., R. Ali, and N. A. Trayanova. The role of personalized atrial modeling in understanding atrial fibrillation mechanisms and improving treatment. Int. J. Cardiol. 287:139–147, 2019.
Berte, B., K. Zeppenfeld, and R. Tung. Impact of micro-, mini-and multi-electrode mapping on ventricular substrate characterisation. Arrhythm. Electrophysiol. Rev. 9(3):128, 2020.
Barkagan, M., J. Sroubek, A. Shapira-Daniels, H. Yavin, J. Jang, R. Nezafat, and E. Anter. A novel multielectrode catheter for high-density ventricular mapping: electrogram characterization and utility for scar mapping. EP Europace. 22(3):440–449, 2020.
Octaray\(^{{\rm TM}}\) mapping catheter with trueref\(^{{\rm TM}}\) technology. Accessed February 21, 2023. https://www.jnjmedtech.com/en-US/product/octaray-mapping-catheter-trueref-technology.
Dodeja, A. K., Y. Tan, T. Ackley, J. Russell, N. Kertesz, C. J. Daniels, and A. Kamp. Pentaray® multielectrode mapping catheter for atrial tachyarrhythmia in adults with congenital heart disease. Tex. Heart Inst. J. 49(5):207535, 2022.
Advisor HD Grid\(^{{\rm TM}}\) mapping catheter, sensor enabled\(^{{\rm TM}}\). Accessed February 21, 2023. https://www.cardiovascular.abbott/us/en/hcp/products/electrophysiology/diagnostic-catheters/advisor-hd-grid.html
Jiang, R., A. D. Beaser, Z. Aziz, G. A. Upadhyay, H. M. Nayak, and R. Tung. High-density grid catheter for detailed mapping of sinus rhythm and scar-related ventricular tachycardia: comparison with a linear duodecapolar catheter. Clin. Electrophysiol. 6(3):311–323, 2020.
Tan, J. L., G. S. Guandalini, M. C. Hyman, J. Arkles, P. Santangeli, R. D. Schaller, F. Garcia, G. Supple, D. S. Frankel, S., Nazarian, et al. Substrate and arrhythmia characterization using the multi-electrode optrell mapping catheter for ventricular arrhythmia ablation—a single-center experience. J. Interv. Cardiac Electrophysiol. 1–11, 2023.
Yavin, H. D., Z. P. Bubar, K. Higuchi, J. Sroubek, J. Yarnitsky, and E. Anter. Propagation vectors facilitate differentiation between conduction block, slow conduction, and wavefront collision. Circulation.14(8):010081, 2021.
Deno, D. C., A. Bhaskaran, D. J. Morgan, F. Goksu, K. Batman, G. K. Olson, K. Magtibay, S. Nayyar, A. Porta-Sánchez, M. A. Laflamme, et al. High-resolution, live, directional mapping. Heart Rhythm. 17(9):1621–1628, 2020.
Dittrich, S., C. Scheurlen, J.-H. van den Bruck, K. Filipovic, J. Wörmann, S. Erlhöfer, J.-H. Schipper, J. Lüker, D. Steven, and A. Sultan, The omnipolar mapping technology—a new mapping tool to overcome “bipolar blindness” resulting in true high-density maps. J. Interv. Cardiac Electrophysiol. 1–10, 2023.
Ruiperez-Campillo, S., F. Castells, M. Crespo, L. Pancorbo, A. Guill, F. Chorro, J. Merino, R. Casado-Arroyo, and J. Millet. Study of the omnipolar egm reconstruction for robustness against wavefront propagation in epicardial signals. Europace. 25(Suppl 1):122–662, 2023.
Iacopino, S., F. Cecchini, A. Tripodi, P. Sorrenti, G. Fabiano, and A. Petretta. Epicardial multisite conduction blocks detected by equispaced electrode array and omnipolar technology in brugada syndrome. Heart Rhythm Case Rep. 9(1):12–16, 2023.
Van Schie, M. S., P. Knops, L. Zhang, F. Van Schaagen, Y. J. Taverne, and N. De Groot. Detection of endo-epicardial atrial low-voltage areas using unipolar and omnipolar voltage mapping. Front. Physiol. 13:1030025, 2022.
Yeo, C., V. H. Tan, and Y. Wang. Omnipolar activation egm to identify the earliest breakout site of atrial tachycardia. J. Arrhythm. 38(5):801–804, 2022.
Karatela, M. F., R. S. Dowell, D. Friedman, K. P. Jackson, and J. P. Piccini. Omnipolar versus bipolar electrode mapping in patients with atrial fibrillation undergoing catheter ablation. Clin. Electrophysiol. 8(12):1539–1552, 2022.
Burg, M. R., R. D. Anderson, S. Massé, and K. Nanthakumar. Cardiac mapping with irreverence to time: replacing isochrones with omnipolar vectors. Heart Rhythm. 19(11):1802–1803, 2022.
van Schie, M. S., R. K. Kharbanda, C. A. Houck, E. A. Lanters, Y. J. Taverne, A. J. Bogers, and N. M. de Groot. Identification of low-voltage areas: a unipolar, bipolar, and omnipolar perspective. Circulation.14(7):009912, 2021.
Haldar, S. K., K. Magtibay, A. Porta-Sanchez, S. Massé, N. Mitsakakis, P. F. Lai, M. A. Azam, J. Asta, M. Kusha, P. Dorian, et al. Resolving bipolar electrogram voltages during atrial fibrillation using omnipolar mapping. Circulation.10(9):005018, 2017.
Ollitrault, P., L. Champ-Rigot, V. Ferchaud, A. Pellissier, O. Coffin, and P. Milliez. Vascular entrapment of a multipolar basket catheter (oriontm) during catheter ablation. J. Cardiovasc. Electrophysiol. 32(2):545–546, 2021.
Pathik, B., G. Lee, F. Sacher, M. Haïssaguerre, P. Jaïs, G. Massoullié, N. Derval, P. Sanders, P. Kistler, and J. M. Kalman. Epicardial-endocardial breakthrough during stable atrial macroreentry: evidence from ultra-high-resolution 3-dimensional mapping. Heart Rhythm. 14(8):1200–1207, 2017.
Oesterlein, T., D. Frisch, A. Loewe, G. Seemann, C. Schmitt, O. Dössel, and A. Luik, Basket-type catheters: diagnostic pitfalls caused by deformation and limited coverage. BioMed Res. Int., 2016.
Ruipérez-Campillo, S., M. Crespo, Á. Tormos, A. Guill, A. Cebrián, A. Alberola, J. Heimer, F. J., Chorro, J., Millet, and F. Castells, Evaluation and assessment of clique arrangements for the estimation of omnipolar electrograms in high density electrode arrays: an experimental animal model study. Phys. Eng. Sci. Med. 1–12, 2023.
Jeong, C., J. Park, and H. Yu. Random access in millimeter-wave beamforming cellular networks: issues and approaches. IEEE Commun. Mag. 53(1):180–185, 2015.
Wang, M., F. Gao, S. Jin, and H. Lin. An overview of enhanced massive mimo with array signal processing techniques. IEEE J. Sel. Topics Signal Process. 13(5):886–901, 2019.
Trayanova, N. A., D. M. Popescu, and J. K. Shade. Machine learning in arrhythmia and electrophysiology. Circ. Res. 128(4):544–566, 2021.
Alhusseini, M. I., F. Abuzaid, A. J. Rogers, J. A. Zaman, T. Baykaner, P. Clopton, P. Bailis, M. Zaharia, P. J. Wang, W.-J. Rappel, et al. Machine learning to classify intracardiac electrical patterns during atrial fibrillation: machine learning of atrial fibrillation. Circulation.13(8):008160, 2020.
Corrado, C., S. Williams, C. Roney, G. Plank, M. O’Neill, and S. Niederer. Using machine learning to identify local cellular properties that support re-entrant activation in patient-specific models of atrial fibrillation. EP Europace. 23(Suppl 1):12–20, 2021.
Acknowledgements
Acknowledgments are not compulsory. Where included they should be brief. Grant or contribution numbers may be acknowledged. Please refer to Journal-level guidance for any specific requirements.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest.
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Additional information
Associate Editor Derek J. Dosdall, Ph.D. oversaw review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, S., Linz, D., Saha, D. et al. Overcoming Uncertainties in Electrogram-Based Atrial Fibrillation Mapping: A Review. Cardiovasc Eng Tech 15, 52–64 (2024). https://doi.org/10.1007/s13239-023-00696-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-023-00696-w