Abstract
The exponentially increasing population poses a serious threat to global food security. Concurrently, the climate change condition also limits crop productivity by enhancing the effect of biotic and abiotic stressors. The traditional crop improvement programs are not enough to meet the food and nutritional requirements of such a progressive population. Recently, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) based genome editing tool adopted from bacterial adaptive immune system against invading foreign DNA has demonstrated its tremendous potential in the sector of crop improvement. CRISPR/Cas-mediated targeting can activate, repress, or completely abolish the gene function. CRISPR/Cas-mediated interventions can produce biofortified crops by targeting negative regulators or activating positive regulators for nutrients. Thus, it can address nutritional security concerns. The advancement in CRISPR/Cas-mediated genome editing, encompassing base and prime editing has paved the way to modify an organism’s genome in a predictable and precise manner. The use of morphogenetic regulators can omit the problem of tissue culture stages, which is one of the major bottlenecks in plant genome editing. CRISPR/Cas-based genome editing has been performed in many crop plants to induce biotic and abiotic stress tolerance, increase quality and nutritional values, enhance productivity, and prevent post-harvest losses. In this review article, we summarize the progress, challenges opportunities and regulatory landscape of genome editing for the improvement of various traits in crop plants.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that by the year 2050, environment, agriculture, and food systems will face major challenges worldwide [118]. As the global population will reach around 9.7 billion, there will be a huge rise in agricultural products demand compared to 2012. Despite substantial investments in agricultural and technological progress, crop production has not made significant progress over the past thirty years mainly due to declining agricultural lands and an increasing population burden. The annual growth in yields of staple crops worldwide has averaged a little over 1% since the 1990s, a notably slower pace compared to the 1960s [43]. The necessity for an accelerated increase in crop productivity and quality becomes imperative due to factors such as climate change, ever-growing population, depletion of resources, loss of biodiversity and the emergence of plant pests and diseases [43].
Throughout the extensive history of crop domestication, various methods such as conventional plant breeding, mutation breeding, insertion of transgenes, and genome editing have been utilized for the introduction of various traits. Traditional hybridization and mutation-based breeding although considered effective, often require prolonged periods, significant labor input, and also carry unwanted traits due to less precision. The development of transgenic gained rapid momentum in the past century, emerging as a promising technology for incorporating multiple desirable traits into a variety. However, certain limitations were imposed initially because of the disputes that arose from the safety and ethical considerations of transgenic plants [33]. The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) system (CRISPR/Cas) has become widely acclaimed and is an extensively utilized genome editing tool due to its affordability, adaptability, and precise genetic manipulation [121]. Its successful application has been implemented in numerous economically significant crops. Recent developments in CRISPR/Cas-based editing have directed new avenues for improving relatively complicated traits in various crop plants.
Genome editing assisted by sequence-specific nucleases (SSNs)
The emergence of sequence-specific nucleases (SSNs) facilitates the precise manipulation of a particular gene sequence using genome editing technologies (Fig. 1). The SSNs demonstrated to achieve efficient gene editing involve meganuclease, zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and CRISPR/Cas. Genome editing through the CRISPR/Cas system holds the substantial potential to enhance crop genetics and advance molecular breeding, primarily because of its cost-effectiveness, accuracy, and less time-consuming process.
Overview of different genome editing techniques. a Meganucleases or homing endonucleases. b Zinc finger nucleases comprise with zinc finger DNA binding domain and FokI endonuclease. c TALE effector nucleases involving TAL effectors fused to FokI endonuclease. d CRISPR/Cas, adopted from bacterial adaptive immune system. e Base editing is a modification of CRISPR/Cas by fusing deaminase to catalytically altered Cas protein. f Prime editing- a search and replace tool for genome editing
Meganucleases, commonly denoted as endonucleases, interrogate a large site spanning approximately 12–40 base pairs (bp). This unique characteristic renders meganucleases highly effective carriers for all types of vectors, including those associated with plant RNA viruses. Conversely, meganuclease poses challenges when it comes to reengineering in comparison with alternative genome-targeting strategies, as their DNA-binding and catalytic domains are often intricately linked and not easily separable [121]. ZFN, an early genome editing technology developed in the 1990s, enables targeted alterations at specific genomic locus. It encompasses a zinc finger protein that identifies and binds to particular DNA sequences, along with the non-specific DNA-cutting nuclease, FokI. ZFN operates as a dimer, requiring a DNA-binding domain to facilitate attachment to the target, followed by FokI-mediated endonuclease activity for double-strand DNA breakage, initiating the endogenous DNA repair response [71]. Despite its high target binding efficiency, ZFN's limitations include a constrained zinc finger protein repertoire, a costly design and a restricted pool of recognizable genomic sequences. These factors collectively curtail the efficiency of genome editing, leading to a decline in its use. In 2009, the TALEN emerged as an advancement from ZFN, boasting enhanced design flexibility. TALEN comprises two components, a cleavage domain featuring the restriction endonuclease FokI and a DNA binding domain to recognize specific DNA sequences. The binding domain incorporates repetitive conserved sequences derived from the TALE protein present in the Xanthomonas spp. This domain contains 34 highly conserved amino acids, having 12th and 13th variable residues imparting specific recognizing capability. After recruitment on target loci, TALEN forms a dimer to induce double-stranded breaks (DSBs) in the sequence spacer of TALEN. This prompts the DNA repair mechanism to perform gene editing [197]. Unlike ZFN, TALEN's doublets of variable amino acid residues can recognize diverse nucleic bases, simplifying design and facilitating screening. However, implementing the TALEN in plant genome editing is considered technically difficult, resulting in lower editing efficiency and higher costs compared to ZFN.
The CRISPR/Cas system employs a Cas9 endonuclease in conjunction to a complex of RNA consisting of CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) to facilitate precise DNA cleavage at target sites. The Cas9 protein initiates the double-stranded DNA cleavage before a protospacer-adjacent motif (PAM). For instance, NGG is the PAM sequence for Streptococcus pyogenes (Sp) Cas9 at locations that align with the crRNA sequence. The DSBs amended by DNA repair processes of cells lead to editing at target loci (Fig. 2).
Major DNA damage repair pathways in plants. I Non-homologous end joining (NHEJ) is an error-prone DNA damage repair that introduces insertion or deletion (InDels) randomly, hence employed in gene knockouts. II Homology-dependent repair (HDR) pathway which relies on the presence of repair templates for gene knock-in/replacement
CRISPR/Cas: a bacterial adaptation against invading foreign DNA
CRISPR is an adaptive defense mechanism found in the majority of described archaea and bacteria. CRISPR/Cas systems have been classified into two main groups based on their components and mechanisms. In class 1 (types I, III, and IV), the process of RNA-guided target cleavage requires a complex of multiple effector proteins. While class 2 (types II, V, and VI) operate with just one RNA-guided endonuclease. For instance, Cas9 belongs to type II and Prevotella and Francisella 1 (Cpf1) in type V are responsible for carrying out the target cleavage process [113].
CRISPR/Cas-mediated immunity involves three phases [144]. The acquisition of foreign DNA sequences known as protospacer at the CRISPR locus initiates adaptive immunity. The synthesis of Cas proteins and transcription of the CRISPR array leads to the generation of pre-crRNA which is matured in subsequent steps. Then finally Cas protein in conjunction with mature crRNA performs cleavage at the target locus [39]. The absence of PAM near the crRNA target site in the host genome CRISPR locus protects themselves from the self-cleavage in type I and type II CRISPR systems [67].
Reprogramming of the CRISPR/Cas system for targeted genome editing
Cas endonuclease can be targeted to any genomic loci just by changing the protospacer region specific to the loci of interest. The combination of Cas endonuclease and sgRNA has the remarkable ability to target virtually any genomic location, leading to the creation of DSBs [116]. These DSBs can be repaired via two main natural repair pathways, the less precise non-homologous end-joining (NHEJ) repair or the more accurate homology-directed repair (HDR) pathways [170]. NHEJ often results in gene knockouts, while HDR allows for precise insertion-based modifications of DNA sequences. In higher plants, NHEJ is more commonly observed compared to HDR [163]. The HDR pathway needs a repair template for homologous recombination to resolve the DSBs. HDR-based repairing property presents several opportunities such as introducing precise single-base changes, diversifying localized sequences, creating novel versions of proteins and expediting the evolution of specific proteins to develop cultivars with enhanced abiotic/biotic tolerance.
Crop improvement with CRISPR/Cas-based genome editing
Since CRISPR/Cas-based genome editing offers immense potential to modify genomes in a very efficient and well-predicted manner, it has been employed in many crops to improve abiotic and biotic stress, nutritional and quality-related traits. However, some of the regulatory and ethical concerns limit the worldwide use of genome editing in better exploration and public accountability (Fig. 3). The advancements in genome editing technologies are stepping forward to overcome these stumbling stones. The recent developments involving the utilization of genome editing for various trait improvements are explored in the following sections and summarized in Table 1.
Improving salinity stress tolerance
Salinity stress is a critical abiotic challenge impacting both fertile lands and crop productivity and ranked second in severity [46]. The overuse of chemicals, comprising pesticides and fertilizers is turning the cultivable lands into saline. Notably, the salt stress resilience of rice was enhanced using CRISPR/Cas9 technology to edit the B-type response regulator 22 (RR22) gene encoding a transcription factor pivotal in cytokinin signaling and metabolism. This genetic alteration resulted in improved salt tolerance observed across two successive generations, without discernible differences between the edited and unedited lines [218]. Similarly, introducing paraquat tolerance-3 mutations (PQT3) through CRISPR/Cas9 led to substantial salt tolerance in rice [6]. The potential of miR535, a miRNA gene implicated in salt stress response was explored. The CRISPR/Cas9-mediated disruption of miR535 showed rice plants ability to withstand salinity. Additionally, a 5 bp deletion within the miR535 coding region emerged as a viable target for elevating salt tolerance in rice [212].
Further progress in salt stress tolerance was achieved through CRISPR/Cas9-mediated modification of various genes. By eliminating the basic helix-loop-helix 024 (bHLH024) gene and enhancing the ion transporter expression including high-affinity potassium transporter 1 (HKT1), HKT3, high-affinity K+ transporter 7 (HAK7) and salt overly sensitive 1 (SOS1), endure salt stress capacity in rice [5]. Modifying the gene related to ABI3 and VP1 2 (RAV2) using CRISPR/Cas technology enabled rice plants to face saline conditions [99]. Enhancements in salt stress resilience were observed in tomatoes by altering the 8-cysteine motif (8CM) and proline-rich domain (PRD) of the hybrid proline-rich protein 1 (HyPRP1) coding gene [177]. Moreover, CRISPR/Cas9 targeting of genes like drought and salt tolerance (DST) in rice [153], NAC041 [190] and inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) in barley [181] holds substantial potential for enhancing salt stress tolerance.
Improving drought tolerance
Drought stress stands as one of the most significant threats to global food security, leading to substantial losses in agricultural production and productivity. Solely, drought can cause yield reduction ranging from 50 to 70% across various crop species [77]. Following the emergence of genome editing techniques, strategies are being devised to alter genes linked to drought tolerance.
Recent breakthroughs include the identification of novel abscisic acid (ABA)-induced transcription repressors (AITRs) family, playing a crucial role in the regulation of ABA signaling and contributing to drought and salinity stress resistance in Arabidopsis thaliana [34]. CRISPR/Cas9-mediated targeting of the open stomata 2 (OST2) gene has shown drought resistance in Arabidopsis [135]. Additionally, knocking out the miR169a gene has demonstrated notable improvement in drought tolerance in Arabidopsis [220]. Moreover, CRISPR/Cas9-assisted activation of the vacuolar H + -pyrophosphate (AVP1) regulating gene has been applied to enhance drought tolerance in Arabidopsis [137]. Further, activating the abscisic acid-responsive element binding gene 1 (AREB1) [149] and silencing the trehalase 1 (TRE1) gene [130] have been shown to induce drought resistance in Arabidopsis.
In rice plants, the modification of enhanced response to the ABA1 (ERA1) gene through CRISPR/Cas9 has led to increased drought stress tolerance [132]. Likewise, CRISPR/Cas9-mediated knockouts of the semi-rolled leaf (SRL) 1, SRL2 and ERA1 genes in rice have shown potential for improved drought resistance [94]. The mutation in the pyrabactin resistance-like 9 (PYL9) gene was proposed to enhance rice yield and drought tolerance [179]. By modifying the DST gene, the rice cultivar MTU1010 has been developed with broader leaves, reduced stomatal density and improved leaf water retention under drought-stress conditions [153]. In rice, genes downstream of stress-activated protein kinase 2 (SAPK2), including OsOREB1 (an ABRE binding TF), OsRab16b, OsRab21, OsbZIP23, OsLEA3, OsSLAC1 and OsSLAC7 have been modulated using CRISPR/Cas technology for enhancement of drought stress resistance [104].
In chickpea, genes like reveille 7 (RVE7) and 4-coumarate ligase (4CL), linked to drought tolerance have been edited through CRISPR/Cas9 [13]. Genome editing has been employed in maize to alter the gene to replace the gene involved in organ size 2 (GOS2) promoter with an auxin-regulated GOS8 (ARGOS8) promoter sequence, aiming to boost production under drought stress [161]. In tomato, CRISPR/Cas9 has targeted genes such as gibberellin insensitive dwarf 1 (GID1), lateral organ boundaries domain 40 (LBD40) and mitogen activating protein kinase 3 (MAPK3), leading to increased drought tolerance and altered water content in tomato [103, 183]. In maize crops, the mutation in gibberellic acid biosynthetic enzyme ZmGA20ox3 by CRISPR/cas9 results in semi-dwarf phenotype and drought tolerance [98]. Knockout of the gene non-expressor of pathogenesis-related 1 (NPR1) has not only improved drought tolerance in tomato but also down-regulated drought-related genes [89].
The advancement of drought resistance in wheat has been achieved by editing ethylene response factor 3 (ERF3) and dehydration-responsive element binding protein 2 (DREB2) genes [70]. Likewise, CRISPR/Cas9 has been employed to modify a negative regulator of drought tolerance 3′(2′),5′-bisphosphate nucleotidase (Sal1) gene in wheat, resulting in increased drought resistance in the seedling stage [1]. CRISPR/Cas-mediated targeting of the homeobox 12 (HB12) gene has been reported to enhance drought resistance in cotton [48]. Furthermore, CRISPR/Cas9-mediated modification of the repressor of GA1-3 (RGA) gene in Brassica napus has significantly enhanced rapeseed capacity to endure drought conditions [199].
In summary, the potential of genome editing, particularly through CRISPR/Cas technology, to enhance drought tolerance in various crops is becoming increasingly evident. These efforts promise to mitigate the substantial challenges posed by drought stress to global food security. However, the ethical, regulatory and ecological aspects of genetically engineered crops need to be considered appropriately alongside these developments.
Reducing temperature sensitivity
Plants exhibit a preferred temperature range and any deviation from this range whether higher or lower can significantly hinder their growth and productivity. The response to heat stress, causing a buildup of reactive oxygen species (ROS) is regulated by heat shock proteins (HSPs) and heat shock transcription factors (HSFs). Consequently, tolerance to temperature stress in plants can be enhanced by increasing their ability to counter reactive oxygen species (ROS) [11].
The utilization of CRISPR/Cas9 technology has facilitated the creation of a cultivable rice mutant with increased heat-inducible characteristics [124]. In tomatoes, modifications using CRISPR/Cas were made to orthologs of MAPK3 and agamous-like 6 (AGL6) genes to enhance heat stress sensitivity, while ADP-ribosylation factor 4 (ARF4) was used to improve sensitivity to salinity shock [22]. A positive role in heat tolerance was attributed to the brassinazole-resistant 1 (BZR1) gene which promotes ROS generation in the apoplastic space of tomatoes. BZR1 was proposed to induce the respiratory burst oxidase homolog 1 (RBOH1) gene to induce hydrogen peroxide signaling for heat stress tolerance response. The mutations in BZR1 and RBOH1 resulted in decreased apoplastic hydrogen peroxide production and reduction in temperature tolerance showing their crucial role in heat stress tolerance [208]. In tomato mutants with reduced heat stress sensitivity are achieved through CRISPR/Cas-mediated alterations in the heat-stress-sensitive albino 1 (HSA1) gene [143]. In maize, the CRISPR/Cas-mediated alteration of the thermosensitive genic male sterile gene was employed to generate plants that are sensitive to temperature-induced male sterility [91]. In lettuce, the knockout of 9-cis-epoxycarotenoid dioxygenase (NCED4), a pivotal ABA biosynthetic enzyme allowed to germinate the seeds at a relatively higher temperature. This implies that nced4 mutants of lettuce could hold significance in industries operating under elevated temperatures [19]. The stomatal density and photosynthesis capacity of rice have been altered by editing of the epidermal patterning factor (OsEPF1) by CRISPR/Cas9 in the rice variety ASD 16 and showed temperature sensitivity by modulating the transpiration process [145]. Calcium-dependent protein kinases (CPK) sense Ca2+ and are crucial for plants to exert rapid stress response against a variety of stimuli. CRISPR/Cas mediated editing of CPK28 generated thermosensitive tomato, supporting its role in stress response [53].
To enhance the resilience against cold temperatures in plants, editing of the MYB30 transcription factor was reported to enhance cold tolerance in rice [108]. To ascertain the precise roles of the TIFY1a and TIFY1b genes to resist cold stress, CRISPR/Cas9-mediated modification of these genes has been shown to improve yield as well as temperature resilience in rice [58]. Proline-rich proteins (PRPs) are known to have crucial roles in plants. For instance, they assist in coping with lower temperatures and also diminish the loss of nutrients, enhance the effectiveness of antioxidants, and contribute to the synthesis of chlorophyll. By utilizing CRISPR/Cas9 technology, the knockout of the proline-rich protein 1 (PRP1) gene compromised rice to withstand cold conditions [125]. CRISPR/Cas9 mediated targeting of three rice-specific genes viz., PIN5b (an auxin efflux carrier), grain size 3 (GS3) and MYB30 showed an increase in length of the spike, larger grain and improved tolerance to cold-induced stress, respectively [215]. CRISPR/Cas9 mediated editing of genes related to the G-complex namely, Rice Gα (RGA1), GS3, dense and erect panicle 1 (DEP1) and putative extra-large G protein 4 (PXLG4) demonstrated resistance to chilling stress in rice [36]. Due to the susceptibility to chilling stress, the fruits are prone to cold-induced damage in tomatoes. CRISPR/Cas9-based mutations in the C-repeat binding factor 1 (CBF1) gene shielded tomato from cold damage by reducing electrolyte leakage [92].
Combating heavy metals stress
Oxidative stress is induced by heavy metals by stimulating the production of superoxide radicals, hydroxyl radicals (OH) and hydrogen peroxide (H2O2). The application of CRISPR/Cas-based genetic modification to the 5-oxoprolinase 1 (OXP1) gene in Arabidopsis increased resilience to cadmium exposure [14]. The natural resistance-associated macrophage protein 1 (NRAMP1) in rice was disabled through CRISPR/Cas9 which exhibited reduced quantities of cadmium (Cd) and lead (Pb) [35]. The CRISPR/Cas9 mediated silencing of a transcription factor arsenite-responsive MYB1 (ARM1), prevented the uptake and movement of arsenic (As) in rice [184]. A novel Indica rice variety with minimal Cd accumulation in the grains has been developed by CRISPR/Cas mediated targeting of NRAMP5 [172]. The HAK1 (Cs+-permeable K+ transporter) gene governs the absorption and movement of cesium (Cs+) in rice. By utilizing the CRISPR/Cas9 methodology, the activity of the HAK1 was suppressed in rice [128].
Creating herbicide tolerance
The control of weed proliferation is essential to increase crop productivity. The most commonly employed method involves the use of herbicides. Herbicides not only eliminate unintended plants but also induce stress in the desired plants and weed species by disrupting or altering their metabolism. Additionally, they leave residues posing environmental risks. A primary objective in enhancing agricultural productivity is to create crop plants that possess increased tolerance to herbicides. Utilizing CRISPR/Cas9 technology to modify the acetolactate synthase (ALS) gene, has been demonstrated to develop herbicide-resistant rice, maize and watermelon [76, 166, 175, 193]. Glyphosate is a herbicide that hinders the activity of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) which is an enzyme involved in aromatic amino acid biosynthesis in plants. CRISPR/Cas-based targeting of the EPSPS gene has resulted in a glyphosate-tolerant phenotype in rice and flax [85, 155]. Recently, precise alterations through CRISPR/Cas9 in EPSPS, phytoene desaturase (PDS) and ALS genes in tomato plants have successfully induced herbicide resistance [206].
Inducing biotic stress resistance
The occurrence of plant diseases can lead to a significant reduction in both yield and the quality of crops, fruits and other edible plant products. Biotic stressors including viruses, fungi, bacteria and oomycetes are usually responsible for various plant diseases. Fungal diseases such as powdery mildew and late blight represent a severe threat to crops and significantly reduce crop yield. Many pathogens depend on distinct host genes termed susceptibility genes (S genes) for recognition, penetration and evasion of host defense. Mutations in S genes can lead to sustained, wide-ranging and heritable resistance. The mildew resistance locus (Mlo) gene encodes a protein that is associated with the cell membrane and possesses seven transmembrane domains. By employing the CRISPR/Cas9 technology, mutation of the Mlo has led to powdery mildew-resistant wheat and tomato [126, 158, 185]. Further, precise mutation in the enhanced disease resistance 1 (EDR1) gene developed the powdery mildew resistance in wheat [217]. The knockout of powdery mildew resistance 4 (PMR4) enhanced the powdery mildew resistance in tomato [152]. CRISPR/Cas9-based knockout of ethylene response factor 922 (ERF922) [191] and subunit of the exocyst complex 3A (SEC3A) [110] genes in rice improved resistance to rice blast, but mutation in SEC3A gene also reported elevation of salicylic acid that caused dwarfism in rice plant. Resistance against tomato late blight (caused by Phytophthora infestans) was induced by CRISPR/Cas9-based multiplex targeting of miR482b and miR482c [52]. It uncovered a novel mechanism where miRNAs can be targeted by genome editing to regulate fungal resistance. CRISPR/Cas9-induced knockout of pectate lyase substantially reduced gray mold infection in tomato fruits [162].
Viruses present another serious threat to plants and are capable of inducing diseases in several economically important crops. CRISPR/Cas9-mediated knockout of the eukaryotic translation initiation factor 4E (eIF4E) gene created resistance against ipomoviruses and potyviruses in cucumber (Cucumis sativus) [28] and Arabidopsis [141]. The CRISPR/Cas9-mediated alteration of the eIF4G locus in rice showed resistance against rice tungro spherical virus (RTSV) [111]. Further, CRISPR/Cas-based targeting of eIF4e led to homozygous mutation and exhibited resistance against viruses belonging to the potyviridae family such as cucumber vein yellowing virus (CVYV), papaya ring spot mosaic virus-W (PRSV-W) and zucchini yellow mosaic virus (ZYMV) [141]. In a study, designing and targeting of 43 different guide RNAs specific to bean yellow dwarf virus (BeYDV) and beet severe curly top virus (BSCTV) provided resistance against these viruses in Nicotiana benthamiana and Arabidopsis [16, 62]. Tomato yellow leaf curl virus (TYLCV) specific gRNAs from different regions like coat protein (CP), intergenic region (IR) and replication protein (Rep) were delivered in Cas9 expressing N. benthamiana and showed resistance to TYLCV [7]. Recently, diverse CRISPR/Cas9 tools were developed to specifically target cotton leaf curl Kokhran virus (CLCuKoV) and TYLCV [8]. The tomato mosaic virus (ToMV) resistance was induced by editing the Dicer-like 2 (DCL2) gene in tomato [186]. It is reported that simultaneous editing of DCL2a and DCL2b genes enhanced resistance against the potato virus X (PVX) and ToMV [187].
Bacterial diseases also pose a remarkable threat to crop productivity. CRISPR/Cas9-based knockout of sucrose transporter SWEET13 (S gene) enhanced bacterial blight resistance caused by Xanthomonas oryzae pv. oryzae in rice [221]. Citrus bacterial canker (CBC), caused by Xanthomonas citri subspecies citri, stands as the most prevalent bacterial threat in citrus. The alteration of lateral organ boundary 1 (LOB1) promoter in Duncan grapefruit enhanced canker disease resistance [63]. The targeted CRISPR/Cas9 alteration of the LOB1 promoter at the effector binding site (i.e., EBEPthA4 which enables binding of Xanthomonas citri subspecies citri efferctor PthA4), enhanced the resistance to canker disease in Wanjincheng orange (Citrus sinensis Osbeck) [139]. Jasmonate-ZIM domain protein 2 (JAZ2) is crucial for Pseudomonas syringae infection in bacterial leaf spot disease. CRISPR/Cas9-based editing of JAZ2 imparted bacterial leaf spot resistance in tomato [134]. The downy mildew resistance 6 (DMR6) orthologues have been precisely modified in the banana by using CRISPR/Cas9 technology. It showed control of the pathogenicity in edited lines caused by Xanthomonas wilt [178]. Similarly, DMR6 editing in tomato imparted tolerance against bacteria, oomycetes and fungi [174]. The CRISPR/Cas editing of the DMR6 in the elite cultivar ‘Italiko’ belonging to Ocimum basilicum provides wide-spectrum resistance to downy mildew [80]. CRISPR/Cas-based mutagenesis of the DspA/E-interacting proteins from Malus (DIPM) gene within apple protoplasts provided resistance against fire blight disease [115].
Crop yield and quality are negatively impacted by pests which cause harm through both physical destruction and the spread of plant illnesses. Over the past few years, the warming climate has led to greater agricultural losses due to pests. In addition, the extensive application of pesticides can harm the environment, posing a potential drawback. The inhibition of serotonin biosynthesis through the disruption of tryptamine 5-hydroxylase led to an increase in salicylic acid concentrations enhancing defense against plant hoppers and stem borers in rice [106]. The utilization of CRISPR/Cas9 to create a gmcdpk38 mutant with a Hap3 knockout in soybean resulted in significant resistance to common cutworms [82].
Improvement of crop quality-related traits
The post-harvest loss is a major concern for a consistent supply chain throughout the year. The exploration of CRISPR/Cas9 potential is a promising way to extend the shelf life of crops. CRISPR/Cas-assisted HDR-mediated precise alteration in the alcobaca (ALC) gene has demonstrated improved tomato storage properties [79]. Similarly, employing CRISPR/Cas9 to disrupt the pectate lyase gene resulted in firmer tomatoes with an extended shelf life without compromising sensory and nutritional qualities [211]. Ethylene significantly influences post-harvest preservation and shelf life in climacteric fruits. Therefore, beyond targeting cell wall-degrading genes, the other effective strategy involves reducing endogenous ethylene production to delay fruit softening. CRISPR/Cas-based mutagenesis of 1-aminocyclopropane-1-carboxylate oxidase1 (ACO1) gene in banana delayed the ripening process by 2 days following ethephon treatment [54]. Furthermore, it was also noted to increase vitamin C and sugar content without compromising fruit quality in banana [54]. In Solanum melongena (eggplant), mutating the CuA-domain of the polyphenol oxidase2 (PPO2) gene showed reduced browning and also increased shelf life of the genome-edited eggplant [73]. Mutation of the squamosa promoter binding protein-like 13 (SPL13) gene in lettuce plants has been shown to increase biomass and leaf density [18]. The red-colored ‘Ailsa Craig’ cultivar of tomato was recolored into different hues including brown, yellow, pink, pink-brown, light-yellow and yellow-green by CRISPR/Cas mediated editing of three distinct genes (PSY1, MYB12, and SGR1) [205]
The CRISPR/Cas9 system has been employed to fine-tune the size/shape of crops in alignment with consumer preferences. Various quantitative trait loci (QTLs) and genes involved in determining the shape/size of crops have been identified. The initial QTL found to control grain length, known as GS3, has been effectively deactivated in five different japonica rice cultivars using CRISPR/Cas9 [160, 214] and the outcome showed elevation in grain length. Further, targeting negative regulators of grain weight (GW2, GW5 and GW6) in rice and GW7 in wheat enhanced the grain weight compared to the wild type [189, 201]. CRISPR/Cas mediated editing of OVATE, CLAVATA (CLV), WUSCHEL (WUS) and excessive number of floral organs (ENO) has been demonstrated to improves the shape and size of tomato fruits [213, 223].
The Waxy (Wx) gene known for encoding granule binding starch synthase (GBSS) is responsible for amylose biosynthesis. CRISPR/Cas9-based mutagenesis of the Wx gene has successfully demonstrated low amylose deposition while safeguarding other favorable attributes in japonica rice accessions [200]. In a parallel effort, wx maize mutants have been generated across twelve high-quality inbred lines through the alteration of the Wx gene using CRISPR/Cas9 [44]. The diminished palatability of rice can be due to elevated grain protein content (GPC), which inversely affects eating and cooking qualities (ECQ). CRISPR/Cas-assisted editing of amino acid permease 6 (AAP6) and AAP10 within the GPC-related QTL have demonstrated the capability to swiftly reduce GPC and enhance the ECQ of rice [194]. Numerous crops possess a notable abundance of the compound 2-acetyl-1-pyrroline (2AP), imparting them aroma [183]. The betaine aldehyde dehydrogenase (BADH) plays a role in transforming γ-aminobutyraldehyde (GABald) into γ-aminobutyric acid (GABA). Therefore, BADH2 gene mutation created through CRISPR/Cas9 has been reported to divert the conversion of GABald into 2AP and subsequently non-fragrant rice variety named ASD16 was effectively transformed into a new aromatic rice variant [10].
Improving nutrition value
Carotenoids play a role in preventing eye-related diseases and lowering the chances of cancer and cardiovascular diseases. The CRISPR/Cas9-based knock-in of phytoene synthase (PSY) genes modulated the flow of carbon into the carotenoid biosynthesis pathway resulting in enhanced β-carotene levels in rice [41]. CRISPR/Cas mediated knockout of lycopene epsilon cyclase (LCYε) and carotenoid cleavage dioxygenase 4 (CCD4) genes in banana enriched the β-carotene levels in context to unedited wild type plants [12, 69]. Similarly, in tomatoes five genes related to carotenoid metabolism (stay-green 1 or SGR1, LCYe, beta-lycopene cyclase or BLC, lycopene β-cyclase 1 or LCY-B1 and LCY-B2) were targeted, resulting in five-fold enrichment of lycopene [88]. GABA serves as an inhibitory neurotransmitter, playing roles in anti-anxiety responses and blood pressure regulation. The enzyme glutamate decarboxylase (GAD) plays a pivotal role in catalyzing the conversion of glutamate to GABA. GAD features an inhibitory domain at its C-terminal, limiting GABA accumulation. To elevate GABA content, CRISPR/Cas9 assisted silencing of the glutamate decarboxylase (GAD) gene resulted in enhanced GABA accumulation in tomato and rice [4, 129]. Further, CRISPR/Cas9-mediated simultaneous targeting of GABA-Ts and SSADH resulted in an approximately 20-fold increase in GABA levels but compromised tomato fruit size and yield [83]. Monounsaturated fatty acids (MUFA) have favorable cardiovascular benefits. CRISPR/Cas-mediated mutagenesis of fatty acid desaturase 2 (FAD2) has been shown to improve fatty acid composition in soybean, rapeseed and camelina [40, 65, 133]. Recently, the gene-edited soybean variety with elevated oleic acid content has been introduced for commercial availability in the United States market [26]. Enriching crop plants with micronutrients through biofortification presents a sustainable solution for individuals who are devoid of a balanced diet. CRISPR/Cas9 mediated suppression of the vacuolar iron transporter (VIT) gene elevates the iron (Fe) content in the rice grains [31]. Furthermore, the arsenite tolerant 1 (astol1) rice mutant which possesses a gain-of-function characteristic, notably elevated the selenium (Se) content in the grains [165]. Recently, multiple homologs of glucosinolate transporter 1 (GTR1) and GTR2 were targeted in Brassica juncea (oilseed mustard) using the CRISPR/Cas system [117]. This targeted approach resulted in oilseed mustard with low-seed glucosinolate levels but high-leaf glucosinolate content while maintaining normal phenotypic attributes [154]. This study demonstrates the potential of the CRISPR/Cas system for multiplexed genome editing in oilseed mustard.
Phytic acid acts as an antinutrient by binding to minerals and proteins to form complexes. CRISPR/Cas-based editing of inositol 1, 3, 4, 5, 6- pentakisphosphate 2-kinase (ITPK) gene reduces phytic acid content in rapeseed [151]. Gluten-intolerant individuals develop coeliac disease because of gluten in wheat. CRISPR/Cas-mediated transgene-free editing of the α-gliadin gene imparts low gluten content in wheat grains [74].
Base editing and prime editing: modifying genome with CRISPR/Cas without DSB induction
Some of the traits bear a single-nucleotide polymorphism and can be improved by single-nucleotide alteration at the locus in the plant genome. Base editing is an innovative CRISPR/Cas tool for modifying target genes precisely either by gain-of-function or loss-of-function mutations. This can speed up the process of annotating gene functions, crop improvement and the domestication process of wild-type plants. The deaminase domain fusion version of catalytically altered Cas9 is known as the base editor that can change specific bases (A to G/C to T/C to G) directly within the genome. Hence, this process is devoid of DSBs in the genome. The base and prime editing tools along with application in plants have been described in the following sections.
Cytosine base editing
Initial cytidine base editor (CBE), CBE1 was developed through the fusion of rat cytidine deaminase (rAPOBEC1) with the N-terminus of an altered Cas9 (dCas9) containing mutations in the catalytic domain [74]. Uracil N-glycosylase (UNG) in base excision repair (BER) limits the CBE1 efficiency by removing U-G (a product of C-deamination) mismatch. UNG inhibitor (UGI) fusion to the C-terminal of CBE1 resulted in CBE2 with better editing outcomes [74]. Replacing dCas9 with nicking Cas9 (nCas9) having one catalytic domain generated CBE3 which further improved the editing efficiency in comparison to CBE2. An additional UGI molecule fusion to CBE3 increased the UNG inhibition potential of CBE3 and was called CBE4. The protein Mu Gam from Mu bacteriophage when fused to CBE4, reduced the indel occurrence. In addition to rAPOBEC1, other cytidine deaminases such as human activation-induced cytidine deaminase (hAID), cytidine deaminase 1 (CDA1) from Petromyzon marinus, human APOBEC3A (hA3A), evoFERNY based editor PhieCBE and phage assisted evolved TadA-8E (an evolved version of adenine deaminase) are explored to strengthen the repertoire of CBE [150].
APOBEC1-derived CBEs were utilized for editing the rice starch branching enzyme IIb (SBEIIb) gene to disrupt an intron–exon junction and subsequently developed amylose rich rice variety [87]. Likewise, the editing of the nitrate transporter (NRT1.1B) gene led to improved nitrogen utilization efficiency, while edits to the slender 1 (SLR1) gene caused significant dwarfing of rice plants [107]. Additionally, a targeted alteration in the squamosa promoter binding protein-like (SPL14) locus enhanced rice grain yield [222]. Notably, the ALS gene editing in wheat, rice, potato and tomato conferred resistance to herbicides in these crops [180, 219]. The Pi-d2 (an R-gene) was effectively edited using CBE3 containing hAID and yielded blast-resistant rice [148]. The CBE3 with engineered nCas9-NG assisted alteration in BZR1 and somatic embryogenesis receptor kinase 2 (SERK2) genes showed enhanced grain quality of rice [147]. In allotetraploid cotton, the CBE3 system was used for precise point mutations in CLA (chloroplast biosynthetic gene) and phosphatidylethanolamine-binding proteins (PEBP) genes [142]. Further, the amylopectin-rich rice has been developed by Wx gene editing through PmCDA1 fused with catalytically altered Cas9 from Streptococcus canis [101].
Adenine base editing
The adenine base editor (ABE) is nCas9 (D10A) and adenosine deaminase enzyme fusion which facilitates the conversion of adenine (A) to inosine (I) within the target DNA sequence. I:T base pair is finally converted to G: C base pairs in subsequent repair and replication events. First ABE (ABE7.10) was composed of TadA and TadA7.10 (engineered adenine deaminase) dimer fused to nCas9. A nuclear localizing signal (NLS) was added to ABE7.10 to improve its efficiency. Another NLS when added to both ends, ABEmax was generated which has been shown to efficiently edit Acetyl-CoA carboxylase (ACC) [93], MAPK6, SERK2 and WRKY45 genes in rice [203]. Targeted modification of A·T to G·C base pair in the SPL14 gene increased grain yield in rice [57]. Targeted ABE-assisted editing of SPL14 and ALS genes in rice was reported to enhance yield and herbicide tolerance, respectively [56]. Subsequent advancement included the more efficient editing of Wx and ALS genes in rice, achieving editing frequencies up to 100% through the utilization of TadA8e-based ABE8e [195]. The engineered version of TadA8e (having a single-stranded DNA-binding domain) in PhieABE showed efficient editing in the broader window in comparison to other ABEs [169]. A more potent TadA9 was engineered from TadA8e and has been demonstrated to efficiently edit challenging endogenous targets [204]. These successful advancements and applications of ABEs in plants showcase immense potential in advancing biological research and the engineering of crop plants, leading to the development of improved traits.
The C to G base editing
The C to G base editor (CGBE) introduces a novel dimension to the existing landscape of base editing. The CBE and ABE primarily perform transitions of bases rather than transversions. However, CGBE is considered a recent innovation that has overcome the limitations of CBE and ABE.
The CGBE is comprised of nCas9 (D10A), rAPOBEC1 cytidine deaminase and UNG which can efficiently introduce base transversions [78]. A CGBE composed of the codon-optimized UNG (OsCGBE03) facilitates C-G editing at five different endogenous loci in rice including ideal plant architecture 1 (IPA1), bZIP5, SLR1, ALS1 and NRT1.1 [176]. Further, engineering of the TadA-8e enzyme (N46L) diminished its adenine deaminase activity and has the potential to generate precise C to G base editing. Overall, CGBE expands the base editor repertoire and is considered a potent tool in the context of precise crop breeding and improvement.
Prime editing: search and replace
A revolutionary search and replace genome editing strategy called "prime editing" enables precise modifications in the genome without needing DSB. The fact that prime editors can incorporate point mutation, transversion, transition, deletion and insertion mutations of up to 50–80 bp without additional donor DNA templates. A modified nCas9 (D10A) C-terminal fusion with reverse transcriptase (RT) forms prime editor (PE). Moloney murine leukemia virus reverse transcriptase (M-MLV-RT) has been specifically used in the designing of PE. In prime editing, a guide RNA termed prime editing guide RNA (pegRNA) is involved which consists of a traditional single-guide RNA (sgRNA) known as reverse transcript encoding the desired edit (RTT) and a primer binding site (PBS) that initiates the reverse transcription process. The PE interacts with the target DNA and introduces a nick on the non-target strand. The 3' terminal of DNA aligns with the PBS, initiating reverse transcription and incorporating the intended edit into the genomic DNA. Subsequently the edited DNA is replicated and repaired. The first generation of PE (PE1) was the fusion of nCas9 (H840A) and M-MLV-RT. Further, the replacement of M-MLV-RT with its engineered version led to the formation of PE2. The use of addition nicking gRNAs further improved the efficiency in PE3 and PE3b. In PE4 and PE5 for suppression of DNA mismatch repair, the dominant negative mismatch repair protein was fused to PE that significantly improved its activity. Engineering of nCas9 in PEmax surprisingly improved editing outcomes. For deletion and insertion of large fragments, TwinPE and GRAND editor were composed of two specifically designed pegRNA [9, 188].
The Plant prime editors (PPE) viz., PPE2 and PPE3 (or PPE3b) have shown efficient editing at CDC48-locus in wheat and CDC46 as well as ALS in rice. This breakthrough has significant implications for developing herbicide-tolerant crops and advancing functional genomics studies [96]. The rice genes ACC2 to ACC4, PDS2 to PDS6 and Wx are also targeted by using the prime editors. ACC2 to ACC4 and PDS2 to PDS6 genes have shown promising mutations but in the Wx gene, mutation was not detected [96]. These studies have shown variation in mutation efficiency at various targeted loci, thus a need to validate pegRNA by using in-vitro/in-vivo assays. The improved editing efficiency in maize has been shown by enhancing the expression of pegRNA targeting for the ALS gene [64]. In this case, a PE system with two pegRNA was developed for editing ALS1 and ALS2 in maize and demonstrated herbicide resistance carrying the P165S or the W542L/S621I mutation in ALS1 and ALS2, respectively. This showcases the adaptability of prime editing in plants for studying gene functions and enhancing crop resilience and yield.
In addition to monocots, prime editors have also been employed in dicot plant species (tomatoes and potatoes) [51]. The potential of prime editors can also be explored to edit other genes for improving the nutritional (high amylose, β-carotene and oleic acid contents) and agronomic (yield, texture, etc.) traits simultaneously.
Challenges associated with genome editing
Genome editing holds significant promise for advancing plant trait development. However, it necessitates improvement to overcome several inherent limitations. Some of the major bottlenecks in genome editing are discussed here.
Factors hampering CRISPR/Cas-based genome editing
In the realm of plant genetic engineering, the common practice involves introducing foreign genes into plants through Agrobacterium-mediated transformation, where these genes become stably integrated into the plant genome [45]. However, the removal of transgenes following genome editing is typically limited to sexually propagated crops. The integration and sustained expression of genome editors may contribute to the occurrence of off-target mutations [122]. Additionally, the presence of foreign DNA raises concerns related to biosafety and regulatory considerations.
It's worth noting that many elite crop varieties and inbred lines, which are commonly used in commercial breeding, exhibit resistance to conventional transformation methods. This poses a challenge for techniques such as biolistic bombardment and regeneration from protoplasts, which are commonly employed in the genome editing process [146] The transformation protocols independent of different genotypes are needed to explore the potential of genome editors in plants. As a result, finding effective means of introducing genetic modifications in these elite crops remains a notable obstacle in the field of genome editing-mediated engineering of plants.
Low efficiency of HDR-based gene knock-in
CRISPR/Cas-based gene replacement practices are limited to plant systems due to naturally lower rates of homologous recombination. NHEJ resulting in gene knockouts is the predominant repair mechanism in plants over the HDR pathway, the presence of donor template in the S and G2 phase of the cell cycle is crucial for successful gene knock-in [61, 140]. Also, inefficient delivery methods for knock-in reagents limit the gene knock-in using the HDR pathway. Indeed, the optimization of donor DNA and optimal expression of gRNA and Cas9 are also essential requirements for effective gene replacement by using HDR-mediated gene knock-in [167].
Challenges associated with base and prime editors
Besides the great potential, base editors led to the generation of off-target mutations. Moreover, base editors can only induce transition mutations and cannot facilitate base transversions. Their limitation of strict editing window is also a major concern for their restricted applicability [84]. Prime editing, a noteworthy development in plant genome editing, addresses this limitation by enabling base transversions/insertions/deletions at target loci. However, prime editing exhibits relatively low efficiency, particularly in dicot species with lower editing frequencies observed for insertions compared to deletions and substitutions [95, 202].
Recent advancements and opportunities for addressing bottlenecks in plant genome editing
CRISPR/Cas-based genome editing has tremendous potential to modify plant genomes with precision. Recent advancements help in addressing longstanding bottlenecks limiting the widespread applications of the CRISPR/Cas system.
Designing and screening of gRNA
The effectiveness of editing constructs can vary significantly, specially when multiple genes are simultaneously targeted using more than one guide RNA (gRNA). Further, the editing efficiency of each gRNA while multiplexing is not uniform [60]. Various systems have been devised to assess the validity of constructs before their stable editing in plant tissues. These systems include in-vitro and in-vivo cleavage of the target DNA sequence in various plant tissues (protoplasts, cell suspension, hairy roots, leaf epidermis) with ribonucleoprotein (RNP) complex using biolistic, electroporation and polyethylene glycol (PEG) mediated delivery methods [24, 72, 127]. Testing gRNA efficiency before employing it in stable genome editing of crop plants not only improves editing efficiency but also saves time and resources.
Emerging Cas variants for advanced genome editing
Cas9 nuclease stands as the most widely used Cas effector in genome editing. However, recent studies have shown the emergence of new Cas variants such as Cas12a/Cpf1 for DNA targeting and Cas13 for RNA targeting. These Cas variants displayed advantageous features and overcame several limitations of traditional CRISPR/Cas9 system. Some of the important Cas variants belonging to different classes are described here along with their promising applications in plant genome editing.
Cas12a/Cpf1
Cas12a/Cpf1 is derived from Prevotella and Francisella bacteria. It is smaller than Cas9 and possesses nuclease (NUC) lobe with two RuvC-like domains. Upon activation through base pairing, it cleaves both target/non-target DNA strands, producing staggered ends in a T-rich PAM-dependent manner. Cas12a, the pioneering Cas12 nuclease for genome editing, processes pre-crRNA into mature crRNA independently of tracrRNA [216]. The CRISPR/Cas12a system facilitates gene insertion, deletion, tagging, and base editing in economically vital plants. Comparative studies in rice targeting the epidermal patterning factor like-9 (EPFL9) gene indicated that the CRISPR/LbCpf1 (LbCas12a) system outperforms Cas9 by increasing mutation percentages and larger deletions [207]. The single transcript unit (STU)-Cas12a system has been designed for single/multiplexed rice genome editing [173]. Additionally, the catalytically dead Cpf1 serves as a transcriptional repressor in plants and bacteria, indicating its potential to regulate plant transcriptomes [171]. Recently, Cas12a orthologs (Hs1Cas12a and Ev1Cas12a) have shown their tremendous potential in both monocot and dicot plant genome editing [90]. The efficient editing of multiple loci and the generation of heritable mutations using the CRISPR/Cas12a system hold promise for developing crops with enhanced yields, disease and pest resistance and other desirable traits.
Cas12b
Cas12b possesses a conserved RuvC-like and a putative NUC domain that are significantly different from those found in Cas12a. CRISPR/Cas12b/C2c1 utilizes a hybridized gRNA formed through the combination of crRNA and tracrRNA to guide its endonuclease activity [97]. Moreover, Cas12b is a more compact, efficient and convenient tool dependent on VTTV (where V is A/G/C) PAM, contributing to editing efficiency exceeding 50%. Notably, Cas12b stands out as the sole Cas protein that generates the longest nucleotide overhangs (6 to 8 nucleotides) in the staggered end, instead of the 1 to 3 nucleotides observed in Cas9 [182]. This characteristic proves advantageous as it decreases errors while NHEJ-mediated repairing. The versatility of Cas12b in functioning across a wide range of temperatures and pH levels facilitates more effective functional studies, especially for robust crops with heat- and salinity-tolerant traits. Cas12b proteins from various bacteria, particularly AaCas12b (Alicyclobacillus acidiphilus), demonstrated improved mutation specificity in rice [120]. AaCas12b has shown effective functionality at high temperatures, making it a promising candidate for developing heat-tolerant crops, as observed in cotton [182]. Other Cas12b types, like BhCas12b v4 (Bacillus hisashii) and BvCas12b (Bacillus sp. V3-13) in Arabidopsis, exhibited high potential for multiplex genome editing and heritable mutations [198].
Cas13
Cas13 represents a category of RNA-guided ribonucleases with a specific focus on targeting RNA. Unlike Cas9 or Cas12, which rely on a PAM for target recognition, certain Cas13 proteins exhibit a preference for a protospacer flanking site (PFS) [131]. All identified Cas13 nucleases feature two distinct higher eukaryote and prokaryote nucleotide-binding (HEPN) domains in the NUC lobe for precise RNA cleavage [131]. Cas13 holds significant potential in diverse applications within plant research, including targeted RNA knockdown, defense against RNA viruses, and modification of the epitranscriptome. LwaCas13a in rice protoplasts resulted in more than 50% knockdown for seven out of nine tested gRNAs [2]. LshCas13a has shown promise in conferring immunity against RNA viruses in both monocot and dicot plants, offering a potential avenue for developing disease-resistant crops using CRISPR technology [159]. Notably, Cas13d, a subtype targeting RNA molecules without a strict PFS preference, recognizes the uracil base within the target RNA, allowing it to target a broader range of RNA molecules. Cas13d is found to effectively function across a wide temperature range, making it suitable for highly sensitive nucleic acid detection methods like reverse transcription recombinase polymerase amplification (RT-RPA). Additionally, the inactivated form, dCas13d retains its target-RNA-binding capacity and when fused with a modified plant APEX2, it enables the detection of RNA–protein interactions [21]. These properties collectively position Cas13d as a potent tool in transcriptome engineering.
Class 1 type 1 CRISPR/Cas system
Cas3, the distinctive protein associated with the type I CRISPR system, functions as a helicase-nuclease with a histidine-aspartate (HD) nuclease domain [196]. Although Cascade-Cas3 has been extensively utilized for prokaryotic genome modification, its application in eukaryotes faced challenges due to the requirement for multiple-subunit effectors, necessitating the simultaneous/sequential expression of multiple genes. However, the advancements led repurposing of Cascade-Cas3, overcoming this limitation. In the well-studied type I-E CRISPR/Cas system, the Cascade complex includes five Cas proteins (Cas5e, Cas6e, Cas7e, Cas8e and Cas11e) and crRNA. Upon PAM interrogation by Cas8, crRNA and the target DNA form an R-loop, followed by the recruitment of the specific nuclease Cas3, resulting in cleavage and degradation of the target DNA [50]. While the type I-E system has been applied for transcriptional control in maize [209]. Recently explored type I-D CRISPR/Cas system, TiD, from Microcystis aeruginosa has been optimized for eukaryotic genomic editing. TiD exhibits a unique combination of type I and type III effector modules, featuring a hybrid helicase (Cas3’) and an HD nuclease domain (Cas3”) fused with Cas10d. This hybrid nature qualifies TiD as a robust genome editing tool for complex crop genomes, offering potential benefits in crops like cassava, wheat, Brassica and potato for improving traits such as enhanced nutritional value and disease resistance [68, 109]. The distinct features of class I CRISPR/Cas prevalent in complex prokaryotes, make them promising and advantageous for enhancing plant traits through DNA editing.
CasΦ
CasΦ system represents a highly compact tool for genome editing. It is characterized by a reduced number of spacers in its CRISPR array, lacking the CRISPR spacer acquisition machinery (Cas1, Cas2 and Cas4) [138]. The CasΦ protein, with its small size (70–80 kDa) is conveniently packaged in a viral vector, facilitating the straightforward and effective transgene expression [138]. In the realm of plant genome editing, CasΦ-2 demonstrated the ability to induce 8–10 bp deletions in the phytoene desaturase 3 (PDS3) gene in Arabidopsis, indicating robust editing capability [138]. Subsequent enhancements of the CRISPR-CasΦ-2 system in tobacco and Arabidopsis have resulted in better specificity and efficiency of genome editing [25]. This optimized system holds promise for application in economically significant crops to enhance their desirable traits.
Addressing the challenges in base and prime editing
Efficient strategies for improving base editing include mitigating gRNA-independent off-target effects by the utilization of alternative deaminases or implementing modifications to the deaminase protein [210]. Improved CBE variants, like YEE-BE3 have been proposed as potential solutions to reduce off-target edits in plants [66]. Despite advancements, base editing faces challenges such as restricted target selection due to PAM site compatibility and editing window length limitations [84]. To overcome these constraints, different Cas orthologs and modified variants with changed PAM specificities have been utilized [147, 192].
Researchers have developed strategies including engineered prime-editing proteins, manipulation of the mismatch repair pathway, improved guide RNA design, and optimization of delivery methods to enhance prime editing efficiency [95]. The careful design of the pegRNA, emphasizing the selection of a suitable combination of the PBS and RT template is crucial for achieving high efficiency [55]. Despite these efforts, challenges persist in target gene selection and navigating plant transformation steps, particularly in the context of large plant genomes with duplicated regions and genes. In addition, the optimizations of genome editing reagents, gene delivery systems and transformation protocols are essential for achieving efficient prime editing in plants.
Improving delivery method for CRISPR/Cas-based genome editing reagents
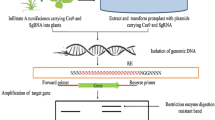
The efficient delivery of GE reagents may enhance the editing efficiency in desired plant systems. Till now, two types of delivery methods for CRISPR components have been reported which are mainly physical (microinjection, electroporation, PEG, mechanical cell deformation) and biological (Agrobacterium-mediated, viral vector-based transformation systems [164]. Agrobacterium-based binary vectors are widely used for plant cell transformation to achieve knock-out and knock-in mutations. In Arabidopsis, HDR-based CRISPR/Cas editing was achieved at repressor of silencing 1 (ROS1) and Demeter (DME1) loci via sequential Agrobacterium-mediated transformation in Cas9 overexpressing plants [119]. In viral vector-mediated transformation, geminivirus replicons (GVR) are used as gene editing vector. GVR is a single-stranded DNA-based vector having a genome size of 2.5 kb including replication proteins (Rep/RepA), histone protein, long intergenic region (LIR), short intergenic region (SIR) and the origin of replication (ori) that is triggered by a single bidirectional promoter [37]. The CRISPR/Cas gene editing by the bean yellow dwarf viral vector (BeYDV) delivery system was achieved in tobacco by targeting the ALS gene [15]. In tomato, BeYDV vector used for targeted insertion of cauliflower mosaic virus 35S strong promoter (CaMV35S) at the upstream of the anthocyanin I (ANTI) mutant gene has shown purple coloration in tomato [27]. The tobacco rattle virus (TRV) is used to deliver gRNAs into tomato cultivar Micro-Tom expressing Cas9 or co-delivery of Cas9 and sgRNAs by potato virus X (PVX) vector targeting PDS gene. The cotton leaf crumple viral (CLCrV) vector is used to edit GL2, BRI1, PDS genes and GUS transgene in Arabidopsis by designing gRNA fusion with mobile sequences like Flowering Locus T (FT) mRNA at the 5′ end [81]. Furthermore, the multiplex editing using the TRV vector in Arabidopsis has been optimized by targeting magnesium-chelatase subunit 1 and subunit 2 (AtCHLI1, AtCHLI2) genes simultaneously [123]. Another tripartite RNA virus, barley stripe mosaic virus (BSMV) has been engineered to deliver editing reagents by agroinfiltration methods in maize and wheat crop plants. In wheat crops, the editing of the histidine-rich calcium-binding (HRC) gene for Fusarium head blight (FHB) resistance, improved FHB resistance in wheat [32]. In barley crops, virus-induced genome editing (VIGE) mediated by BSMV vector in transgenic barley overexpressing Cas9 plants is also shown. The CRISPR/Cas editing at target locus albostrians gene (CMF7) by BSMV vector resulted in transgenic barley having variegated/albino chloroplast phenotypic mutation. Furthermore, MUS81 (a DNA structure selective endonuclease), ASY1 (an axis-localized HORMA domain protein) and ZYP1 (a transverse filament protein of the synaptonemal complex) are also edited by CRISPR/Cas mediated editing by BSMV vector [168].
The TRV RNA viral vector is also used for base-editing where gRNAs targeting to cloroplastos alterados 1 (CLA1) and PDS3 genes were targeted using cytidine deaminase base-editor in Arabidopsis plants [100]. These examples have shown the immense potential of viral vector-mediated efficient genome editing in plants.
Potential use of morphogenetic regulators in plant genome editing
Some developmental regulators (DRs) such as WUSCHEL (WUS) and BABYBOOM (BBM) are demonstrated to induce somatic embryos when ectopically expressed and expand the scope of genome editing in recalcitrant plant species. The co-expression of Wuschel 2 (Wus2) and isopentenyl transferase (ipt) morphogenetic genes along with genome editing components in soil-grown plants led to induced de-novo meristem formation that subsequently rose to genome-edited shoot in tobacco, grape and potato plants [112]. Hence, the escaping of the tissue culture practice in this way can simplify the genome editing process. The co-delivery of morphogenetic regulators with genome editing reagents significantly improves the regeneration of transgenic plants [47]. In the B104 public maize inbred variety, the co-expression of WUS and BBM genes by using stage-specific promoters (phospholipid transfer protein and auxin-inducible promoters) resulted in enhanced somatic embryo formation. For the controlled expression of DRs, Cre/LoxP recombination system along with the selection marker ALS gene resulted in enhanced transformation efficiency. Moreover, editing by CRISPR/Cas system was also validated with DRs system by targeted mutation in virescent yellow-like (VYL) gene [49]. The seedlings with an increased number of leaf trichomes were produced by editing of the teosinate branched 1/cycloidea/proliferationg cell factor 4b (TCP4b) gene in a WUSa overexpressing transgenic Brassica rapa plant [102].
Further, due to the pleiotropic nature of developmental regulators, their prolonged-expression may cause abnormalities in plants. However, controlling the expression of these regulators by using a chemical inducible system and stage-specific promoter offers a more sustainable and effective approach to achieving effective regeneration of genome-edited progeny [105]. In poplar genome editing, the activation of endogenous morphogenetic genes, like WOX 11 and WUS via CRISPR/Cas showed enhanced regeneration efficiency [136]. Recently, recombination-based transgene removal followed by selection marker activation has demonstrated a sustainable method for boosting regeneration in sorghum genome editing [30]. In the Sorghum crop, the combinatorial effect of growth-stimulating factor 4 (GRF4) fusion with GRF-interacting factor1 (GIF1) along with helper plasmid pVS1-VIR2 resulted in maximum transformation efficiency up to 38.28%. [86]. Furthermore, the repertoire is expanded by recently discovered morphogenetic regulators, WOX6 and GRF4-GRF-fusion [29, 38]. Moreover, GRF5 expression improves maize and sugar beet regeneration efficiency [75]. Therefore, the expression of morphogenetic genes increases the efficiency of regeneration and offers a straightforward method to increase the current capacity of genome editing in recalcitrant plant species.
CRISPR/Cas assisted chromosomal engineering
Plant breeding relies on genetic variation and the ability to manipulate genetic linkages between traits. However, these linkages pose a significant challenge to transferring desirable traits from wild species to cultivated relatives. The development of CRISPR/Cas technology has empowered breeders to introduce genetic variability in a controlled and site-specific manner, enhancing traits with high efficiency. Recent studies in Arabidopsis and maize have reported the successful induction of large-scale chromosomal rearrangements [20, 157]. The targeted DSB induction using Cas9 has induced recombination between homologous chromosomes in somatic cells of tomatoes, resulting in gene conversions and putative crossovers [17]. Moreover, the targeted inversion of up to 18 kb was successfully induced in Arabidopsis using Cas9 from Staphylococcus aureus under egg-cell-specific expression [156]. Further evolutionary-derived inversion in Arabidopsis and an elite maize inbred line were reversed using the CRISPR/Cas system. This CRISPR/Cas-mediated inversion in a crop plant, specifically in an elite maize inbred line, spanned nearly one-third of chromosome 2 [157]. Also, the large translocations commonly found in crops can reduce meiotic recombination. The first targeted induction of reciprocal translocations in plants was in Arabidopsis between chromosomes 1 and 2, and between chromosomes 1 and 5 [20]. These translocations were heritable with fragments around 1 Mb and 0.5 Mb in size. Despite these challenges, the CRISPR/Cas system holds tremendous potential in chromosomal engineering, paving the way for the production of designer crops with desired chromosomal structures shortly.
Regulatory landscape of genome editing crops: current status and future
The environmental release of genome-edited crop plants will be determined by the adoption of appropriate biosafety regulatory guidelines or policies in various countries. The regulatory framework for genome-edited crops is rapidly changing worldwide by considering science-based policies and the legality of releasing the crops onto the market. The current status of the global regulatory landscape for genome editing crops can easily be accessed from a publicly available resource “Global gene editing regulation tracker” (https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org/). In India, genome editing-derived plant products free from exogenous foreign DNA and fall under SDN-1/SDN-2 categories are exempted from strict biosafety assessment (https://pib.gov.in/PressReleasePage.aspx?PRID=1871153). However, SDN-3 involves the precise insertion of a donor DNA repair template or foreign gene into the genome of crop plants is considered under the genetically modified organisms (GMOs) category and regulations [140]. The United States of America (USA), Canada, and South American countries also classified genome-edited crops into three categories and considered SDN-1 and SDN-2 as conventional breed crops. The USA has secured no unique regulation status and biosafety assessment for these two categories. Recently, in 2023 Environmental Protection Authority (EPA) in the USA added safety requirements to the current SECURE Biotechnology regulations of the United States Department of Agriculture (USDA). In a similar direction, several other countries including Israel, Argentina, Brazil, Chile, Colombia, Paraguay, Ecuador, Japan and Australia have chosen to exempt genome-edited plants from GMO laws by the case-to-case study as long as no foreign DNA is incorporated into the plant genome [23].
Contrarily in the case of the European Union (EU), the European Court of Justice (ECJ) classified genome-edited lines as GMOs, prohibited for cultivation and consumption. However, the EU is reassessing its genome editing regulation status and has proposed new regulatory guidelines where the genome-edited plants with no foreign DNA (new genomic technology 1: NGT1) will not be regulated, while plants with foreign DNA (NGT2) will be treated as transgenic [23]. New Zealand is regulating genome editing by considering them under GM biosafety rules. Although the EPA of New Zealand took interest in the formulation of genome editing regulations there is still no clear path for the cultivation and commercial release of relevant products [23]. The United Kingdom has exempted genome-edited crops from the GMO definition, and possibly allowed field trials to the commercial release of these crop plants [42]. The Ministry of Agriculture of China published guidelines in January 2022 for the safety assessment of genome-edited plants that are free from exogenous DNA [114]. In summary, most of the countries have considered less strict regulation of genome-edited crops with the requirements of key information such as targeting trait/gene, stability of trait, possible associated risks and benefits, method of generation, and evidence of lacking vector backbone/foreign DNA. Moreover, the legislation and regulations about gene-edited crops are rapidly evolving and adapting to new technologies. These developments are crucial in facilitating the entry of gene-edited products into the market (Table 2) and raising public awareness about the benefits of this technology.
Conclusion and future perspective
The capability to edit multiple genes makes CRISPR/Cas an attractive option for enhancing various traits simultaneously. CRISPR/Cas9 mediated new breeding tool provides substantial benefits compared to traditional plant breeding methods. It allows a short duration for the introduction of desirable traits in a precise manner, while conventional approaches usually take a long time (around 6 to 7 years) and also carry undesirable traits/effects [59]. Therefore, to meet the quality food requirements of an exponentially growing population in changing climatic conditions, CRISPR/Cas9-based editing holds high promise. Plant tissue culture is one of the crucial limiting factors to genome-editing experiments. Further, the long exposure to culture under in-vitro conditions may also induce somaclonal variations, which hampers the widespread application of crop improvement practices. Recent reports showed that the implication of morphogenetic regulators allows direct regeneration of edited shoots [112]. The implication of plant morphogenetic regulators such as BBM, WUS and IPT have been used for improving plant transformation efficiency [29, 112]. Newly emerging tools such as CRISPR-Combo can activate and suppress gene expression simultaneously and show potential application in plant metabolic engineering [136]. The incidence of unintended off-targets also limits the potential of the CRISPR/Cas system. Engineering of Cas9 endonuclease as well as exploration of robust and highly specific Cas orthologs, such as FnCas9 may be the solution to this stumbling block [3]. Moreover, genome editing outcome mostly relies on NHEJ for repairing DSBs instead of HDR repair which in turn results in random insertion or deletions. Using strategies like inhibiting NHEJ and overexpressing HDR components may improve HDR-based precision genome editing [163]. The comprehensive knowledge of genomic sequences and annotation helps to predict the editing outcomes at the loci of interest. As of now, only certain crop plant genomes have been fully sequenced. The innovations in omics technologies are certainly adding information to databases that will be crucial for crop improvement programs. The evolving regulations worldwide are attracting researchers towards genome editing application for crop improvement programs.
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
Abdallah NA, Elsharawy H, Abulela HA, Thilmony R, Abdelhadi AA, Elarabi NI. Multiplex CRISPR/Cas9-mediated genome editing to address drought tolerance in wheat. GM Crops Food. 2022;6:1–17. https://doi.org/10.1080/21645698.2022.2120313.
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, Cox DBT, Kellner MJ, Regev A, Lander ES, Voytas DF, Ting AY, Zhang F. RNA targeting with CRISPR-Cas13. Nature. 2017;550(7675):280–4. https://doi.org/10.1038/nature24049.
Acharya S, Mishra A, Paul D, Ansari AH, Azhar M, Kumar M, Rauthan R, Sharma N, Aich M, Sinha D, Sharma S, Jain S, Ray A, Jain S, Ramalingam S, Maiti S, Chakraborty D. Francisella novicida Cas9 interrogates genomic DNA with very high specificity and can be used for mammalian genome editing. Proc Natl Acad Sci USA. 2019;116(42):20959–68. https://doi.org/10.1073/pnas.1818461116.
Akama K, Akter N, Endo H, Kanesaki M, Endo M, Toki S. An in vivo targeted deletion of the calmodulin-binding domain from rice glutamate decarboxylase 3 (OsGAD3) increases γ-aminobutyric acid content in grains. Rice. 2020;13(1):20. https://doi.org/10.1186/s12284-020-00380-w.
Alam MS, Kong J, Tao R, Ahmed T, Alamin M, Alotaibi SS, Abdelsalam NR, Xu JH. CRISPR/Cas9 mediated knockout of the OsbHLH024 transcription factor improves salt stress resistance in Rice (Oryza sativa L.). Plants (Basel). 2022;11(9):1184. https://doi.org/10.3390/plants11091184.
Alfatih A, Wu J, Jan SU, Zhang ZS, Xia JQ, Xiang CB. Loss of rice PARAQUAT TOLERANCE 3 confers enhanced resistance to abiotic stresses and increases grain yield in field. Plant Cell Environ. 2020;43(11):2743–54. https://doi.org/10.1111/pce.13856.
Ali Z, Abulfaraj A, Idris A, Ali S, Tashkandi M, Mahfouz MM. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 2015;16:238. https://doi.org/10.1186/s13059-015-0799-6.
Ali Z, Ali S, Tashkandi M, Zaidi SS, Mahfouz MM. CRISPR/Cas9-mediated immunity to geminiviruses: differential interference and evasion. Sci Rep. 2016;6:26912. https://doi.org/10.1038/srep26912.
Anzalone AV, Gao XD, Podracky CJ, Nelson AT, Koblan LW, Raguram A, Levy JM, Mercer JAM, Liu DR. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat Biotechnol. 2022;40(5):731–40. https://doi.org/10.1038/s41587-021-01133-w.
Ashokkumar S, Jaganathan D, Ramanathan V, Rahman H, Palaniswamy R, Kambale R, Muthurajan R. Creation of novel alleles of fragrance gene OsBADH2 in rice through CRISPR/Cas9 mediated gene editing. PLoS ONE. 2020;15(8):e0237018. https://doi.org/10.1371/journal.pone.0237018.
Awasthi R, Bhandari K, Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Front Environ Sci. 2015;3:11. https://doi.org/10.3389/fenvs.2015.00011.
Awasthi P, Khan S, Lakhani H, Chaturvedi S, Shivani KN, Singh J, Kesarwani AK, Tiwari S. Transgene-free genome editing supports CCD4 role as a negative regulator of β-carotene in banana. J Exp Bot. 2022;73:erac042. https://doi.org/10.1093/jxb/erac042.
Badhan S, Ball AS, Mantri N. First report of CRISPR/Cas9 mediated DNA-free editing of 4CL and RVE7 genes in chickpea protoplasts. Int J Mol Sci. 2021;22(1):396. https://doi.org/10.3390/ijms22010396.
Baeg GJ, Kim SH, Choi DM, Tripathi S, Han YJ, Kim JI. CRISPR/Cas9-mediated mutation of 5-oxoprolinase gene confers resistance to sulfonamide compounds in Arabidopsis. Plant Biotechnol Rep. 2021;15:753–64. https://doi.org/10.1007/s11816-021-00718-w.
Baltes NJ, Gil-Humanes J, Cermak T, Atkins PA, Voytas DF. DNA replicons for plant genome engineering. Plant Cell. 2014;26(1):151–63. https://doi.org/10.1105/tpc.113.119792.
Baltes NJ, Hummel AW, Konecna E, Cegan R, Bruns AN, Bisaro DM, Voytas DF. Conferring resistance to geminiviruses with the CRISPR/Cas prokaryotic immune system. Nat Plants. 2015;1(10):15145. https://doi.org/10.1038/nplants.2015.145.
Ben Shlush I, Samach A, Melamed-Bessudo C, Ben-Tov D, Dahan-Meir T, Filler-Hayut S, Levy AA. CRISPR/Cas9 induced somatic recombination at the CRTISO locus in tomato. Genes (Basel). 2020;12(1):59. https://doi.org/10.3390/genes12010059.
Beracochea V, Stritzler M, Radonic L, Bottero E, Jozefkowicz C, Darqui F, Ayub N, Bilbao ML, Soto G. CRISPR/Cas9-mediated knockout of SPL13 radically increases lettuce yield. Plant Cell Rep. 2023;42(3):645–7. https://doi.org/10.1007/s00299-022-02952-0.
Bertier LD, Ron M, Huo H, Bradford KJ, Britt AB, Michelmore RW. High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9-induced modifications of NCED4 in lettuce (Lactuca sativa). G3 Genes Genomes Genet. 2018;8(5):1513–21. https://doi.org/10.1534/g3.117.300396.
Beying N, Schmidt C, Pacher M, Houben A, Puchta H. CRISPR-Cas9-mediated induction of heritable chromosomal translocations in Arabidopsis. Nat Plants. 2020;6(6):638–45. https://doi.org/10.1038/s41477-020-0663-x.
Bharathkumar N, Sunil A, Meera P, Aksah S, Kannan M, Saravanan KM, Anand T. CRISPR/Cas-based modifications for therapeutic applications: a review. Mol Biotechnol. 2022;64(4):355–72. https://doi.org/10.1007/s12033-021-00422-8.
Bouzroud S, Gasparini K, Hu G, Barbosa MAM, Rosa BL, Fahr M, Bendaou N, Bouzayen M, Zsögön A, Smouni A, Zouine M. Down regulation and loss of auxin response factor 4 function using CRISPR/Cas9 alters plant growth, stomatal function and improves tomato tolerance to salinity and osmotic stress. Genes (Basel). 2020;11(3):272. https://doi.org/10.3390/genes11030272.
Buchholzer M, Frommer WB. An increasing number of countries regulate genome editing in crops. New Phytol. 2023;237(1):12–5. https://doi.org/10.1111/nph.18333.
Budhagatapalli N, Schedel S, Gurushidze M, Pencs S, Hiekel S, Rutten T, Kusch S, Morbitzer R, Lahaye T, Panstruga R, Kumlehn J, Hensel G. A simple test for the cleavage activity of customized endonucleases in plants. Plant Methods. 2016;12:18. https://doi.org/10.1186/s13007-016-0118-6.
Cai Q, Guo D, Cao Y, Li Y, Ma R, Liu W. Application of CRISPR/CasΦ2 system for genome editing in plants. Int J Mol Sci. 2022;23(10):5755. https://doi.org/10.3390/ijms23105755.
Calyxt, I.: First commercial sale of calyxt high oleic soybean oil on the U.S. Market. https://calyxt.com/first-commercial-sale-of-calyxt-high-oleic-soybean-oil-on-the-u-s-market/. (2019). Accessed 01 July 2023.
Čermák T, Baltes NJ, Čegan R, Zhang Y, Voytas DF. High-frequency, precise modification of the tomato genome. Genome Biol. 2015;16:232. https://doi.org/10.1186/s13059-015-0796-9.
Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal-On A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol. 2016;17(7):1140–53. https://doi.org/10.1111/mpp.12375.
Chaudhary R, Singh S, Kaur K, Tiwari S. Genome-wide identification and expression profiling of WUSCHEL-related homeobox (WOX) genes confer their roles in somatic embryogenesis, growth and abiotic stresses in banana. 3 Biotech. 2022;12(11):321. https://doi.org/10.1007/s13205-022-03387-w.
Che P, Wu E, Simon MK, Anand A, Lowe K, Gao H, Sigmund AL, Yang M, Albertsen MC, Gordon-Kamm W, Jones TJ. Wuschel2 enables highly efficient CRISPR/Cas-targeted genome editing during rapid de novo shoot regeneration in sorghum. Commun Biol. 2022;5(1):344. https://doi.org/10.1038/s42003-022-03308-w.
Che J, Yamaji N, Ma JF. Role of a vacuolar iron transporter OsVIT2 in the distribution of iron to rice grains. New Phytol. 2021;230(3):1049–62. https://doi.org/10.1111/nph.17219.
Chen H, Su Z, Tian B, Liu Y, Pang Y, Kavetskyi V, Trick HN, Bai G. Development and optimization of a Barley stripe mosaic virus-mediated gene editing system to improve Fusarium head blight resistance in wheat. Plant Biotechnol J. 2022;20(6):1018–20. https://doi.org/10.1111/pbi.13819.
Chen K, Wang Y, Zhang R, Zhang H, Gao C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol. 2019;70:667–97. https://doi.org/10.1146/annurev-arplant-050718-100049.
Chen S, Zhang N, Zhou G, Hussain S, Ahmed S, Tian H, Wang S. Knockout of the entire family of AITR genes in Arabidopsis leads to enhanced drought and salinity tolerance without fitness costs. BMC Plant Biol. 2021;21(1):137. https://doi.org/10.1186/s12870-021-02907-9.
Chu C, Huang R, Liu L, Tang G, Xiao J, Yoo H, Yuan M. The rice heavy-metal transporter OsNRAMP1 regulates disease resistance by modulating ROS homoeostasis. Plant Cell Environ. 2022;45(4):1109–26. https://doi.org/10.1111/pce.14263.
Cui Y, Jiang N, Xu Z, Xu Q. Heterotrimeric G protein are involved in the regulation of multiple agronomic traits and stress tolerance in rice. BMC Plant Biol. 2020;20(1):90. https://doi.org/10.1186/s12870-020-2289-6.
Dahan-Meir T, Filler-Hayut S, Melamed-Bessudo C, Bocobza S, Czosnek H, Aharoni A, Levy AA. Efficient in planta gene targeting in tomato using geminiviral replicons and the CRISPR-Cas9 system. Plant J. 2018;95(1):5–16. https://doi.org/10.1111/tpj.13932.
Debernardi JM, Tricoli DM, Ercoli MF, Hayta S, Ronald P, Palatnik JF, Dubcovsky J. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat Biotechnol. 2020;38(11):1274–9. https://doi.org/10.1038/s41587-020-0703-0.
Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–7. https://doi.org/10.1038/nature09886.
Do PT, Nguyen CX, Bui HT, Tran LTN, Stacey G, Gillman JD, Zhang ZJ, Stacey MG. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2-1A and GmFAD2-1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol. 2019;19(1):311. https://doi.org/10.1186/s12870-019-1906-8.
Dong OX, Yu S, Jain R, Zhang N, Duong PQ, Butler C, Li Y, Lipzen A, Martin JA, Barry KW, Schmutz J, Tian L, Ronald PC. Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR/Cas9. Nat Commun. 2020;11(1):1178. https://doi.org/10.1038/s41467-020-14981-y.
Entine J, Felipe MSS, Groenewald JH, Kershen DL, Lema M, McHughen A, Nepomuceno AL, Ohsawa R, Ordonio RL, Parrott WA, Quemada H, Ramage C, Slamet-Loedin I, Smyth SJ, Wray-Cahen D. Regulatory approaches for genome edited agricultural plants in select countries and jurisdictions around the world. Transgenic Res. 2021;30(4):551–84. https://doi.org/10.1007/s11248-021-00257-8.
FAO. The future of food and agriculture—trends and challenges. Rome: FAO. (2017).
Gao H, Gadlage MJ, Lafitte HR, Lenderts B, Yang M, Schroder M, Farrell J, Snopek K, Peterson D, Feigenbutz L, Jones S, St Clair G, Rahe M, Sanyour-Doyel N, Peng C, Wang L, Young JK, Beatty M, Dahlke B, Hazebroek J, Greene TW, Cigan AM, Chilcoat ND, Meeley RB. Superior field performance of waxy corn engineered using CRISPR/Cas9. Nat Biotechnol. 2020;38(5):579–81. https://doi.org/10.1038/s41587-020-0444-0.
Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67(1):16–37. https://doi.org/10.1128/MMBR.67.1.16-37.2003.
Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, Chao DY, Li J, Wang PY, Qin F, Li J, Ding Y, Shi Y, Wang Y, Yang Y, Guo Y, Zhu JK. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63(5):635–74. https://doi.org/10.1007/s11427-020-1683-x.
Gordon-Kamm B, Sardesai N, Arling M, Lowe K, Hoerster G, Betts S, Jones AT. Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants (Basel). 2019;8(2):38. https://doi.org/10.3390/plants8020038.
He X, Luo X, Wang T, Liu S, Zhang X, Zhu L. GhHB12 negatively regulates abiotic stress tolerance in Arabidopsis and cotton. Environ Exp Bot. 2020;176:104087. https://doi.org/10.1016/j.envexpbot.2020.104087.
Hernandes-Lopes J, Pinto MS, Vieira LR, Monteiro PB, Gerasimova SV, Nonato JVA, Bruno MHF, Vikhorev A, Rausch-Fernandes F, Gerhardt IR, Pauwels L, Arruda P, Dante RA, Yassitepe JECT. Enabling genome editing in tropical maize lines through an improved, morphogenic regulator-assisted transformation protocol. Front Genome Ed. 2023;5:1241035. https://doi.org/10.3389/fgeed.2023.1241035.
Hochstrasser ML, Taylor DW, Bhat P, Guegler CK, Sternberg SH, Nogales E, Doudna JA. CasA mediates Cas3-catalyzed target degradation during CRISPR RNA-guided interference. Proc Natl Acad Sci USA. 2014;111(18):6618–23. https://doi.org/10.1073/pnas.1405079111.
Hofvander P, Andreasson E, Andersson M. Potato trait development going fast-forward with genome editing. Trends Genet. 2022;38(3):218–21. https://doi.org/10.1016/j.tig.2021.10.004.
Hong Y, Meng J, He X, Zhang Y, Liu Y, Zhang C, Qi H, Luan Y. Editing miR482b and miR482c simultaneously by CRISPR/Cas9 enhanced tomato resistance to phytophthora infestans. Phytopathology. 2021;111(6):1008–16. https://doi.org/10.1094/phyto-08-20-0360-r.
Hu Z, Li J, Ding S, Cheng F, Li X, Jiang Y, Yu J, Foyer CH, Shi K. The protein kinase CPK28 phosphorylates ascorbate peroxidase and enhances thermotolerance in tomato. Plant Physiol. 2021;186(2):1302–17. https://doi.org/10.1093/plphys/kiab120.
Hu C, Sheng O, Deng G, He W, Dong T, Yang Q, Dou T, Li C, Gao H, Liu S, Yi G, Bi F. CRISPR/Cas9-mediated genome editing of MaACO1 (aminocyclopropane-1-carboxylate oxidase 1) promotes the shelf life of banana fruit. Plant Biotechnol J. 2021;19(4):654–6. https://doi.org/10.1111/pbi.13534.
Hua K, Jiang Y, Tao X, Zhu JK. Precision genome engineering in rice using prime editing system. Plant Biotechnol J. 2020;18(11):2167–9. https://doi.org/10.1111/pbi.13395.
Hua K, Tao X, Liang W, Zhang Z, Gou R, Zhu JK. Simplified adenine base editors improve adenine base editing efficiency in rice. Plant Biotechnol J. 2020;18(3):770–8. https://doi.org/10.1111/pbi.13244.
Hua K, Tao X, Yuan F, Wang D, Zhu JK. Precise A·T to G·C base editing in the rice genome. Mol Plant. 2018;11(4):627–30. https://doi.org/10.1016/j.molp.2018.02.007.
Huang X, Zeng X, Li J, Zhao D. Construction and analysis of tify1a and tify1b mutants in rice (Oryza sativa) based on CRISPR/Cas9 technology. J Agric Biotechnol. 2017;25(6):1003–12.
Hussain B, Lucas SJ, Budak H. CRISPR/Cas9 in plants: at play in the genome and at work for crop improvement. Brief Funct Genomics. 2018;17(5):319–28. https://doi.org/10.1093/bfgp/ely016.
Jacobs TB, Zhang N, Patel D, Martin GB. Generation of a collection of mutant tomato lines using pooled CRISPR libraries. Plant Physiol. 2017;174(4):2023–37. https://doi.org/10.1104/pp.17.00489.
Javaid D, Ganie SY, Hajam YA, Reshi MS. CRISPR/Cas9 system: a reliable and facile genome editing tool in modern biology. Mol Biol Rep. 2022;49(12):12133–50. https://doi.org/10.1007/s11033-022-07880-6.
Ji X, Zhang H, Zhang Y, Wang Y, Gao C. Establishing a CRISPR/Cas-like immune system conferring DNA virus resistance in plants. Nat Plants. 2015;1:15144. https://doi.org/10.1038/nplants.2015.144.
Jia H, Zhang Y, Orbović V, Xu J, White FF, Jones JB, Wang N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J. 2017;15(7):817–23. https://doi.org/10.1111/pbi.12677.
Jiang YY, Chai YP, Lu MH, Han XL, Lin Q, Zhang Y, Zhang Q, Zhou Y, Wang XC, Gao C, Chen QJ. Prime editing efficiently generates W542L and S621I double mutations in two ALS genes in maize. Genome Biol. 2020;21(1):257. https://doi.org/10.1186/s13059-020-02170-5.
Jiang WZ, Henry IM, Lynagh PG, Comai L, Cahoon EB, Weeks DP. Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol J. 2017;15(5):648–57. https://doi.org/10.1111/pbi.12663.
Jin S, Zong Y, Gao Q, Zhu Z, Wang Y, Qin P, Liang C, Wang D, Qiu JL, Zhang F, Gao C. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science. 2019;364(6437):292–5. https://doi.org/10.1126/science.aaw7166. (Epub 2019 Feb 28 PMID: 30819931).
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. https://doi.org/10.1126/science.1225829.
Juma BS, Mukami A, Mweu C, Ngugi MP, Mbinda W. Targeted mutagenesis of the CYP79D1 gene via CRISPR/Cas9-mediated genome editing results in lower levels of cyanide in cassava. Front Plant Sci. 2022;26(13):1009860. https://doi.org/10.3389/fpls.2022.1009860.PMID:36388608;PMCID:PMC9644188.
Kaur N, Alok A, Shivani KP, Kaur N, Awasthi P, Chaturvedi S, Pandey P, Pandey A, Pandey AK, Tiwari S. CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit. Metab Eng. 2020;59:76–86. https://doi.org/10.1016/j.ymben.2020.01.008.
Kim D, Alptekin B, Budak H. CRISPR/Cas9 genome editing in wheat. Funct Integr Genomics. 2018;18(1):31–41. https://doi.org/10.1007/s10142-017-0572-x.
Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to FokI cleavage domain. Proc Natl Acad Sci USA. 1996;93(3):1156–60. https://doi.org/10.1073/pnas.93.3.1156.
Kim H, Kim ST, Ryu J, Kang BC, Kim JS, Kim SG. CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat Commun. 2017;8:14406. https://doi.org/10.1038/ncomms14406.
Kodackattumannil P, Lekshmi G, Kottackal M, Sasi S, Krishnan S, Al Senaani S, Amiri KMA. Hidden pleiotropy of agronomic traits uncovered by CRISPR-Cas9 mutagenesis of the tyrosinase CuA-binding domain of the polyphenol oxidase 2 of eggplant. Plant Cell Rep. 2023;42(4):825–8. https://doi.org/10.1007/s00299-023-02987-x.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533(7603):420–4. https://doi.org/10.1038/nature17946.
Kong J, Martin-Ortigosa S, Finer J, Orchard N, Gunadi A, Batts LA, Thakare D, Rush B, Schmitz O, Stuiver M, Olhoft P, Pacheco-Villalobos D. Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocotspecies. Front Plant Sci. 2020;11:572319. https://doi.org/10.3389/fpls.2020.572319.
Kuang Y, Li S, Ren B, Yan F, Spetz C, Li X, Zhou X, Zhou H. Base-editing-mediated artificial evolution of OsALS1 in planta to develop novel herbicide-tolerant rice germplasms. Mol Plant. 2020;13(4):565–72. https://doi.org/10.1016/j.molp.2020.01.010.
Kumar S. Abiotic stresses and their effects on plant growth, yield and nutritional quality of agricultural produce. Int J Food Sci Agric. 2020;4:367–78. https://doi.org/10.26855/ijfsa.2020.12.002.
Kurt IC, Zhou R, Iyer S, Garcia SP, Miller BR, Langner LM, Grünewald J, Joung JK. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol. 2021;39(1):41–6. https://doi.org/10.1038/s41587-020-0609-x.
Kwabena Osei M, Danquah A, Adu-Dapaah H, Danquah E, Blay E, Massoudi M, Maxwell D. Marker assisted backcrossing of alcobaca gene into two elite tomato breeding lines. Front Hortic. 2022;22(1):1024042.
Laura M, Forti C, Barberini S, Ciorba R, Mascarello C, Giovannini A, Pistelli L, Pieracci Y, Lanteri AP, Ronca A, et al. Highly efficient CRISPR/Cas9 mediated gene editing in Ocimum basilicum ‘FT Italiko’ to induce resistance to Peronospora belbahrii. Plants. 2023;12(13):2395. https://doi.org/10.3390/plants12132395.
Lei J, Dai P, Li Y, Zhang W, Zhou G, Liu C, Liu X. Heritable gene editing using FT mobile guide RNAs and DNA viruses. Plant Methods. 2021;17(1):1–20. https://doi.org/10.1186/s13007-021-00719-4.
Li X, Hu D, Cai L, Wang H, Liu X, Du H, Yang Z, Zhang H, Hu Z, Huang F, Kan G, Kong F, Liu B, Yu D, Wang H. CALCIUM-DEPENDENT PROTEIN KINASE38 regulates flowering time and common cutworm resistance in soybean. Plant Physiol. 2022;190(1):480–99. https://doi.org/10.1093/plphys/kiac260.
Li R, Li R, Li X, Fu D, Zhu B, Tian H, Luo Y, Zhu H. Multiplexed CRISPR/Cas9-mediated metabolic engineering of γ-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol J. 2018;16(2):415–27. https://doi.org/10.1111/pbi.12781.
Li Y, Liang J, Deng B, Jiang Y, Zhu J, Chen L, Li M, Li J. Applications and prospects of CRISPR/Cas9-mediated base editing in plant breeding. Curr Issues Mol Biol. 2023;45(2):918–35. https://doi.org/10.3390/cimb45020059.
Li J, Meng X, Zong Y, Chen K, Zhang H, Liu J, Li J, Gao C. Gene replacements and insertions in rice by intron targeting using CRISPR/Cas9. Nat Plants. 2016;2:16139. https://doi.org/10.1038/nplants.2016.139.
Li J, Pan W, Zhang S, Ma G, Li A, Zhang H, Liu L. A rapid and highly efficient sorghum transformation strategy using GRF4-GIF1/ternary vector system. Plant J. 2023;28(9):1998–2015. https://doi.org/10.1105/tpc.16.00124.
Li J, Sun Y, Du J, Zhao Y, Xia L. Generation of targeted point mutations in rice by a modified CRISPR/Cas9 system. Mol Plant. 2017;10(3):526–9. https://doi.org/10.1016/j.molp.2016.12.001.
Li X, Wang Y, Chen S, Tian H, Fu D, Zhu B, Luo Y, Zhu H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front Plant Sci. 2018;9:559. https://doi.org/10.3389/fpls.2018.00559.
Li R, Wang L, Chen L, Yu W, Zhang S, Sheng J, Shen L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 2019;19(1):38. https://doi.org/10.1186/s12870-018-1627-4.
Li G, Zhang Y, Dailey M, Qi Y. Hs1Cas12a and Ev1Cas12a confer efficient genome editing in plants. Front Genome Ed. 2023;5:1251903. https://doi.org/10.3389/fgeed.2023.1251903.
Li J, Zhang H, Si X, Tian Y, Chen K, Liu J, Chen H, Gao C. Generation of thermosensitive male-sterile maize by targeted knockout of the ZmTMS5 gene. J Genet Genomics. 2017;44(9):465–8. https://doi.org/10.1016/j.jgg.2017.02.002.
Li R, Zhang L, Wang L, Chen L, Zhao R, Sheng J, Shen L. Reduction of tomato-plant chilling tolerance by CRISPR/Cas9-mediated SlCBF1 mutagenesis. J Agric Food Chem. 2018;66(34):9042–51. https://doi.org/10.1021/acs.jafc.8b02177.
Li C, Zong Y, Wang Y, Jin S, Zhang D, Song Q, Zhang R, Gao C. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 2018;19(1):59. https://doi.org/10.1186/s13059-018-1443-z.
Liao S, Qin X, Luo L, Han Y, Wang X, Usman B, Nawaz G, Zha N, Liu Y, Li R. CRISPR/Cas9-induced mutagenesis of Semi-rolled leaf1, 2 Confers curled leaf phenotype and drought tolerance by influencing protein expression patterns and ROS scavenging in rice (Oryza sativa L.). Agronomy. 2019;9(11):728. https://doi.org/10.3390/agronomy9110728.
Lin Q, Jin S, Zong Y, Yu H, Zhu Z, Liu G, Kou L, Wang Y, Qiu JL, Li J, Gao C. High-efficiency prime editing with optimized, paired pegRNAs in plants. Nat Biotechnol. 2021;39(8):923–7. https://doi.org/10.1038/s41587-021-00868-w.
Lin Q, Zong Y, Xue C, Wang S, Jin S, Zhu Z, Wang Y, Anzalone AV, Raguram A, Doman JL, Liu DR, Gao C. Prime genome editing in rice and wheat. Nat Biotechnol. 2020;38(5):582–5. https://doi.org/10.1038/s41587-020-0455-x.
Liu L, Chen P, Wang M, Li X, Wang J, Yin M, Wang Y. C2c1-sgRNA complex structure reveals RNA-guided DNA cleavage mechanism. Mol Cell. 2017;65(2):310–22. https://doi.org/10.1016/j.molcel.2016.11.040.
Liu Y, Chen Z, Zhang C, Guo J, Liu Q, Yin Y, Hu Y, Xia H, Li B, Sun X, Li Y, Liu X. Gene editing of ZmGA20ox3 improves plant architecture and drought tolerance in maize. Plant Cell Rep. 2023;43(1):18. https://doi.org/10.1007/s00299-023-03090-x.
Liu X, Wu D, Shan T, Xu S, Qin R, Li H, Negm M, Wu D, Li J. The trihelix transcription factor OsGTγ-2 is involved in adaption to salt stress in rice. Plant Mol Biol. 2020;103(4–5):545–60. https://doi.org/10.1007/s11103-020-01010-1.
Liu D, Xuan S, Prichard LE, Donahue LI, Pan C, Nagalakshmi U, Ellison EE, Starker CG, Dinesh-Kumar SP, Qi Y, Voytas DF. Heritable base-editing in Arabidopsis using RNA viral vectors. Plant Physiol. 2022;189(4):1920–4. https://doi.org/10.1093/plphys/kiac206.
Liu T, Zeng D, Zheng Z, Lin Z, Xue Y, Li T, Xie X, Ma G, Liu YG, Zhu Q. The ScCas9++ variant expands the CRISPR toolbox for genome editing in plants. J Integr Plant Biol. 2021;63(9):1611–9. https://doi.org/10.1111/jipb.13164.
Liu Y, Zhang L, Li C, Yang Y, Duan Y, Yang Y, Sun X. Establishment of Agrobacterium-mediated genetic transformation and application of CRISPR/Cas9 genome-editing system to Brassica rapa var. rapa. Plant Methods. 2022;18(1):98. https://doi.org/10.1186/s13007-022-00931-w.
Liu L, Zhang J, Xu J, Li Y, Guo L, Wang Z, Zhang X, Zhao B, Guo YD, Zhang N. CRISPR/Cas9 targeted mutagenesis of SlLBD40, a lateral organ boundaries domain transcription factor, enhances drought tolerance in tomato. Plant Sci. 2020;301:110683. https://doi.org/10.1016/j.plantsci.2020.110683.
Lou D, Wang H, Liang G, Yu D. OsSAPK2 Confers abscisic acid sensitivity and tolerance to drought stress in rice. Front Plant Sci. 2017;8:993. https://doi.org/10.3389/fpls.2017.00993.
Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho MJ, Scelonge C, Lenderts B, Chamberlin M, Cushatt J, Wang L, Ryan L, Khan T, Chow-Yiu J, Hua W, Yu M, Banh J, Bao Z, Brink K, Igo E, Rudrappa B, Shamseer PM, Bruce W, Newman L, Shen B, Zheng P, Bidney D, Falco C, Register J, Zhao ZY, Xu D, Jones T, Gordon-Kamm W. Morphogenic regulators baby boom and wuschel improve monocot transformation. Plant Cell. 2016;28(9):1998–2015. https://doi.org/10.1105/tpc.16.00124.
Lu Y, Tian Y, Shen R, Yao Q, Wang M, Chen M, Dong J, Zhang T, Li F, Lei M, Zhu JK. Targeted, efficient sequence insertion and replacement in rice. Nat Biotechnol. 2020;38(12):1402–7. https://doi.org/10.1038/s41587-020-0581-5.
Lu Y, Zhu JK. Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol Plant. 2017;10(3):523–5. https://doi.org/10.1016/j.molp.2016.11.013.
Lv Y, Yang M, Hu D, Yang Z, Ma S, Li X, Xiong L. The OsMYB30 transcription factor suppresses cold tolerance by interacting with a JAZ protein and suppressing β-amylase expression. Plant Physiol. 2017;173(2):1475–91. https://doi.org/10.1104/pp.16.01725.
Ly DNP, Iqbal S, Fosu-Nyarko J, Milroy S, Jones MGK. Multiplex CRISPR-Cas9 gene-editing can deliver potato cultivars with reduced browning and acrylamide. Plants (Basel). 2023;12(2):379. https://doi.org/10.3390/plants12020379.
Ma J, Chen J, Wang M, Ren Y, Wang S, Lei C, Cheng Z, Sodmergen. Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J Exp Bot. 2018;69(5):1051–64. https://doi.org/10.1093/jxb/erx458.
Macovei A, Sevilla NR, Cantos C, Jonson GB, Slamet-Loedin I, Čermák T, Voytas DF, Choi IR, Chadha-Mohanty P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol J. 2018;16(11):1918–27. https://doi.org/10.1111/pbi.12927.
Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF. Plant gene editing through de novo induction of meristems. Nat Biotechnol. 2020;38(1):84–9. https://doi.org/10.1038/s41587-019-0337-2.
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJ, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. An updated evolutionary classification of CRISPR/Cas systems. Nat Rev Microbiol. 2015;13(11):722–36. https://doi.org/10.1038/nrmicro3569.
Mallapaty S. China’s approval of gene-edited crops energizes researchers. Nature. 2022;602(7898):559–60. https://doi.org/10.1038/d41586-022-00395-x.
Malnoy M, Viola R, Jung MH, Koo OJ, Kim S, Kim JS, Velasco R, Nagamangala KC. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci. 2016;7:1904. https://doi.org/10.3389/fpls.2016.01904.
Manghwar H, Li B, Ding X, Hussain A, Lindsey K, Zhang X, Jin S. CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv Sci (Weinh). 2020;7(6):1902312. https://doi.org/10.1002/advs.201902312.
Mann A, Kumari J, Kumar R, Kumar P, Pradhan AK, Pental D, Bisht NC. Targeted editing of multiple homologues of GTR1 and GTR2 genes provides the ideal low-seed, high-leaf glucosinolate oilseed mustard with uncompromised defence and yield. Plant Biotechnol J. 2023. https://doi.org/10.1111/pbi.14121.
Metje-Sprink J, Menz J, Modrzejewski D, Sprink T. DNA-free genome editing: past, present and future. Front Plant Sci. 2019;9:1957. https://doi.org/10.3389/fpls.2018.01957.
Miki D, Zhang W, Zeng W, Feng Z, Zhu JK. CRISPR-Cas9 mediated gene targeting in Arabidopsis using sequential transformation. Nat Commun. 2018;9(1):1967. https://doi.org/10.1038/s41467-018-04416-0.
Ming M, Ren Q, Pan C, He Y, Zhang Y, Liu S, Zhong Z, Wang J, Malzahn AA, Wu J, Zheng X, Zhang Y, Qi Y. CRISPR-Cas12b enables efficient plant genome engineering. Nat Plants. 2020;6(3):202–8. https://doi.org/10.1038/s41477-020-0614-6.
Mishra R, Zhao K. Genome editing technologies and their applications in crop improvement. Plant Biotechnol Rep. 2018;12:57–68. https://doi.org/10.1007/s11816-018-0472-0.
Modrzejewski D, Hartung F, Lehnert H, Sprink T, Kohl C, Keilwagen J, Wilhelm R. Which factors affect the occurrence of off-target effects caused by the use of CRISPR/Cas: a systematic review in plants. Front Plant Sci. 2020;11:574959. https://doi.org/10.3389/fpls.2020.574959.
Nagalakshmi U, Meier N, Liu JY, Voytas DF, Dinesh-Kumar SP. High-efficiency multiplex biallelic heritable editing in Arabidopsis using an RNA virus. Plant Physiol. 2022;189(3):1241–5. https://doi.org/10.1093/plphys/kiac159.
Nandy S, Pathak B, Zhao S, Srivastava V. Heat-shock-inducible CRISPR/Cas9 system generates heritable mutations in rice. Plant Direct. 2019;3(5):e00145. https://doi.org/10.1002/pld3.145.
Nawaz G, Han Y, Usman B, Liu F, Qin B, Li R. Knockout of OsPRP1, a gene encoding proline-rich protein, confers enhanced cold sensitivity in rice (Oryza sativa L.) at the seedling stage. 3 Biotech. 2019;9(7):254. https://doi.org/10.1007/s13205-019-1787-4.
Nekrasov V, Wang C, Win J, Lanz C, Weigel D, Kamoun S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci Rep. 2017;7(1):482. https://doi.org/10.1038/s41598-017-00578-x.
Nguyen DV, Hoang TT, Le NT, Tran HT, Nguyen CX, Moon YH, Chu HH, Do PT. An efficient hairy root system for validation of plant transformation vector and CRISPR/Cas construct activities in cucumber (Cucumis sativus L.). Front Plant Sci. 2022;202212:770062. https://doi.org/10.3389/fpls.2021.770062.
Nieves-Cordones M, Mohamed S, Tanoi K, Kobayashi NI, Takagi K, Vernet A, Guiderdoni E, Périn C, Sentenac H, Véry AA. Production of low-Cs+ rice plants by inactivation of the K+ transporter OsHAK1 with the CRISPR/Cas system. Plant J. 2017;92(1):43–56. https://doi.org/10.1111/tpj.13632.
Nonaka S, Arai C, Takayama M, Matsukura C, Ezura H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci Rep. 2017;7(1):7057. https://doi.org/10.1038/s41598-017-06400-y.
Nuñez-Muñoz L, Vargas-Hernández B, Hinojosa-Moya J, Ruiz-Medrano R, Xoconostle-Cázares B. Plant drought tolerance provided through genome editing of the trehalase gene. Plant Signal Behav. 2021;16(4):1877005. https://doi.org/10.1080/15592324.2021.1877005.
O’Connell MR. Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR-Cas systems. J Mol Biol. 2019;431(1):66–87. https://doi.org/10.1016/j.jmb.2018.06.029.
Ogata T, Ishizaki T, Fujita M, Fujita Y. CRISPR/Cas9-targeted mutagenesis of OsERA1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE. 2020;15(12):e0243376. https://doi.org/10.1371/journal.pone.0243376.
Okuzaki A, Ogawa T, Koizuka C, Kaneko K, Inaba M, Imamura J, Koizuka N. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol Biochem. 2018;131:63–9. https://doi.org/10.1016/j.plaphy.2018.04.025.
Ortigosa A, Gimenez-Ibanez S, Leonhardt N, Solano R. Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol J. 2019;17(3):665–73. https://doi.org/10.1111/pbi.13006.
Osakabe Y, Watanabe T, Sugano SS, Ueta R, Ishihara R, Shinozaki K, Osakabe K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci Rep. 2016;26(6):26685. https://doi.org/10.1038/srep26685.
Pan C, Li G, Malzahn AA, Cheng Y, Leyson B, Sretenovic S, Gurel F, Coleman GD, Qi Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat Plants. 2022;8(5):513–25. https://doi.org/10.1038/s41477-022-01151-9.
Park JJ, Dempewolf E, Zhang W, Wang ZY. RNA-guided transcriptional activation via CRISPR/dCas9 mimics overexpression phenotypes in Arabidopsis. PLoS ONE. 2017;12(6):e0179410. https://doi.org/10.1371/journal.pone.0179410.
Pausch P, Al-Shayeb B, Bisom-Rapp E, Tsuchida CA, Li Z, Cress BF, Knott GJ, Jacobsen SE, Banfield JF, Doudna JA. CRISPR-CasΦ from huge phages is a hypercompact genome editor. Science. 2020;369(6501):333–7. https://doi.org/10.1126/science.abb1400.
Peng A, Chen S, Lei T, Xu L, He Y, Wu L, Yao L, Zou X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol J. 2017;15(12):1509–19. https://doi.org/10.1111/pbi.12733.
Pixley KV, Falck-Zepeda JB, Paarlberg RL, Phillips PWB, Slamet-Loedin IH, Dhugga KS, Campos H, Gutterson N. Genome-edited crops for improved food security of smallholder farmers. Nat Genet. 2022;54(4):364–7. https://doi.org/10.1038/s41588-022-01046-7.
Pyott DE, Sheehan E, Molnar A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol Plant Pathol. 2016;17(8):1276–88. https://doi.org/10.1111/mpp.12417.
Qin L, Li J, Wang Q, Xu Z, Sun L, Alariqi M, Manghwar H, Wang G, Li B, Ding X, Rui H, Huang H, Lu T, Lindsey K, Daniell H, Zhang X, Jin S. High-efficient and precise base editing of C•G to T•A in the allotetraploid cotton (Gossypium hirsutum) genome using a modified CRISPR/Cas9 system. Plant Biotechnol J. 2020;18(1):45–56. https://doi.org/10.1111/pbi.13168.
Qiu Z, Kang S, He L, Zhao J, Zhang S, Hu J, Zeng D, Zhang G, Dong G, Gao Z, Ren D, Chen G, Guo L, Qian Q, Zhu L. The newly identified heat-stress sensitive albino 1 gene affects chloroplast development in rice. Plant Sci. 2018;267:168–79. https://doi.org/10.1016/j.plantsci.2017.11.015.
Rath D, Amlinger L, Rath A, Lundgren M. The CRISPR/Cas immune system: biology, mechanisms, and applications. Biochimie. 2015;117:119–28. https://doi.org/10.1016/j.biochi.2015.03.025.
Rathnasamy SA, Kambale R, Elangovan A, Mohanavel W, Shanmugavel P, Ramasamy G, Alagarsamy S, Marimuthu R, Rajagopalan VR, Manickam S, Ramanathan V, Muthurajan R, Vellingiri G. Altering stomatal density for manipulating transpiration and photosynthetic traits in rice through CRISPR/Cas9 mutagenesis. Curr Issues Mol Biol. 2023;45(5):3801–14. https://doi.org/10.3390/cimb45050245.
Reed KM, Bargmann BOR. Protoplast regeneration and its use in new plant breeding technologies. Front Genome Ed. 2021;3:734951. https://doi.org/10.3389/fgeed.2021.734951.
Ren B, Liu L, Li S, Kuang Y, Wang J, Zhang D, Zhou X, Lin H, Zhou H. Cas9-NG greatly expands the targeting scope of the genome-editing toolkit by recognizing Ng and other atypical PAMs in rice. Mol Plant. 2019;12(7):1015–26. https://doi.org/10.1016/j.molp.2019.03.010.
Ren B, Yan F, Kuang Y, Li N, Zhang D, Zhou X, Lin H, Zhou H. Improved base editor for efficiently inducing genetic variations in rice with CRISPR/Cas9-guided hyperactive hAID mutant. Mol Plant. 2018;11(4):623–6. https://doi.org/10.1016/j.molp.2018.01.005.
Roca Paixão JF, Gillet FX, Ribeiro TP, Bournaud C, Lourenço-Tessutti IT, Noriega DD, Melo BP, de Almeida-Engler J, Grossi-de-Sa MF. Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a histone AcetylTransferase. Sci Rep. 2019;9(1):8080. https://doi.org/10.1038/s41598-019-44571-y.
Saber Sichani A, Ranjbar M, Baneshi M, Torabi Zadeh F, Fallahi J. A review on advanced CRISPR-based genome-editing tools: base editing and prime editing. Mol Biotechnol. 2023;65(6):849–60. https://doi.org/10.1007/s12033-022-00639-1.
Sánchez-León S, Gil-Humanes J, Ozuna CV, Giménez MJ, Sousa C, Voytas DF, Barro F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J. 2018;16(4):902–10. https://doi.org/10.1111/pbi.12837.
Santillán Martínez MI, Bracuto V, Koseoglou E, Appiano M, Jacobsen E, Visser RGF, Wolters AA, Bai Y. CRISPR/Cas9-targeted mutagenesis of the tomato susceptibility gene PMR4 for resistance against powdery mildew. BMC Plant Biol. 2020;20(1):284. https://doi.org/10.1186/s12870-020-02497-y.
Santosh Kumar VV, Verma RK, Yadav SK, Yadav P, Watts A, Rao MV, Chinnusamy V. CRISPR/Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol Mol Biol Plants. 2020;26(6):1099–110. https://doi.org/10.1007/s12298-020-00819-w.
Sashidhar N, Harloff HJ, Potgieter L, Jung C. Gene editing of three BnITPK genes in tetraploid oilseed rape leads to significant reduction of phytic acid in seeds. Plant Biotechnol J. 2020;18(11):2241–50. https://doi.org/10.1111/pbi.13380.
Sauer NJ, Mozoruk J, Miller RB, Warburg ZJ, Walker KA, Beetham PR, Schöpke CR, Gocal GF. Oligonucleotide-directed mutagenesis for precision gene editing. Plant Biotechnol J. 2016;14(2):496–502. https://doi.org/10.1111/pbi.12496.
Schmidt C, Pacher M, Puchta H. Efficient induction of heritable inversions in plant genomes using the CRISPR/Cas system. Plant J. 2019;98(4):577–89. https://doi.org/10.1111/tpj.14322.
Schwartz C, Lenderts B, Feigenbutz L, Barone P, Llaca V, Fengler K, Svitashev S. CRISPR-Cas9-mediated 75.5-Mb inversion in maize. Nat Plants. 2020;6(12):1427–31. https://doi.org/10.1038/s41477-020-00817-6.
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu JL, Gao C. Targeted genome modification of crop plants using a CRISPR/Cas system. Nat Biotechnol. 2013;31(8):686–8. https://doi.org/10.1038/nbt.2650.
Sharma SK, Gupta OP, Pathaw N, Sharma D, Maibam A, Sharma P, Sanasam J, Karkute SG, Kumar S, Bhattacharjee B. CRISPR-Cas-Led revolution in diagnosis and management of emerging plant viruses: new avenues toward food and nutritional security. Front Nutr. 2021;8:751512. https://doi.org/10.3389/fnut.2021.751512.
Shen L, Wang C, Fu Y, Wang J, Liu Q, Zhang X, Yan C, Qian Q, Wang K. QTL editing confers opposing yield performance in different rice varieties. J Integr Plant Biol. 2018;60(2):89–93. https://doi.org/10.1111/jipb.12501.
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE. ARGOS8 variants generated by CRISPR/Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J. 2017;15(2):207–16. https://doi.org/10.1111/pbi.12603.
Silva CJ, van den Abeele C, Ortega-Salazar I, Papin V, Adaskaveg JA, Wang D, Casteel CL, Seymour GB, Blanco-Ulate B. Host susceptibility factors render ripe tomato fruit vulnerable to fungal disease despite active immune responses. J Exp Bot. 2021;72(7):2696–709. https://doi.org/10.1093/jxb/eraa601.
Singh S, Chaudhary R, Deshmukh R, Tiwari S. Opportunities and challenges with CRISPR-Cas mediated homologous recombination based precise editing in plants and animals. Plant Mol Biol. 2023;111(1–2):1–20. https://doi.org/10.1007/s11103-022-01321-5.
Song X, Liu C, Wang N, Huang H, He S, Gong C, Wei Y. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy. Adv Drug Deliv Rev. 2021;168:158–80. https://doi.org/10.1016/j.addr.2020.04.010.
Sun SK, Xu X, Tang Z, Tang Z, Huang XY, Wirtz M, Hell R, Zhao FJ. A molecular switch in sulfur metabolism to reduce arsenic and enrich selenium in rice grain. Nat Commun. 2021;12(1):1392. https://doi.org/10.1038/s41467-021-21282-5.
Svitashev S, Young JK, Schwartz C, Gao H, Falco SC, Cigan AM. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 2015;169(2):931–45. https://doi.org/10.1104/pp.15.00793.
Taleei R, Nikjoo H. Biochemical DSB-repair model for mammalian cells in G1 and early S phases of the cell cycle. Mutat Res. 2013;756:206–12. https://doi.org/10.1016/j.mrgentox.2013.06.004.
Tamilselvan-Nattar-Amutha S, Hiekel S, Hartmann F, Lorenz J, Dabhi RV, Dreissig S, Hensel G, Kumlehn J, Heckmann S. Barley stripe mosaic virus-mediated somatic and heritable gene editing in barley (Hordeum vulgare L.). Front Plant Sci. 2023;14:1201446. https://doi.org/10.3389/fpls.2023.1201446.
Tan J, Zeng D, Zhao Y, Wang Y, Liu T, Li S, Xue Y, Luo Y, Xie X, Chen L, Liu YG, Zhu Q. PhieABEs: a PAM-less/free high-efficiency adenine base editor toolbox with wide target scope in plants. Plant Biotechnol J. 2022;20(5):934–43. https://doi.org/10.1111/pbi.13774.
Tang XD, Gao F, Liu MJ, Fan QL, Chen DK, Ma WT. Methods for enhancing clustered regularly interspaced short palindromic repeats/Cas9-mediated homology-directed repair efficiency. Front Genet. 2019;10:551. https://doi.org/10.3389/fgene.2019.00551.
Tang X, Lowder LG, Zhang T, Malzahn AA, Zheng X, Voytas DF, Zhong Z, Chen Y, Ren Q, Li Q, Kirkland ER, Zhang Y, Qi Y. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants. 2017;3:17018. https://doi.org/10.1038/nplants.2017.18.
Tang L, Mao B, Li Y, Lv Q, Zhang L, Chen C, He H, Wang W, Zeng X, Shao Y, Pan Y, Hu Y, Peng Y, Fu X, Li H, Xia S, Zhao B. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci Rep. 2017;7(1):14438. https://doi.org/10.1038/s41598-017-14832-9.
Tang X, Ren Q, Yang L, Bao Y, Zhong Z, He Y, Liu S, Qi C, Liu B, Wang Y, Sretenovic S, Zhang Y, Zheng X, Zhang T, Qi Y, Zhang Y. Single transcript unit CRISPR 20 systems for robust Cas9 and Cas12a mediated plant genome editing. Plant Biotechnol J. 2019;17(7):1431–45. https://doi.org/10.1111/pbi.13068.
Thomazella DPT, Seong K, Mackelprang R, Dahlbeck D, Geng Y, Gill US, Qi T, Pham J, Giuseppe P, Lee CY, Ortega A, Cho MJ, Hutton SF, Staskawicz B. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/pnas.2026152118.
Tian S, Jiang L, Cui X, Zhang J, Guo S, Li M, Zhang H, Ren Y, Gong G, Zong M, Liu F, Chen Q, Xu Y. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018;37(9):1353–6. https://doi.org/10.1007/s00299-018-2299-0.
Tian Y, Shen R, Li Z, Yao Q, Zhang X, Zhong D, Tan X, Song M, Han H, Zhu JK, Lu Y. Efficient C-to-G editing in rice using an optimized base editor. Plant Biotechnol J. 2022;20(7):1238–40. https://doi.org/10.1111/pbi.13841.
Tran MT, Doan DTH, Kim J, Song YJ, Sung YW, Das S, Kim EJ, Son GH, Kim SH, Van Vu T, Kim JY. CRISPR/Cas9-based precise excision of SlHyPRP1 domain(s) to obtain salt stress-tolerant tomato. Plant Cell Rep. 2021;40(6):999–1011. https://doi.org/10.1007/s00299-020-02622-z.
Tripathi JN, Ntui VO, Shah T, Tripathi L. CRISPR/Cas9-mediated editing of DMR6 orthologue in banana (Musa spp.) confers enhanced resistance to bacterial disease. Plant Biotechnol J. 2021;19(7):1291–3. https://doi.org/10.1111/pbi.13614.
Usman B, Nawaz G, Zhao N, Liao S, Liu Y, Li R. Precise editing of the OsPYL9 gene by RNA-guided Cas9 nuclease confers enhanced drought tolerance and grain yield in rice (Oryza sativa L.) by regulating circadian rhythm and abiotic stress responsive proteins. Int J Mol Sci. 2020;21(21):7854. https://doi.org/10.3390/ijms21217854.
Veillet F, Perrot L, Guyon-Debast A, Kermarrec MP, Chauvin L, Chauvin JE, Gallois JL, Mazier M, Nogué F. Expanding the CRISPR toolbox in P. patens using SpCas9-NG variant and application for gene and base editing in Solanaceae crops. Int J Mol Sci. 2020;21(3):1024. https://doi.org/10.3390/ijms21031024.
Vlčko T, Ohnoutková L. Allelic variants of CRISPR/Cas9 induced mutation in an Inositol Trisphosphate 5/6 Kinase gene manifest different phenotypes in barley. Plants (Basel). 2020;9(2):195. https://doi.org/10.3390/plants9020195.
Wang Q, Alariqi M, Wang F, Li B, Ding X, Rui H, Li Y, Xu Z, Qin L, Sun L, Li J, Zou J, Lindsey K, Zhang X, Jin S. The application of a heat-inducible CRISPR/Cas12b (C2c1) genome editing system in tetraploid cotton (G. hirsutum) plants. Plant Biotechnol J. 2020;18(12):2436–43. https://doi.org/10.1111/pbi.13417.
Wang L, Chen L, Li R, Zhao R, Yang M, Sheng J, Shen L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J Agric Food Chem. 2017;65(39):8674–82. https://doi.org/10.1021/acs.jafc.7b02745.
Wang FZ, Chen MX, Yu LJ, Xie LJ, Yuan LB, Qi H, Xiao M, Guo W, Chen Z, Yi K, Zhang J, Qiu R, Shu W, Xiao S, Chen QF. OsARM1, an R2R3 MYB transcription factor, is involved in regulation of the response to arsenic stress in rice. Front Plant Sci. 2017;8:1868. https://doi.org/10.3389/fpls.2017.01868.
Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu JL. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol. 2014;32(9):947–51. https://doi.org/10.1038/nbt.2969.
Wang T, Deng Z, Zhang X, Wang H, Wang Y, Liu X, Liu S, Xu F, Li T, Fu D, Zhu B, Luo Y, Zhu H. Tomato DCL2b is required for the biosynthesis of 22-nt small RNAs, the resulting secondary siRNAs, and the host defense against ToMV. Hortic Res. 2018;5:62. https://doi.org/10.1038/s41438-018-0073-7.
Wang Z, Hardcastle TJ, Canto Pastor A, Yip WH, Tang S, Baulcombe DC. A novel DCL2-dependent miRNA pathway in tomato affects susceptibility to RNA viruses. Genes Dev. 2018;32(17–18):1155–60. https://doi.org/10.1101/gad.313601.118.
Wang J, He Z, Wang G, Zhang R, Duan J, Gao P, Lei X, Qiu H, Zhang C, Zhang Y, Yin H. Efficient targeted insertion of large DNA fragments without DNA donors. Nat Methods. 2022;19(3):331–40. https://doi.org/10.1038/s41592-022-01399-1.
Wang W, Pan Q, Tian B, He F, Chen Y, Bai G, Akhunova A, Trick HN, Akhunov E. Gene editing of the wheat homologs of TONNEAU1-recruiting motif encoding gene affects grain shape and weight in wheat. Plant J. 2019;100(2):251–64. https://doi.org/10.1111/tpj.14440.
Wang H, La Russa M, Qi LS. CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem. 2016;85:227–64. https://doi.org/10.1146/annurev-biochem-060815-014607.
Wang F, Wang C, Liu P, Lei C, Hao W, Gao Y, Liu YG, Zhao K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the erf transcription factor gene OsERF922. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0154027.
Wang M, Xu Z, Gosavi G, Ren B, Cao Y, Kuang Y, Zhou C, Spetz C, Yan F, Zhou X, Zhou H. Targeted base editing in rice with CRISPR/ScCas9 system. Plant Biotechnol J. 2020;18(8):1645–7. https://doi.org/10.1111/pbi.13330.
Wang F, Xu Y, Li W, Chen Z, Wang J, Fan F, Tao Y, Jiang Y, Zhu QH, Yang J. Creating a novel herbicide-tolerance OsALS allele using CRISPR/Cas9-mediated gene editing. Crop J. 2021;9:305–12. https://doi.org/10.1016/j.cj.2020.06.001.
Wang S, Yang Y, Guo M, Zhong C, Yan C, Sun S. Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system. Crop J. 2020;8:457–64. https://doi.org/10.1016/j.cj.2020.02.005.
Wei C, Wang C, Jia M, Guo HX, Luo PY, Wang MG, Zhu JK, Zhang H. Efficient generation of homozygous substitutions in rice in one generation utilizing an rABE8e base editor. J Integr Plant Biol. 2021;63(9):1595–9. https://doi.org/10.1111/jipb.13089.
Westra ER, van Erp PB, Künne T, Wong SP, Staals RH, Seegers CL, Bollen S, Jore MM, Semenova E, Severinov K, de Vos WM, Dame RT, de Vries R, Brouns SJ, van der Oost J. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol Cell. 2012;46(5):595–605. https://doi.org/10.1016/j.molcel.2012.03.018.
Wright DA, Li T, Yang B, Spalding MH. TALEN-mediated genome editing: prospects and perspectives. Biochem J. 2014;462(1):15–24. https://doi.org/10.1042/BJ20140295.
Wu F, Qiao X, Zhao Y, Zhang Z, Gao Y, Shi L, Du H, Wang L, Zhang YJ, Zhang Y, Liu L, Wang Q, Kong D. Targeted mutagenesis in Arabidopsis thaliana using CRISPR-Cas12b/C2c1. J Integr Plant Biol. 2020;62(11):1653–8. https://doi.org/10.1111/jipb.12944.
Wu J, Yan G, Duan Z, Wang Z, Kang C, Guo L, Liu K, Tu J, Shen J, Yi B, Fu T, Li X, Ma C, Dai C. Roles of the Brassica napus DELLA protein BnaA6.RGA, in modulating drought tolerance by interacting with the ABA signaling component BnaA10.ABF2. Front Plant Sci. 2020;11:577. https://doi.org/10.3389/fpls.2020.00577.
Xu Y, Lin Q, Li X, Wang F, Chen Z, Wang J, Li W, Fan F, Tao Y, Jiang Y, Wei X, Zhang R, Zhu QH, Bu Q, Yang J, Gao C. Fine-tuning the amylose content of rice by precise base editing of the Wx gene. Plant Biotechnol J. 2021;19(1):11–3. https://doi.org/10.1111/pbi.13433.
Xu R, Yang Y, Qin R, Li H, Qiu C, Li L, Wei P, Yang J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J Genet Genomics. 2016;43(8):529–32. https://doi.org/10.1016/j.jgg.2016.07.003.
Xu W, Yang Y, Yang B, Krueger CJ, Xiao Q, Zhao S, Zhang L, Kang G, Wang F, Yi H, Ren W, Li L, He X, Zhang C, Zhang B, Zhao J, Yang J. A design optimized prime editor with expanded scope and capability in plants. Nat Plants. 2022;8(1):45–52. https://doi.org/10.1038/s41477-021-01043-4.
Yan F, Kuang Y, Ren B, Wang J, Zhang D, Lin H, Yang B, Zhou X, Zhou H. Highly efficient A·T to G·C base editing by Cas9n-guided tRNA adenosine deaminase in rice. Mol Plant. 2018;11(4):631–4. https://doi.org/10.1016/j.molp.2018.02.008.
Yan D, Ren B, Liu L, Yan F, Li S, Wang G, Sun W, Zhou X, Zhou H. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Mol Plant. 2021;14(5):722–31. https://doi.org/10.1016/j.molp.2021.02.007.
Yang T, Ali M, Lin L, Li P, He H, Zhu Q, Sun C, Wu N, Zhang X, Huang T, Li CB, Li C, Deng L. Recoloring tomato fruit by CRISPR/Cas9-mediated multiplex gene editing. Hortic Res. 2022;10(1):uhac214. https://doi.org/10.1093/hr/uhac214.
Yang SH, Kim E, Park H, Koo Y. Selection of the high efficient sgRNA for CRISPR-Cas9 to edit herbicide related genes, PDS, ALS, and EPSPS in tomato. Appl Biol Chem. 2022;65(1):13.
Yin X, Biswal AK, Dionora J, Perdigon KM, Balahadia CP, Mazumdar S, Chater C, Lin HC, Coe RA, Kretzschmar T, Gray JE, Quick PW, Bandyopadhyay A. CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep. 2017;36(5):745–57. https://doi.org/10.1007/s00299-017-2118-z.
Yin Y, Qin K, Song X, Zhang Q, Zhou Y, Xia X, Yu J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018;59(11):2239–54. https://doi.org/10.1093/pcp/pcy146.
Young JK, Gasior SL, Jones S, Wang L, Navarro P, Vickroy B, Barrangou R. The repurposing of type I-E CRISPR-Cascade for gene activation in plants. Commun Biol. 2019;18(2):383. https://doi.org/10.1038/s42003-019-0637-6.
Yu Y, Leete TC, Born DA, Young L, Barrera LA, Lee SJ, Rees HA, Ciaramella G, Gaudelli NM. Cytosine base editors with minimized unguided DNA and RNA off-target events and high on-target activity. Nat Commun. 2020;11(1):2052. https://doi.org/10.1038/s41467-020-15887-5.
Yu QH, Wang B, Li N, Tang Y, Yang S, Yang T, Xu J, Guo C, Yan P, Wang Q, Asmutola P. CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Sci Rep. 2017;7(1):11874. https://doi.org/10.1038/s41598-017-12262-1.
Yue E, Cao H, Liu B. OsmiR535, a potential genetic editing target for drought and salinity stress tolerance in Oryza sativa. Plants (Basel). 2020;9(10):1337. https://doi.org/10.3390/plants9101337.
Yuste-Lisbona FJ, Fernández-Lozano A, Pineda B, Bretones S, Ortíz-Atienza A, García-Sogo B, Müller NA, Angosto T, Capel J, Moreno V, Jiménez-Gómez JM, Lozano R. ENO regulates tomato fruit size through the floral meristem development network. Proc Natl Acad Sci USA. 2020;117(14):8187–95. https://doi.org/10.1073/pnas.1913688117.
Yuyu C, Aike Z, Pao X, Xiaoxia W, Yongrun C, Beifang W, Yue Z, Liaqat S, Shihua C, Liyong C, Yingxin Z. Effects of GS3 and GL3.1 for grain size editing by CRISPR/Cas9 in rice. Rice Sci. 2020;27(5):405–13.
Zeng Y, Wen J, Zhao W, Wang Q, Huang W. Rational improvement of rice yield and cold tolerance by editing the three genes OsPIN5b, GS3, and OsMYB30 with the CRISPR/Cas9 system. Front Plant Sci. 2020;10:1663. https://doi.org/10.3389/fpls.2019.01663.
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–71. https://doi.org/10.1016/j.cell.2015.09.038.
Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017;91(4):714–24. https://doi.org/10.1111/tpj.13599.
Zhang A, Liu Y, Wang F, Li T, Chen Z, Kong D, Bi J, Zhang F, Luo X, Wang J, Tang J, Yu X, Liu G, Luo L. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol Breed. 2019;39:47. https://doi.org/10.1007/s11032-019-0954-y.
Zhang Y, Qi Y. CRISPR enables directed evolution in plants. Genome Biol. 2019;20(1):83. https://doi.org/10.1186/s13059-019-1693-4.
Zhao Y, Zhang C, Liu W, Gao W, Liu C, Song G, Li WX, Mao L, Chen B, Xu Y, Li X, Xie C. An alternative strategy for targeted gene replacement in plants using a dual-sgRNA/Cas9 design. Sci Rep. 2016;6:23890. https://doi.org/10.1038/srep23890.
Zhou J, Peng Z, Long J, Sosso D, Liu B, Eom JS, Huang S, Liu S, Vera Cruz C, Frommer WB, White FF, Yang B. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015;82(4):632–43. https://doi.org/10.1111/tpj.12838.
Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, Qiu JL, Wang D, Gao C. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat Biotechnol. 2017;35(5):438–40. https://doi.org/10.1038/nbt.3811.
Zsögön A, Čermák T, Naves ER, Notini MM, Edel KH, Weinl S, Freschi L, Voytas DF, Kudla J, Peres LEP. De novo domestication of wild tomato using genome editing. Nat Biotechnol. 2018. https://doi.org/10.1038/nbt.4272.s.
Acknowledgements
The authors express their gratitude to the National Agri-Food Biotechnology Institute (NABI), Department of Biotechnology (DBT) for research facilities and support. Thankful to DBT for a junior research fellowship to SS and Council of Scientific and Industrial Research (CSIR) for a junior research fellowship to RC. The present work was also supported by the Biotechnology Industry Research Assistance Council (BIRAC) for a banana biofortification project grant to ST. SS and RC are thankful to the Regional Centre for Biotechnology for PhD registration. The authors would like to acknowledge the DBT-eLibrary Consortium (DelCON) for providing access to online journals.
Funding
This work was supported by the Core Grant of National Agri-Food Biotechnology Institute [IN].
Author information
Authors and Affiliations
Contributions
ST conceived and designed the idea. SS and RC performed the literature survey. SS, RC, VL and ST wrote the manuscript. ST contributed to the editing of the manuscript. All authors have read and agreed to the present version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Corresponding Editor: Kutubuddin Ali Molla; Reviewers: Praveen Awasthi, Muntazir Mushtaq
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Chaudhary, R., Lokya, V. et al. Genome editing based trait improvement in crops: current perspective, challenges and opportunities. Nucleus 67, 97–126 (2024). https://doi.org/10.1007/s13237-024-00472-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-024-00472-8