Abstract
Purpose
Robust experimental data for performing validation of fluid–structure interaction (FSI) simulations of the transport of deformable solid bodies in internal flow are currently lacking. This in vitro experimental study characterizes the clot trapping efficiency of a new generic conical-type inferior vena cava (IVC) filter in a rigid anatomical model of the IVC with carefully characterized test conditions, fluid rheological properties, and clot mechanical properties.
Methods
Various sizes of spherical and cylindrical clots made of synthetic materials (nylon and polyacrylamide gel) and bovine blood are serially injected into the anatomical IVC model under worst-case exercise flow conditions. Clot trapping efficiencies and their uncertainties are then quantified for each combination of clot shape, size, and material.
Results
Experiments reveal the clot trapping efficiency increases with increasing clot diameter and length, with trapping efficiencies ranging from as low as approximately 42% for small 3.2 mm diameter spherical clots up to 100% for larger clot sizes. Because of the asymmetry of the anatomical IVC model, the data also reveal the iliac vein of clot origin influences the clot trapping efficiency, with the trapping efficiency for clots injected into the left iliac vein up to a factor of 7.5 times greater than that for clots injected into the right iliac (trapping efficiencies of approximately 10% versus 75%, respectively).
Conclusion
Overall, this data set provides a benchmark for validating simulations predicting IVC filter clot trapping efficiency and, more generally, low-Reynolds number FSI modeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inferior vena cava (IVC) filters are medical devices that are intended to capture blood clots generated by lower extremity deep vein thrombosis before they reach the lungs and occlude the pulmonary circulation, a potentially fatal condition known as pulmonary embolism. There are a number of preclinical tests that are necessary to assess IVC filter safety and effectiveness that include clot trapping, biocompatibility, simulated deployment, durability, vessel perforation, filter migration resistance, and magnetic resonance imaging (MRI) compatibility.13, 24 Of these, evaluating the clot trapping efficiency is one of the most burdensome preclinical tests performed for these devices.
Historically, preclinical IVC filter clot trapping efficiency has been assessed from in vitro experiments using a mock IVC with either real or synthetic blood clots. Prior in vitro studies have evaluated the trapping efficiency of permanent,22, 28, 31, 33, 43, 48 retrievable,16, 18, 22, 31, 33, 37, 48 and biodegradable IVC filters.11, 55 In general, there exists a trend of increasing trapping efficiency with embolus size11, 16, 18, 19, 28, 31,32,33, 37, 43, 48 and decreasing capture success with increasing IVC diameter.16, 18, 25, 31, 43 The impact of caval orientation with relationship to gravity has also been established, with the majority of studies finding that a vertically oriented (upright) IVC facilitates higher embolus trapping efficiency than an IVC in a horizontal (supine or prone) position.16, 19, 25, 28, 36, 43, 48 Additionally, centered IVC filter placement generally leads to a higher in vitro clot trapping efficiency compared to tilted placement,18, 25, 33, 36, 37 which correlates with clinical findings that indicate an increased risk of pulmonary embolism with tilted filters.42
Though in vitro experiments are the historical precedent, computational modeling may also be used to evaluate IVC filter clot trapping efficiency provided that the simulations are rigorously verified and validated. A number of investigators have performed computational fluid dynamics (CFD) simulations of the hemodynamics in mock IVCs that range in complexity from a simple straight tube to anatomically accurate patient-specific models.2, 5, 9, 30, 38,39,40, 44,45,46,47, 49, 54 Modeling IVC hemodynamics is a first step toward simulating IVC filter clot trapping, which is especially challenging owing to the complexity of modeling the coupled fluid–structure interaction (FSI) that occurs between relatively large deformable clots and the flow in the IVC. Recent progress has been made in the development of methods to predict IVC filter trapping efficiency for rigid spherical clots,3, 4 and there is ongoing work to extend these capabilities to simulate realistic, deformable clots. In principle, such FSI simulations may be used as a complement to, or potentially in lieu of, traditional in vitro experiments to predict the preclinical clot trapping efficiency of an IVC filter. This would, however, require rigorous verification and validation of the computational modeling.1 Currently, there is a lack of robust experimental data for IVC filter clot trapping that includes sufficient detail and is of the quality necessary for performing such a rigorous validation.
The objective of this study is to characterize the in vitro clot trapping efficiency of a new generic conical-type IVC filter designed for research purposes by U.S. Food and Drug Administration (FDA) scientists and collaborators. We perform experiments in accord with the current international consensus standard for preclinical bench testing of IVC filters, ISO 25539-3:2011 (“Cardiovascular Implants—Endovascular Devices—Part 3: Vena Cava Filters”).24 The trapping efficiency is evaluated for a range of clinically relevant sizes and shapes of both synthetic and whole blood clots in a representative rigid anatomical IVC model in an upright orientation under worst-case physiological flow conditions. Our results elucidate the influence of clot size, shape, and material properties on the in vitro trapping efficiency of the conical-type GENI filter that is generally representative of other conical-type IVC filters that are in clinical use (Montgomery and Kaufman 2016).34 The resultant data set represents an experimental benchmark for validating computer simulations of in vitro IVC filter clot trapping, and more generally, low-Reynolds number FSI modeling.
Methods
Mock IVC
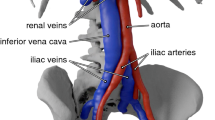
As recommended by ISO 25539-3:2011,24in vitro clot trapping experiments were performed using a “mock vena cava of the appropriate diameter and length”. A rigid IVC model was fabricated using inkjet 3D printing6 and has been described in previous work.9, 15 The model (Fig. 1a) is based on the realistic geometry of Rahbar et al. (2011), who created it from observations of computed tomography (CT) scans of 10 patients with normal IVC anatomies.39 Importantly, unlike more simplified models of the IVC, the present geometry includes all of the essential anatomical features that influence IVC hemodynamics and clot transport; namely the iliac veins, infrarenal IVC curvature, and non-circular vessel cross-sections.
Mock IVC model and IVC filter used for the in vitro clot trapping experiments. (a) The mock IVC was 3D printed and is based on the realistic IVC model of Rahbar et al.39 (images adapted from Gallagher et al.15 with permission). Anatomical right (R) and left (L) iliac veins and the respective angles of the vessel centerlines with respect to the infrarenal IVC are annotated. (b) FDA generic IVC filter, termed the GENI (GEneric NItinol) filter, designed for research purposes by FDA scientists and collaborators. This filter is generally representative of other conical-type filters.
The present anatomical IVC model was modified from the original geometry of Rahbar et al.(2011) in several ways. In accord with ISO 25539-3:2011, the model was uniformly scaled to obtain a worst-case infrarenal IVC diameter for clot trapping of 28 mm, which is characteristic of the maximum indicated diameter for many currently marketed IVC filters. Additionally, as justified by Craven et al. (2018), the model was truncated just upstream of the renal vein confluence.9 This is because IVC filters are most commonly placed in the infrarenal IVC upstream of the renal veins, which generally have an entry angle such that the incoming flow is directed downstream and does not affect infrarenal flow and IVC filter clot trapping (see Craven et al. 2018 for further details). Finally, because the present experiments will comprise a validation benchmark dataset, well-defined inlet conditions were desired that are easily prescribed in a computational model. Thus, the IVC model was modified by extruding the slightly non-circular iliac inlets to a circular cross-section and attaching straight inlet tubes with lengths of 1.22 m to obtain fully developed flow at the worst-case exercise flow condition considered here. Fully developed flow was confirmed from recent particle image velocimetry (PIV) experiments in the same model by Gallagher et al. (2018).15
FDA GENI IVC Filter
In this study, we characterize the in vitro clot trapping efficiency of a generic IVC filter, termed the GENI (GEneric NItinol) filter, designed for research purposes by the FDA authors and collaborators (Fig. 1b). The device was designed to be generally representative of other conical-type IVC filters that are in clinical use (e.g., see Montgomery and Kaufman 2016).34 As illustrated in Fig. 1b, the filter is a conical-type design that resembles the support structure of an umbrella and consists of a central hub with 16 evenly spaced radial struts. The GENI filter was laser cut from SE508 nitinol tubing.
For the in vitro clot trapping experiments, the GENI filter was deployed in the infrarenal section of the mock IVC with the distal tip of the filter hub located approximately 138 mm from the bifurcation of the iliac veins (Fig. 2a). The device was in an approximately centered configuration with no observable mediolateral tilt and a slight 2.5° anteroposterior tilt of the filter hub toward the posterior side of the IVC (Fig. 2a). To prevent clots from dislodging the filter, each strut was adhered to the 3D printed IVC model with cyanoacrylate glue.
Circulatory Flow Loop
The mock IVC was placed in an upright orientation and connected to a circulatory flow loop to test the in vitro clot trapping efficiency of the GENI IVC filter. The flow loop consisted of a fluid reservoir, a variable centrifugal pump (Cole-Parmer, Vernon Hills, IL), and an injection valve for introducing clots upstream of each iliac vein (Fig. 2). As previously mentioned, a straight inlet tube with a length of 1.22 m was attached to each iliac vein to obtain fully developed iliac inflow in each experiment.
A Newtonian blood analog solution comprised of a mixture of water or saline and glycerin was used. As described in the following sections, the proportion of glycerin with water or saline was varied to obtain a clinically relevant clot-to-fluid density ratio of approximately 1.01–1.04.35 In each experiment, a single clot was introduced into the flow loop using an injection valve located upstream of the entrance tubing connected to each iliac vein (Fig. 2). After each trial in which a clot was captured in the IVC filter, flow was reversed to remove the embolus from the flow loop before beginning the next trial.
Experiments were performed at flow conditions corresponding to the average physiological flow rate that occurs in the IVC during exercise.8, 15 Compared to flow rates that occur during resting conditions, exercise flow conditions were chosen as a worst-case for IVC filter clot trapping due to the higher Reynolds number (Re) of the flow that causes more pronounced secondary flow and mixing that affect clot transport (see Refs. 9, 15). Additionally, because clots are transported with greater inertia at higher flow rates, this leads to more pronounced FSI effects when the clots collide with the walls of the IVC and with the struts of the IVC filter.
Specifically, all experiments were conducted at flow conditions that roughly match the exercise condition infrarenal Re number of approximately 1500 from the PIV study of Gallagher et al.15 in the same IVC model. Because the ratio of water or saline with glycerin was varied depending on the specific clot type used in the trapping experiments, the flow rate in each iliac vein was manually adjusted by changing the circuit resistance using a tubing clamp upstream of the clot inject site (see Fig. 2) to obtain an approximately 50/50 flow split between the two iliac veins and the desired infrarenal Re. Importantly, to simplify computational modeling of the present experiment, at this Reynolds number the inlet flow in each iliac vein is fully developed, as previously confirmed by Gallagher et al.15 The fluid temperature, flow rate through each iliac vein, and the pressure at the entrance of the IVC model were measured during each experiment.
Bovine Blood Clots and Synthetic Clots
Spherical and cylindrical clot shapes have been used in previous literature to characterize the trapping efficiency of IVC filters. In summary, three clot materials and two clot shapes were investigated here: (i) rigid nylon clots (spherical only); (ii) deformable bovine blood clots (spherical and cylindrical); and (iii) deformable polyacrylamide (PA) gel clots (cylindrical only). Similar to previous investigations,18, 19, 33, 36, 37, 42, 48 clot diameters of 3.2 mm (small), 4.8 mm (medium), and 6.4 mm (large) were considered. Larger clots were not used because, as will be shown, all of the 6.4 mm clots were captured and, thus, any clots larger than 6.4 mm would certainly have a similar fate. The lengths of the cylindrical clots were one, three, and five times the clot diameter. Given the variability in clot materials, clot densities varied among the clot types considered. Analog blood fluid densities were therefore tuned to obtain clot-to-fluid density ratios in the physiological range of 1.01–1.04.35
Synthetic Nylon Clots (Spherical)
Nylon spheres (McMaster-Carr, Elmhurst, Illinois) of sizes 3.17 ± 0.01 mm, 4.73 ± 0.01 mm, and 6.34 ± 0.01 mm were selected for this study (Fig. 3a). To ensure that the nylon clots were saturated during the experiments, each clot was briefly soaked in the test fluid (Fluid A, 40% glycerin/60% water by weight with 24 g/L NaCl, see “Methods” section “Blood Analog Fluids”) prior to each trial. Sphere mass and diameter were measured, and the density (ρ = 1.14 ± 0.01 g/ml) was calculated. Given their durability, the nylon synthetic clots were reused in this study to perform a total of n = 110 (small, n = 50; medium, n = 30; large, n = 30) clot trapping runs.
Bovine Blood Clots (Spherical and Cylindrical)
Bovine blood was obtained through an approved Institutional Animal Care and Use Committee protocol at the Pennsylvania State University. The whole blood was collected in 500 mL donor bags and anti-coagulated with citrate phosphate dextrose adenine-1 (CPDA-1). The blood was separated into its components using a centrifugation protocol and reconstituted to maintain a consistent 50:50 concentration of red blood cells and platelet-rich plasma. Coagulation of reconstituted bovine blood was initiated with the introduction of CaCl2 at a concentration of 20 mMol in blood. Spherical and cylindrical blood clots were allowed to coagulate overnight at room temperature in custom molds and polyvinylchloride tubing, respectively. The target diameters of the small, medium, and large spherical clots were 3.2, 4.8, and 6.4 mm, respectively (Fig. 3b). For the spherical clots, custom, oversized molds were created to account for the anticipated reduction in blood volume associated with water evaporation and clot contraction during coagulation.53 The average final diameters of the spherical blood clots were measured to be 3.55 ± 0.20 mm, 4.90 ± 0.23 mm, and 6.07 ± 0.30 mm. Cylindrical clots of diameters 3.34 ± 0.09 mm, 4.81 ± 0.06 mm, and 6.50 ± 0.06 mm were cut to lengths of 1×, 3×, and 5× the clot diameter (Fig. 3c). To ensure clot densities did not change during the experiments, the blood clots were briefly soaked in the test fluid (Fluid B, 35% glycerin/65% water by weight solution, see “Methods” section “Blood Analog Fluids”) prior to each trial. Mass, diameter, and lengths were measured, and the clot densities (spherical, ρ = 1.11 ± 0.01 g/mL; cylindrical, ρ = 1.13 ± 0.03 g/mL) were calculated. Each spherical (n = 98) and cylindrical (n = 110) blood clot was introduced to the flow loop only once in this study; no clots were reused.
Synthetic Polyacrylamide Gel Clots (Cylindrical)
Polyacrylamide (PA) gels were prepared with acrylamide solutions from Bio-Rad Laboratories (Hercules, CA). The hydrogels were created from 12 mL of 40% acrylamide solution, 1.8 mL of 2% bisacrylamide solution, 105.5 mL of distilled water, 0.60 mL of ammonium persulfate, and 0.067 mL of tetramethylethylene-diamine.51 This solution was chosen to produce PA gels mimicking the mechanics of physiological blood clots that have been observed to bend and fold during capture by an IVC filter (e.g., Robinson et al 2013).41 The mixed solution was cured for 3 h in 3.2 4.8, and 6.4 mm inner diameter polyvinylchloride tubing. The formed gels had final diameters of 3.27 ± 0.07 mm, 4.82 ± 0.07 mm, and 6.45 ± 0.11 mm. Samples were cut to lengths of 1×, 3×, and 5× the clot diameter (Fig. 3d). The gels were soaked in 0.1% bovine serum albumin overnight and dyed to enhance optical visualization in the flow loop. The synthetic PA gels were removed from the dye solution and subsequently soaked in the test fluid comprised of 35% glycerin/65% water by weight (Fluid B, see “Methods” section “Blood Analog Fluids”) for 24 h to ensure equilibrium to prevent changes in clot density during the experiments. Clot mass, diameter, and length were measured and the density (ρ = 1.12 ± 0.04 g/mL) was calculated. Each cylindrical PA synthetic clot was introduced to the flow loop only once in this study; no clots were reused.
Mechanical Characterization of Deformable Clots
In addition to rigid body translation and rotation, clots deform during their transport through the vasculature and their collision with and potential capture by an IVC filter (e.g., Jaeger et al. 1997, 1998; Robinson et al. 2013).25, 26, 41 Therefore, to facilitate future use of the clot capture data provided herein as a computational validation benchmark, mechanical characterization of the deformable bovine blood and PA gel clots was performed.
Prior to mechanical testing, both bovine blood and PA gels clots were formed with the same protocols describe previously but in 50 mL falcon tubes to increase their sizes for more consistent results. After the clots had coagulated overnight or cured, respectively, samples were cut with a scalpel into disk shapes with thickness less than half of their width to avoid buckling during compressive loading. The thickness and diameter of each individual sample was measured prior to testing using digital calipers. Cyclic compression testing was then performed using a Lloyd Tensile Testing Machine (AMETEK, Inc., Berwyn, PA) with a 5 N load cell. The samples were pre-loaded to 0.01 N and then cyclically compressed to 50% of their original thickness (true strain of approximately 41%) at a rate of 0.1 Hz for four total cycles to allow for preconditioning.23 For each clot material, a total of eight samples were tested and averaged. For blood clots, two separate sets of bovine donor blood were collected (n = 4 for each) and for PA clots, two batches of gels were produced (n = 4 for each).
Blood Analog Fluids
Because the mass density of the clots varied by clot material, it was necessary to adjust the test fluid composition for each to achieve clot-to-fluid density ratios in the targeted physiological range (1.01–1.04; e.g., Nahirnyak et al. 2006).35 The nylon spherical clots had a higher absolute density, whereas the blood and PA clots had a lower and approximately equal density. Therefore, two fluids were used. Fluid A was tuned for use with the synthetic nylon clots and was composed of 40% glycerin and 60% water, by weight, and 24.1 g/L NaCl. Fluid B was used for spherical and cylindrical bovine blood clots and the cylindrical synthetic PA clots and was composed of 35% glycerin and 65% water, by weight. The fluid and clot properties and the experimental flow parameters are summarized in Table 1.
Statistical Analysis
The clot trapping efficiency of the IVC filter and Clopper–Pearson exact binomial confidence intervals at 95% coverage were calculated for each test scenario.7 Differences in trapping efficiencies associated with clot material, clot size, and iliac vein of origin (i.e., left or right) were also assessed using a two-tailed, two-proportion z-test.3
Results
Mechanical Characterization of Deformable Clots
As observed in Fig. 4, the bovine blood clots exhibited hyperelasticity, hysteresis (an indication of underlying viscoelasticity) and cyclic softening.14, 21, 23 Example true stress versus true strain data for a single blood clot is shown in Fig. 4a. Cyclic softening is clearly observed over the initial loading cycles, but an approximately cyclic steady state was achieved by the third cycle. The averaged stress-strain response from both the first and final (converged) loading cycles are shown in Figs. 4b and 4c, respectively (n = 8). Peak true stresses at 50% compression (approximately 41% true strain) were measured to be 6302 ± 1203 Pa during the first loading cycle and 5395 ± 828 Pa in the final converged loading cycle. The PA gel clots also exhibited hysteresis and viscoelastic behavior during cyclic unloading (Figs. 4d, 4e), but their loading behavior was more linear compared to that of the bovine blood clots (Fig. 4d). Cyclic softening was not observed with the PA gel clots (Fig. 4d). Under monotonic compressive loading, both clot materials displayed similar stress-strain behavior up to a true strain of approximately 10% (Figs. 4b, 4e). In this linear region, the blood clots had an elastic modulus of 979 ± 157 Pa, and the PA clots had an elastic modulus of 1260 ± 159 Pa. Stress–strain data for Fig. 4b, c, and e are provided in Supplemental Material 1.
True stress versus true strain data for blood and PA clots. (a) An example cyclic loading curve for a single blood clot. (b, c) Averaged data (n = 8) of the first cycle (b) and the final, converged loading cycle (c), with error bars representing one standard deviation. (d) An example cyclic loading curve for a PA clot. (e) Averaged cyclic loading data (n = 8) with error bars representing one standard deviation. Note the difference in y-axis scales between the blood clot stress–strain data (a–c) and the PA clot stress–strain data (d and e). All strains are compressive.
Clot Trapping Efficiency: Spherical Clots
The observed IVC filter clot trapping efficiencies for spherical nylon and bovine blood clots are presented in Fig. 5 and Table S1 in Supplemental Material 2. Figure 6 and Supplemental Materials 3–6 show the transport of the nylon and blood clots through the mock IVC and their interactions with the placed IVC filter. In summary, the total clot trapping efficiency increases with nominal clot diameter (p ≈ 0.01 to p ≈ 5e−9 comparing smaller to larger clot diameters), ranging from approximately 42% on average for the small 3.2 mm diameter spheres to 100% for the large 6.4 mm diameter spheres. Clot trapping efficiencies for the synthetic nylon clots were relatively close to those observed for the deformable bovine blood clots (Fig. 5 and Table S1 in Supplemental Material 1), with the exception of the medium 4.8 mm diameter nylon clots that were captured at an efficiency 20% higher than the similarly sized deformable bovine blood clots (trapping efficiencies of 100% and 80%, respectively, p ≈ 0.01; Fig. 5). Considering the iliac vein of origin, whenever clots were not uniformly captured at 100% efficiency, clots were trapped at greater efficiency if originating from the left iliac vein compared to the right (p ≈ 5e−10).
Image series of (a) a spherical 4.8 mm diameter nylon clot captured in the IVC filter (Supplemental Material 3), (b) a spherical 3.2 mm diameter nylon clot that escaped the IVC filter (Supplemental Material 4), (c) a spherical 6.1 mm diameter blood clot captured in the IVC filter (Supplemental Material 5), and (d) a spherical 3.5 mm diameter blood clot that escaped the IVC filter (Supplemental Material 6). Arrows annotate the position of the clot at the time of capture or escape.
Clot Trapping Efficiency: Cylindrical Clots
The observed IVC filter clot trapping efficiencies for cylindrical blood and PA gel clots are presented in Fig. 7 and Table S2 in Supplemental Material 2. Figure 8 and Supplemental Material 7–10 show the transport of the cylindrical blood and PA gel clots through the mock IVC and their interactions with the placed IVC filter. In summary, the clot trapping efficiencies increased with both clot length (p ≈ 5e−5 for 1× vs. 5× length 3.2 mm diameter clots) and clot diameter (p ≈ 5e−5 for 1× length 3.2 mm diameter clots versus any length of 4.8 or 6.4 mm diameter clots). Moreover, all medium, 4.8 mm diameter and large, 6.4 mm diameter clots were captured at 100% efficiency regardless of clot length. Clot trapping efficiencies were very similar between the PA gel clots and the bovine blood clots (p > 0.5 comparing PA gel clots versus bovine blood clots; Fig. 7 and Table S2 in Supplemental Material 2).
Image series of (a) a cylindrical 4.8 mm diameter × 24 mm length blood clot captured in the IVC filter (Supplemental Material 7) (b) a cylindrical 3.2 mm diameter × 9.6 mm length blood clot that escaped the IVC filter (Supplemental Material 8), (c) a cylindrical 3.2 mm diameter × 16 mm length polyacrylamide clot captured in the IVC filter (Supplemental Material 9), and (d) a cylindrical 3.2 mm diameter × 3.2 mm length polyacrylamide clot that escaped the IVC filter (Supplemental Material 10). Arrows annotate the position of the clot at the time of capture or escape.
Note that trials of the 3.2 mm cylindrical blood clots of length 1× entering the IVC from the left iliac vein could not be performed in this study. These clots became stalled within the low-velocity region along the wall in the 1.22 m entrance length tubing upstream of the IVC filter and their trapping efficiency could therefore not be determined (Table S2 in Supplemental Material 2, marked “N/A”).
Discussion
The purpose of this study was to characterize the clot trapping efficiency of the GENI IVC filter and generate a benchmark FSI dataset for validation of IVC filter clot trapping simulations. Although a handful of FSI benchmarks are available in the literature, many are numerical only52 and thus lack accompanying experimental data to facilitate rigorous validation. Moreover, the available FSI benchmark studies that provide quantitative empirical data are generally of limited relevance and applicability to IVC filter clot trapping given their focus on flow-induced oscillation of fixed flexible structures.10, 12, 17, 20 Indeed, to our knowledge there are no FSI experimental validation benchmarks that consider the transport of deformable bodies in internal flow. Accordingly, this is the first in vitro clot trapping study to provide well-characterized experimental parameters and inflow boundary conditions necessary for comparison between experimental observations and computational predictions of IVC filter clot trapping efficiency. This study is also the first experimental work to date to investigate the trapping efficiency of an IVC filter in a representative rigid anatomical model of the human IVC.
In brief, the results of this study demonstrate the trapping efficiency of the GENI IVC filter increases with increasing clot diameter and length, in agreement with findings from previous in vitro literature.11, 16, 18, 19, 28, 31,32,33, 37, 43, 48 Clot deformability and iliac vein of origin are also shown to influence clot trapping outcomes. In particular, we found a decreased IVC filter trapping efficiency of small spherical clots (≈ 3.2 mm) originating from the right iliac vein as compared to the left iliac vein (Fig. 5). Relatedly, the smallest cylindrical bovine clots (≈ 3.2 mm diameter and length), when introduced in the left iliac vein, had a tendency to fall to the bottom of the iliac tubing, where they would slowly roll downstream before eventually stalling without reaching the infrarenal IVC or IVC filter (Table S2 in Supplemental Material 2). Similar clot transport behavior in the left iliac vein was observed in previous computational clot transport simulations of small spherical clots in the same anatomical IVC model (Supplemental Video S1 in Aycock et al. 2017b).3 Note that, when the IVC model studied herein is in the upright position, the left iliac vein is oriented more horizontally than the right so that gravitational forces are directed closer to perpendicular to the vein wall (Fig. 1a). This difference between the orientation of the left and right iliac vein axes, and its observed influence on clot transport behavior and capture rates, motivates further use of anatomically relevant models to characterize the trapping efficiency of IVC filters. However, given the known variability in IVC morphology (e.g., Kaufman et al. 1995),29 there is unlikely to be a single geometry that is generally representative of the adult population. Quantitative characterization of IVC morphology is therefore needed to facilitate future investigations of IVC filter clot trapping efficiency that account for IVC anatomical variability.
Additionally, to the authors’ knowledge, there have been no prior in vitro experimental studies to assess IVC filter embolus trapping efficiency under exercise flow conditions (> 4 L/min). Because the relative magnitude of secondary flow increases with flow rate and Reynolds number in the presence of iliac bifurcation and infrarenal vessel curvature, and because increased secondary flow in an upright IVC has been shown to increase the propensity for clot transport toward the vein wall, where IVC filter strut gaps are larger and capture is less likely,3 we anticipate exercise conditions to represent a worst-case for IVC filter clot trapping performance in the vertical orientation. Interestingly, however, Hammer et al. (1994) previously investigated in vitro IVC filter clot trapping performance at two flow rates using an idealized straight, circular IVC in a horizontal orientation and found increasing the IVC flow rate from 1 to 2 L/min increased IVC filter trapping efficiency, although only for small emboli.19 Indeed, in a horizontal orientation, cross-stream flow may be desirable to overcome gravitational forces and move clots away from the bottom vein wall toward the IVC filter trapping site. Thus, the combined influence of IVC orientation and flow rate on clot transport and trapping should be considered in future work to ensure the chosen hemodynamic conditions are appropriate for worst-case characterization of IVC filter clot trapping performance.
This study also found clot trapping efficiency increases with increasing clot stiffness for the medium-sized 4.8 mm diameter spherical clots. That is, the nylon clots of this size and shape are captured more frequently than the corresponding bovine blood clots (Fig. 5). Robinson et al. 2013 previously studied and compared the clot trapping efficiency of cylindrical clots of varying stiffness and instead found filter clot trapping efficiency increases with decreasing clot rigidity.41 The differing trends in the influence of material stiffness on clot trapping efficiency observed between the present study and that of Robinson et al. are likely due to differences in the transport and capture mechanics of spherical and cylindrical clots. Specifically, deformable spherical clots are anticipated to change shape upon collision with IVC filter, elongating in the flow direction such that they can pass more easily through the gaps between the filter struts. Conversely, as observed and noted by Robinson et al., deformable cylindrical clots have a tendency to wrap around filter struts, thus increasing their propensity for capture. Collectively, these findings emphasize the importance of ensuring clot surrogates used for in vitro testing of IVC filters mimic both the geometry and mechanical behavior of physiological human blood clots.
Limitations
The experimental data presented herein include rigorous characterization of inflow boundary conditions, fluid and clot mechanical properties, and the flow domain. However, the initial conditions for each clot trial are not fully characterized. Simulation validation activities will thus require stochastic simulation and assumptions regarding initial clot positions and velocities. Moreover, this dataset does not include quantitative characterization of time-resolved fluid or solid mechanics and is thus only appropriate for validation of model predictions of clot trapping statistics. Though considerably more difficult and resource intensive, characterization of the time resolved, coupled fluid-structure interaction of clot transport and capture could be characterized in future work using more advanced methods.
Although the aim of this study was to generate a well-characterized in vitro dataset for FSI validation and not to closely emulate physiological conditions, there are a number of limitations related to the relevance of this in vitro study to in vivo IVC filter performance that should be mentioned. First, the IVC model used in this study is rigid and thus does not mimic the true IVC compliance or the respiratory-induced changes that occur in vivo during diaphragmatic motion.50 Future work could be performed using a compliant IVC model to more closely simulate IVC hemodynamics, clot transport, and clot capture mechanics. This study is also limited by examining a single flow condition. However, the high flow rate mimicking exercise conditions, in combination with a wide IVC diameter, were chosen intentionally as previously justified to evaluate the filter performance under worst-case clot trapping conditions. Additionally, although venous flow has been previously approximated as steady in most in vitro and computational studies, recent time-resolved phase-contrast MRI data demonstrate the presence of both cardiac and respiratory pulsatility in the infrarenal IVC.27 Such transient flow behavior will influence IVC hemodynamics and secondary flow patterns and may, therefore, affect IVC filter trapping efficiency. The influence of patient-specific IVC morphology on IVC filter clot trapping has also been predicted in a previous computational study3 and, based on this work, in vitro clot trapping characterization using the present (somewhat idealized) IVC model may not be generally representative of the in vivo performance in more complex patient-specific models. We emphasize, however, that the value of the present data for validation of computational clot trapping predictions is relatively unaffected by these limitations. Indeed, a validated computational model could be used to investigate the aforementioned aspects of in vivo IVC filter clot trapping that are extremely difficult to replicate in vitro.
Conclusions
In this study, we characterize the in vitro clot trapping efficiency of a new generic conical-type IVC filter by performing experiments in accord with the current international consensus standard for preclinical bench testing of IVC filters, ISO 25539-3:2011.24 The trapping efficiency is evaluated for a range of clinically relevant sizes and shapes of both synthetic and whole blood clots in a representative rigid anatomical IVC model in an upright orientation under worst-case physiological flow conditions. Our results elucidate the influence of clot size, shape, and material properties on the in vitro trapping efficiency of the GENI filter that is generally representative of other conical-type IVC filters that are in clinical use. The resultant data set represents an experimental benchmark for validating computer simulations of in vitro IVC filter clot trapping, and more generally, low-Reynolds number FSI modeling.
References
ASME V&V 40-2018. Assessing Credibility of Computational Modeling Through Verification and Validation: Application to Medical Devices. New York: American Society of Mechanical Engineers, 2018.
Aycock, K. I., R. L. Campbell, F. C. Lynch, K. B. Manning, and B. A. Craven. The importance of hemorheology and patient anatomy on the hemodynamics in the inferior vena cava. Ann. Biomed. Eng. 44(12):3568–3582, 2016.
Aycock, K. I., R. L. Campbell, F. C. Lynch, K. B. Manning, and B. A. Craven. Computational predictions of the embolus-trapping performance of an IVC filter in patient-specific and idealized IVC geometries. Biomech. Model. Mechanobiol. 16:1957–1969, 2017.
Aycock, K. I., R. L. Campbell, K. B. Manning, and B. A. Craven. A resolved two-way coupled CFD/6-DOF approach for predicting embolus transport and the embolus-trapping efficiency of IVC filters. Biomech. Model. Mechanobiol. 16:851–869, 2017.
Aycock, K. I., R. L. Campbell, K. B. Manning, S. P. Sastry, S. M. Shontz, F. C. Lynch, and B. A. Craven. A computational method for predicting inferior vena cava filter performance on a patient-specific basis. J. Biomech. Eng. 136(8):081003, 2014.
Aycock, K. I., P. Hariharan, and B. A. Craven. Particle image velocimetry measurements in an anatomical vascular model fabricated using inkjet 3D printing. Exp. Fluids 58(11):154, 2017.
Brown, L. D., T. T. Cat, and A. DasGupta. Interval estimation for a proportion. Stat. Sci. 16:101–133, 2001.
Cheng, C. P., R. J. Herfkens, and C. A. Taylor. Inferior vena caval hemodynamics quantified in vivo at rest and during cycling exercise using magnetic resonance imaging. Am. J. Physol. Heart Circ. 284:1161–1167, 2003.
Craven, B. A., K. I. Aycock, and K. B. Manning. Steady flow in a patient-averaged inferior vena cava: Part II—computational fluid dynamics verification and validation. Cardiovasc. Eng. Techn. 9(4):654–673, 2018.
De Nayer, G., A. Kalmbach, M. Breuer, S. Sicklinger, and R. Wüchner. Flow past a cylinder with a flexible splitter plate: A complementary experimental–numerical investigation and a new FSI test case (FSI-PfS-1a). Comput. Fluids. 99:18–43, 2014.
Dria, S. J., and M. D. Eggers. In vitro evaluation of clot capture efficiency of an absorbable vena cava filter. J. Vasc. Surg-Venous L. 4(4):472–478, 2016.
Elbing, B. R., S. D. Young, M. L. Jonson, R. L. Campbell, B. A. Craven, R. F. Kunz, and K. L. Koudela. Experimental characterization of high-amplitude fluid–structure interaction of a flexible hydrofoil at high Reynolds number. J. Vib. Acoust 142(4):041014, 2020.
FDA. (1999) Guidance for industry and FDA staff: Guidance for cardiovascular intravascular filter 510(k) submissions. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm073777.pdf.
Fung, Y. C., and S. C. Cowin. Biomechanics: Mechanical Properties of Living Tissues (2nd ed.). New York: Springer, 1993.
Gallagher, M. B., K. I. Aycock, B. A. Craven, and K. B. Manning. Steady flow in a patient-averaged inferior vena cava—Part I: Particle image velocimetry measurements at rest and exercise conditions. Cardiovasc. Eng. Techn. 9:641–653, 2018.
Gao, X., J. Zhang, B. Chen, H. Yu, J. Li, S. Zhang, Z. Feng, L. Ye, and J. Han. A new self-convertible inferior vena cava filter: Experimental in-vitro and in-vivo evaluation. J. Vasc. Interv. Radiol. 22(6):829–834, 2010.
Gomes, J. P., S. Yigit, H. Lienhart, and M. Schäfer. Experimental and numerical study on a laminar fluid–structure interaction reference test case. J. Fluid. Struct. 27(1):43–61, 2011.
Günther, R. W., J. Neuerburg, A. Mossdorf, J. Pfeffer, R. Høj, A. Mølgaard-Nielsen, A. Bücker, and T. Schmitz-Rode. New optional IVC filter for percutaneous retrieval - In vitro evaluation of embolus capturing efficiency. Rofo. 177(5):632–636, 2005.
Hammer, F. D., H. P. Rousseau, F. G. Joffre, B. P. Sentenac, T. Tran-Van, and R. P. Barthelemy. In vitro evaluation of vena cava filters. J. Vasc. Interv. Radiol. 5(6):869–876, 1994.
Hessenthaler, A., N. R. Gaddum, O. Holub, R. Sinkus, O. Röhrle, and D. Nordsletten. Experiment for validation of fluid–structure interaction models and algorithms. Int. J. Numer. Method. Biomed. Eng. 33(9):e2848, 2017.
Holzapfel, G. A., T. C. Gasser, and M. Stadler. A structural model for the viscoelastic behavior of arterial walls: Continuum formulation and finite element analysis. Eur. J. Mech. A 21(3):441–463, 2002.
Hosaka, J., S. Roy, K. Kvernebo, I. Enge, and F. Laerum. In vitro function of an adjustable temporary venous spring filter: Comparison with the temporary RF02 filter and the permanent Greenfield vena cava filter. Acad. Radiol. 5(9):620–625, 1998.
Humphrey, J. D. Continuum biomechanics of soft biological tissues. Proc. R. Soc. Lond. Series A 459(2029):3–46, 2003.
ISO 25539-3:2011ISO 25539-3:2011. Cardiovascular implants — endovascular devices — part 3: Vena cava filters. Geneva: International Organization for Standardization, 2011.
Jaeger, H. J., S. Kolb, T. Mair, M. Geller, A. Christmann, R. K. Kinne, and K. D. Mathias. In vitro model for the evaluation of inferior vena cava filters: Effect of experimental parameters on thrombus-capturing efficacy of the Vena Tech-LGM filter. J. Vasc. Interv. Radiol. 9(2):295–304, 1998.
Jaeger, H. J., T. Mair, M. Geller, R. K. Kinne, A. Christmann, and K. D. Mathias. A physiologic in vitro model of the inferior vena cava with a computer-controlled flow system for testing of inferior vena cava filters. Invest. Radiol. 32(9):511–522, 1997.
Joseph, A. A., D. Voit, and J. Frahm. Inferior vena cava revisited – Real time flow MRI of respiratory maneuvers. NMR Biomed. 33(4):e4232, 2020.
Katsamouris, A. A., A. C. Waltman, M. A. Delichatsios, and C. A. Athanasoulis. Inferior vena cava filters: In vitro comparison of clot trapping and flow dynamics. Radiology 166(2):351–366, 1988.
Kaufman, J. A., A. C. Waltman, S. M. Rivitz, and S. C. Geller. Anatomical observations on the renal veins and inferior vena cava at magnetic resonance angiography. CardioVasc. Interv. Radiol. 18(3):153–157, 1995.
Lopez, J. M., G. Fortuny, D. Puigjaner, J. Herrero, and F. Marimon. A comparative CFD study of four inferior vena cava filters. Int. J. Numer. Method. Biomed. Eng. 34(7):e2990, 2018.
Lorch, H., A. Dallmann, M. Zwaan, and H. Weiss. Efficacy of permanent and retrievable vena cava filters: experimental studies and evaluation of a new device. Cardiovasc. Intervent. Radiol. 25(3):193–199, 2002.
Lorch, H., M. Zwaan, C. Kulke, and H. D. Weiss. In vitro studies of temporary vena cava filters. Cardiovasc. Intervent Radiol. 21(2):146–150, 1998.
Mahnken, A. H., J. Pfeffer, S. Stanzel, A. Mossdorf, R. W. Günther, and T. Schmitz-Rode. In vitro evaluation of optionally retrievable and permanent IVC filters. Invest Radiol. 42(7):529–535, 2007.
Montgomery, J. P., and J. A. Kaufman. A critical review of available retrievable inferior vena cava filters and future directions. Semin. Intervent. Rad. 33(2):79–87, 2016.
Nahirnyak, V. M., S. W. Yoon, and C. K. Holland. Acousto-mechanical and thermal properties of clotted blood. J. Acoust. Soc. Am. 119(6):3766–3772, 2006.
Neuerburg, J., A. Haupt-Pichler, F. J. Katterbach, R. Eilers, T. Siess, K. Buro, C. von Pichler, K. Mottaghy, and R. W. Günther. Determining the effectiveness of percutaneous cava filters: Experimental studies. Rofo. 164(4):331–337, 1996.
Nicolas, M., M. Malve, E. Peña, M. A. Martinez, and R. Leask. In vitro comparison of Günther Tulip and Celect filters. Testing filtering efficiency and pressure drop. J. Biomech. 48(3):504–511, 2015.
Nicolas, M., V. Palero, E. Pena, M. Arroyo, M. Martinez, and M. Malve. Numerical and experimental study of the fluid flow through a medical device. Int. Commun. Heat Mass 61(2):170–178, 2015.
Rahbar, E., D. Mori, and J. Moore, Jr. Three-dimensional analysis of flow disturbances caused by clots in inferior vena cava filters. J. Vasc. Interv. Radiol. 22(6):835–842, 2011.
Ren, Z., S. L. Wang, and M. A. Singer. Modeling hemodynamics in an unoccluded and partially occluded inferior vena cava under rest and exercise conditions. Med. Biol. Eng. Comput. 50:277–287, 2012.
Robinson, R. A., L. H. Herbertson, S. S. Das, R. A. Malinauskas, W. F. Pritchard, and L. W. Grossman. Limitations of using synthetic blood clots for measuring in vitro clot capture efficiency of inferior vena cava filters. Med. Devices 10(6):49–57, 2013.
Rogers, F. B., G. Strindberg, S. R. Shackford, T. M. Osler, C. S. Morris, M. A. Ricci, K. E. Najarian, R. D’Agostino, and D. B. Pilcher. Five-year follow-up of prophylactic vena cava filters in high-risk trauma patients. Arch. Surg. 133(4):406–412, 1998.
Simon, M., D. J. Rabkin, S. Kleshinski, D. Kim, and B. J. Ransil. Comparative evaluation of inferior vena cava filters with an in vitro physiologic simulation of the vena cava. Radiology 189(3):769–774, 1993.
Singer, M. A., W. D. Henshaw, and S. L. Wang. Computational modeling of blood flow in the TrapEase inferior vena cava filter. J. Vasc. Interv. Radiol. 20(6):799–805, 2009.
Singer, M. A., and S. L. Wang. Modeling blood flow in a tilted inferior vena cava filter: Does tilt adversely affect hemodynamics? J. Vasc. Interv. Radiol. 22(2):229–235, 2011.
Singer, M. A., S. L. Wang, and D. P. Diachin. Design optimization of vena cava filters: An application to dual filtration devices. J. Biomech. Eng. 132(10):101006, 2010.
Stewart, S. F. C., R. A. Robinson, R. A. Nelson, and R. A. Malinauskas. Effects of thrombosed vena cava filters on blood flow: Flow visualization and numerical modeling. Ann. Biomed. Eng. 36(11):1764–1781, 2008.
Stoneham, G. W., B. E. Burbridge, and S. F. Millward. Temporary inferior vena cava filters: In vitro comparison with permanent IVC filters. J. Vasc. Interv. Radiol. 6(5):731–736, 1995.
Swaminathan, T. N., H. H. Hu, and A. A. Patel. Numerical analysis of the hemodynamics and embolus capture of a Greenfield vena cava filter. J. Biomech. Eng. 128(3):360–370, 2006.
Tedaldi, E., C. Montanari, K. I. Aycock, F. Sturla, A. Redaelli, and K. B. Manning. An experimental and computational study of the inferior vena cava hemodynamics under respiratory-induced collapse of the infrarenal IVC. Med. Eng. Phys. 54(4):44–55, 2018.
Tse, J. R., and A. J. Engler. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 47(1):1–16, 2010.
Turek, S., and J. Hron. Proposal for numerical benchmarking of fluid–structure interaction between an elastic object and laminar incompressible flow. In: Fluid–Structure Interaction, edited by H. J. Bungartz, and M. Schäfer. Berlin: Springer, 2006, pp. 371–385.
Tutwiler, V., A. D. Peshkova, I. A. Adrianova, D. R. Khasanova, J. W. Weisel, and R. I. Litvinov. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscl. Thromb. Vasc. Biol. 37(2):271–279, 2017.
Wang, S. L., and M. A. Singer. Toward an optimal position for inferior vena cava filters: Computational modeling of the impact of renal vein inflow with Celect and TrapEase filters. J. Vasc. Interv. Radiol. 21(3):367–374, 2010.
Yang, C., F. Ma, C. Gao, Y. Kang, G. Zhang, P. Liu, H. Jiang, and Z. Chang. Design and evaluation of a novel biodegradable inferior vena cava filter. J. Biomater. Appl. 33(8):1060–1069, 2019.
Acknowledgments
The authors thank Dr. Luke Herbertson for providing the protocol to create the polyacrylamide clots, Sailahari Ponnaluri, who helped with the creation of the polyacrylamide clots, and Dr. Frank Lynch for technical consultation on best practices for IVC filter deployment. The authors also thank Confluent Medical Technologies for manufacturing the generic nitinol IVC filter. This study was funded by the U.S. FDA Center for Devices and Radiological Health (CDRH) Critical Path program. The research was supported in part by an appointment to the Research Participation Program at the U.S. FDA administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and FDA. The findings and conclusions in this article have not been formally disseminated by the FDA and should not be construed to represent any agency determination or policy. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Author Contributions
JMR, NSP, HMS, and BCG performed the experimental work with KIA, BAC, and KBM providing input on experimental design. JMR, BCG, KIA, BAC, and KBM performed data analysis and writing of the manuscript, editing, and approving the manuscript submission.
Data Availability
The data that support the findings of this study are available from the corresponding author, KBM, upon reasonable request.
Funding
This study was funded by the U.S. FDA Center for Devices and Radiological Health (CDRH) Critical Path program.
Conflict of interest
J.M. Riley, N.S. Price, H.M. Saaid, B.C. Good, K.I. Aycock, and B.A. Craven declare that they have no conflict of interest. K.B. Manning serves as a consultant for Cordis Inc.
Research Involving Human and Animal Rights
No human studies were carried out by the authors for this article. Approval from the Penn State IACUC was received to conduct the bovine blood studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Patrick Segers oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 3 (MOV 740 kb)
Supplementary material 4 (MOV 610 kb)
Supplementary material 5 (MOV 856 kb)
Supplementary material 6 (MOV 682 kb)
Supplementary material 7 (MOV 1210 kb)
Supplementary material 8 (MOV 896 kb)
Supplementary material 9 (MOV 957 kb)
Supplementary material 10 (MOV 946 kb)
Rights and permissions
About this article
Cite this article
Riley, J.M., Price, N.S., Saaid, H.M. et al. In Vitro Clot Trapping Efficiency of the FDA Generic Inferior Vena Cava Filter in an Anatomical Model: An Experimental Fluid–Structure Interaction Benchmark. Cardiovasc Eng Tech 12, 339–352 (2021). https://doi.org/10.1007/s13239-021-00524-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-021-00524-z