Abstract
Chronic kidney diseases are described by the decreased ability of the kidney to perform its normal functions, which include removing waste products from the blood, controlling blood pressure, and producing erythropoietin. In the current molecular epidemiological study we aimed to find out the association between the ACE I/D, 4a/b of eNOS, rs1801133 of MTHFR, and T344C of CYP11B2 polymorphism and chronic kidney diseases (CKD) in the population of Jammu region of the north Indian population. Convenient-based random sampling and simple random sampling approach were utilized to draw patients and control respectively. DNA was isolated from the collected blood sample and after target sequence amplification, the PCR–RFLP genotyping method was utilized to detect polymorphism and the result was confirmed by statistical analysis. We observed that risk allele i.e., CC of T344C, 4a/b variation of eNOS and rs1801133 of MTHFR was found to be significantly associated with CKD with an association value of OR 1.33, 95% CIs [1.02–1.72] (p value = 0.007), OR 1.72, 95% CIs 1.72 [1.24–2.38] (p value = 0.001) and OR 5.98, 95% CIs [2.05–17.42] (p value = 0.0002) respectively. In conclusion, this molecular epidemiology study shows the variation in CYP11B2, eNOS, and MTHFR (T344C, 4a/b allele, and rs1801133 respectively) significantly increases the risk of CKD in the region of Jammu of the north Indian population.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Featured with the reduced ability of the kidney to carry out the normal function including filtration of the waste product from the blood, and maintaining blood pressure, erythropoietin productions are used to define Chronic Kidney Diseases (CKD) [58]. National Kidney Foundation (NKF) defines CKD based on the estimated glomerular filtration rate (eGFR) (Fig. 1), which is in turn caused by many risk attributes including pathologic kidney abnormalities, renal haematuria, persistent proteinuria, etc. (Kidney disease|National Kidney Foundation). Global burden disorder- 2019 (GBD-2019) has enlightened the vital statistical estimates including prevalence where Japan showed the highest prevalence rate affecting about 19,325.01 per 100,000 per year (adjusted for both sex and all age group) followed by Mauritius (17,613.18), and Thailand (16,176.81). In India, the highest prevalence has been recorded in Kerela followed by Goa and Tamil Nadu ranging from 12,542. 47, 11,232.34, and 10,808.33 per 10,000 per year (GBD India Compare|IHME Viz Hub (healthdata.org).
Since CKD, by itself, does not exhibit any symptoms during the early stages but epidemiological studies have shown an increased risk for CKD where subjects are suffering from diabetes, hypertension, cardiovascular disease, urinary tract infections, family history of CKD itself, exposure to certain drugs, systemic infections and notably urinary stone [29, 40]. Diseases susceptibility is defined by the influence of risk factors which are largely categorized into environmental and genetic factors. Other than the environmental factor, genetic factors are largely responsible for determining the disease’s susceptibility.
As vascular regulation is affected in patients with CKD and due to its categorization in non-mendelian disorder/complex disorder, numerous candidate genes have been discovered since passed two decades. Some examples include the Angiotensin-converting enzyme (ACE), aldosterone reductase (CYP11B2), and e-NOS (endothelial nitric oxide synthase) (Fig. 2). Other than vascular dysfunctioning, a decreased pool of methyl-THF and hyper-homo-cysteinemia has been linked to increased DNA mutation, and loss in DNA repair activity The candidate protein is the 5-methyl reductase enzyme encoded by MTHFR [57]. These genes are associated with CKD in different populations [19, 20, 26, 47, 51, 64] therefore, in the current molecular epidemiological study, we aimed to find out the association between the CYP11B2 T344C, ACE I/D polymorphism, MTHFR rs1801133, VNTR 4a/b polymorphism of eNOS (Fig. 2) in the population of Jammu region of the north Indian population.
Material and method
Subject enrolment
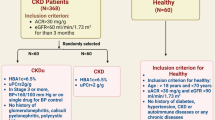
Using a convenient-based sampling technique, subjects were recruited from the Out Patient Department of Nephrology at the Government Medical College and Super-speciality Hospital in Jammu (Jammu and Kashmir) (UT). Following a nephrologist's diagnosis, we identified 250 confirmed instances of chronic kidney disease patients (within a 2-year time frame), and for the comparative study, 250 healthy controls with matched ages and sexes were randomly selected from the general population of same region. 4–5 ml of blood was drawn out from both confirmed patients and controls in the 5 ml EDTA-coated vial only after their consent. Each subject was made aware of the nature and scope of the study. The power of the study was calculated by Genetic Association Study (GAS) Power Calculator (GAS Power Calculator (umich.edu).
Ethical permission
The present study has been performed under the norms of the Ethical Committee and guidelines of the Medical Council of India/University of Jammu and was duly approved by the Institutional Ethical Committee (IEC) of University of Jammu, and Government Medical College, Jammu, Jammu and Kashmir (India) vide notification number: RA/19/3120 and JMC/UG/506 respectively. Every subject was made aware of the nature and scope of the study and their consent or from their guardian (where applicable) was taken before the blood sample collection.
Genomic DNA isolation
Utilizing different DNA isolation protocols (Phenol–chloroform isoamyl alcohol, salting out method)/using commercially available kits (QIAGEN, Cat. Nos. 69504), DNA was isolated from both cases and controls. For the quality assessment, the DNA sample was mixed with 1 μl of 1 × DNA loading dye (Bromo phenol blue, glycerol, and EDTA) and was loaded in the gel. The exact concentration of the isolated DNA was measured by spectrophotometry.
PCR primers and PCR condition
Variations harboring DNA sequences were amplified using different targeted sequence-specific primers (Table 1). Applied Biosystems thermal cycler (Make Veriti by Life Technology, Singapore) system was utilized using 25 μl of final reaction mixture containing different concentrations (Table 2).
RFLP condition
For the MTHFR C677T polymorphism genotyping, the polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) genotyping approach was used to confirm the presence or absence of the polymorphism. Hinf-I (New England Biolabs) restriction endonuclease used for detection of MTHFR C677T polymorphism. For the confirmation of CYP11B2,0.2 μl Hae III restriction enzyme (10U/μl) was used. The restriction digestion was performed by using 0.2 μl of restriction enzyme (10 U/μl), 10 μl of amplified PCR product, 2 μl NEB restriction buffer 4, and 7.8 μl of sterile distilled water to reach a total volume of 20 μl. The restriction digestion mixture was given overnight incubation at 37 °C.
Detection of genotypes
The products of restriction digestion were separated on a 4% agarose gel pre-stained with ethidium bromide and analyzed under UV transilluminator. Gel images of all RFLP-based genotyped variants are presented (Fig. 3A, B, C,D).
(A) Gel image shows the RFLP product of ACE (I/D) where Lane “L” represent the ladder (100 bp), Lane 1,3,5, and 6 shows ID, and Lane 2 and 4 shows II genotype. (B) Gel image shows the RFLP product of CYP11B2–T344C polymorphism Lane “L” represent the ladder, Lane 1,6, and 9 represent the TT genotype, Lane 2–5, 7 and 8 shows CT genotype. (C) Gel image shows the RFLP product of eNOS 4a/b intronic polymorphism: where Lane “L” represent the ladder, Lane 1,4, and 6 shows 420 bp, lane 2,3, and 5 shows 393 + 420 bp (genotype). (D) Gel image shows the RFLP product of MTHFR C677T where Lane “L” represent the ladder, Lane 1,3,4, and 6 showed CC gemotype and Lane 2,5,7, and 8 represent 198 + 175 bp (CT genotype)
Statistical analysis
Genotypic and allelic frequencies were calculated by the gene count method. Genotypic and allelic distribution of ACE I/D, 4a/b of eNOS, rs1801133 of MTHFR, and T344C of CYP11B2 were analysed using Pearson's goodness of fit Chi-square test. The significant association of selected polymorphism with CKD susceptibility was calculated by logistic regression model utilizing Odds ratio (OR) with a 95% confidence interval (CI) and a p-value of < 0.05 was considered to be significant. Due to the user-friendly GUI (graphical user interface of Statistical Package for the Social Sciences (SPSS) tools, the tool was utilized to draw out the estimates.
Result
Demographic features
In the present study, a total of 282 males and 218 females were enrolled. Among CKD cases 53.60% were males (n = 134) and 46.40% (n = 116) were females, while the healthy controls consisted of 59.20% males (n = 148) and 40.80% males (n = 102). The dwelling patterns of study participants showed that the majority of them were urban dwellers (total = 280) in comparison to rural dwellers (total = 220). In the patient group, 52.40% were residing in urban areas (n = 131) and 47.60% were natives of rural areas (n = 119). The controls comprised of 59.60% urban dwellers (n = 149) and 40.40% rural dwellers (n = 101). Regarding educational status, 74.40% were educated (n = 186) and 25.60% were illiterate (n = 64) in patient group whereas in controls 76.40% were literate (n = 191) and 23.60% were uneducated (n = 59). 42.40% of patients (n = 106) were reportedly non-vegetarian, whereas 39.60% controls (n = 99) were also found to be keen for non-vegetarian diet.
In CKD cases 22.40% cases (n = 56) were smokers whereas, among controls 13.20% (n = 33) were smokers. OR analysis revealed that smoking was imparting approximately 1.90 folds risk to the progression of CKD in our population [OR = 1.90 (1.18–3.04); p = 0.01]. It was found that the majority of the controls in comparison to cases were non-alcoholics (n = 223 vs. 188 respectively). The percentage of alcoholics was 24.80% (n = 62) and 10.80% (n = 27) among CKD cases and controls, respectively. OR analysis showed that alcoholism was adding nearly 2.72 folds risk in the development of CKD in our population.
Comparison of anthropometric, clinical, and biochemical characteristics in the study population
The comparison of various anthropometric, clinical, and biochemical parameters between the CKD patients and controls among the study population of the Jammu region is given in (Table 3). The controls were slightly younger than the patients, with a mean age of 52.24 years compared to 54.53 years in the patient group. BMI was significantly higher in patients than in controls (p = 0.0001) whereas no statistical difference was observed in the waist-hip ratio (WHR) (p = 0.9). The psychometric characteristics viz. SBP (Systolic Blood Pressure), DBP (Diastolic Blood Pressure), and PR (Pulse Rate) were higher in patients in comparison to controls in a significant manner. The mean SBP (142.58 ± 19.96 in patients vs. 124.70 ± 7.80 in controls) and DBP (87.64 ± 11.01 in patients vs. 84.01 ± 7.74 in controls) indicated significant (p < 0.0001) differences between the two studies groups. A significant distinction was observed between PR among cases (84.01 ± 10.53) and controls (76.68 ± 7.49) (Table 3).
On comparing biochemical characteristics of CKD cases with controls, statistically significant difference for FBS (p < 0.0001), urea (p < 0.0001), creatinine (p = 0.002), LDL (p = 0.0003) and HDL (p < 0.0001) was observed. However, values for total cholesterol (p = 0.3) and triglycerides (p = 0.7) were not statistically significant among cases and controls. The mean total cholesterol of cases and controls was 152.35 ± 36.41 and 149.09 ± 32.16, those of triglycerides were 155.56 ± 56.32 and 157.05 ± 57.89 and those of LDL-C were 123.38 ± 63.61 and 107.43 ± 28.21 respectively. While comparing CKD patients with controls, significant differences could be observed for HDL-C (42.27 ± 7.23 vs. 45.21 ± 8.75, p < 0.0001). The mean duration of the disease in patients was found to be 0.79 ± 0.67.
Genetic association
ACE I/D
In the present study, the observed genotypic frequencies in patients were 30.8% (77/250) for wild genotype (II), 44% (110/250) for heterozygous genotype (ID), and 25.2% (63/250) for variant genotype (DD). The allelic frequency of a wild allele (I) was 0.53 and for risk allele (D) was 0.47 in CKD cases (Fig. 4B). The prevalence of II, ID, and DD in controls were 34% (85/250), 46.4% (116/250), and 19.6% (49/250) respectively (Fig. 4B). The frequency of a wild allele (I) was 0.57 and the risk allele (D) was 0.43 in controls which were close to the allelic frequencies obtained in CKD cases (Fig. 4A) for the genotypic frequencies for both CKD cases and controls in HWE. The genotypic and allelic frequencies along with chi-square values for Hardy–Weinberg calculation for study participants are shown (Table 4).
Genotypic and allelic frequency of different candidate genes: (A) Allelic frequency of ACE I/D variant in cases and controls where the frequency of “I” is more control group [57%]. (B) Genotypic frequency of ACE I/D variant in both cases and controls. (C) CYP11B2 allelic frequency where the mutant allele was found more in case 40% in comparison to controls [33%]. (D) CYP11B2 genotypic frequency of mutant genotype was more in cases [19.20%] as compared to controls [10%]. (E) Pie diagram shows the allelic frequency of eNOS a/b variant, where the “a” was found more in cases [22%] as compared to control [14%]. (F) Histogram represent the genotypic frequency of a/b polymorphism, where the frequency of mutant “aa” genotype was higher in cases [3.60%] as compared to 1.60% in controls. (G) Pie graphs represents the allelic frequency of MTHFR C677T, where the “T” allele was found more in cases [5%] in compared to controls [1%]. (H) Genotypic frequency of MTHFR C677T
Different genetic models were used since D is the risk allele for ACE, co-dominant (ID vs. II, DD vs. II), dominant (ID + DD vs. II), recessive (DD vs. ID + II) and allelic (D vs. I) models were applied to calculate the possibility of association of ACE I/D polymorphism in the study population. The frequencies of ID versus II with OR 1.05, 95% CI [0.70–1.57], p = 0.8; DD versus II with OR 1.42, 95% CI [0.88–2.30], p = 0.2; ID + DD versus II with OR 1.16, 95% CI [0.80–1.68], p = 0.4; DD versus ID + II with OR 1.38, 95% CI [0.91–2.11], p = 0.1; D versus I with OR 1.20, 95% CI [0.93–1.53], p = 0.2 did not showed any significant association with CKD (Table 4). The allelic model shows that the D-allele (risk) was adding onefold risk to the study population but was unable to reach statistical significance p = 0.2.
CYP11B2 rs4646903
After genotyping of 500 individuals (250 case and 250 controls) the observed genotypic pattern was as follows: 39.2% (98) for wild (TT) genotype, 41.6% (n = 104) for heterozygous (TC) genotype and 19.2% (n = 48) for variant (CC) genotype in cases and 43.2% (n = 108) for wild (TT) genotype, 46.8% (n = 117) for heterozygous (TC) genotype and 10% (n = 25) for variant genotype in controls (Fig. 4D). The overall frequency of the wild (T) allele in CKD cases and controls was 0.6 and 0.67 respectively, whereas the frequency of the risk (C) allele was higher in cases (0.4) as compared to controls (0.33) (Fig. 4C). The genotypic frequency for CKD cases deviated from HWE and that of controls were in HWE. The genotypic and allelic frequencies along with Chi-square values for Hardy–Weinberg calculations for the study participants were presented in (Table 4).
In order to find out the risk association of rare allele, we have taken ‘C’ as a risk allele and ‘T’ as a wild allele. The OR for homozygous co-dominant model (C vs. TT) OR 2.11, 95% CI [1.21–3.69], p = 0.007], recessive model (CC vs. TC + TT) OR 2.14, 95% CI [1.27–3.60], (p value = 0.003) were projecting significant association with the disease. It was concluded that the C-allele was accounting 1.3 folds risk (C vs. T) OR 1.33, 95% CI [1.02–1.72], (p = 0.03) for susceptibility of CKD in the population taken under consideration (Table 5).
eNOS 27 bp VNTR 4b/a gene polymorphism
In patients, the genotypic frequency for wild genotype (bb), heterozygous (ba) and risk genotype (aa) were 58.8% (n = 147), 37.6% (n = 94) and 3.6% (n = 9) respectively. Regarding 250 controls the genotypic frequencies for wild (bb), heterozygous (ba) and risk (aa) genotype were 72.8% (n = 182), 25.6% (n = 64) and 1.6% (n = 4) (Fig. 4F). The allelic frequency of a wild allele (b) obtained in CKD patients was 0.78 and that of risk allele (a) was 0.22 whereas the allelic frequency of a wild allele (b) was 0.86 and that of risk allele (a) was 0.14 in controls (Fig. 4E). The genotypic frequency of both CKD cases and controls was in concordance with HWE. The genotypic and allelic distribution along with chi-square values for Hardy–Weinberg calculation for study participants are shown (Table 4).
A positive association was observed for (ba vs. bb) heterozygous co-dominant with OR 1.82, 95% CI [1.23–2.67] (P = 0.002), (bb + aa vs. bb) dominant OR 1.88, 95% CI [1.29–2.73] (p = 0.001) and (a vs. b) allelic model OR 1.72, 95% CI [1.24–2.38], (p = 0.001) (Table 5). The variant allele (a) was significantly (p < 0.001) adding 1.7 folds risk for the susceptibility of CKD.
MTHFR rs1801133
Genotypic frequencies distribution for wild genotype (CC), heterozygous genotype (CT) and risk genotype (TT) were 91.6% (n = 229), 7.6% (n = 19) and 0.8% (n = 2) respectively. The allelic frequency of the wild allele obtained was 0.95 and that of risk allele was 0.05. The observed genotype frequencies among 250 controls for wild genotype (CC) and heterozygous genotype (CT) were 98.4% (n = 246) and 1.6% (n = 4) respectively. There was a complete absence of risk genotype (TT) in controls (Fig. 4H). The frequency of wild allele (C) was observed to be high in both patients and control group i.e., 0.95 and 0.99 respectively. The frequency of risk allele (T) in patients was 0.05 and that of control group was 0.01 (Fig. 4G). The observed genotype frequencies in control group did not show any deviation from HWE but patient group was showing significant deviation from HWE. The distribution of genotypes and alleles along with chi-square values for Hardy–Weinberg calculation for study participants are shown (Table 4).
As ‘T’ is the risk allele, OR was calculated by comparing wild genotype (CC) which acts as a reference to CT heterozygous genotype forming heterozygous co-dominant model with OR 5.10, 95% CI [1.71–15.22], (p = 0.001). The allelic model suggests that the risk allele ‘T’ was adding approximately sixfold risk OR 5.98, 95% [2.05–17.42], (p = 0.002) towards CKD development. The OR for dominant and recessive genotype frequencies was not possible due to the absence of TT genotype in the control group (Table 5).
Discussion
Homeostasis of vascular structure is an important factor for proper kidney functioning and therefore any disruption can affect negatively its function. Different candidate genes have been found which regulate vascularity such as ACE, CYP11B2, and eNOS. Also, the increased rate of mutation and defects in the DNA repair mechanism has been linked to the folate cycle. Much fame has been grasped by the MTHFR which is responsible for the synthesis of methyl tetrahydrofolate reductase. Different studies have presented the association of such genes with CKD within different demographics. Therefore, the present study aimed to explore the association of the ACE, CYP11B2, eNOS & MTHFR in the population of the Jammu division representing the north Indian population.
The Renin–angiotensin–aldosterone system (RAAS) is a key regulator of both blood pressure and kidney functions and one such example is the Angiotensin-converting gene (ACE). ACE encodes for a “dipeptidyl carboxypeptidase” a membrane-bound ectoenzyme found in different cells epithelial cells of various including heart, kidney but in lungs it is present in abundance. ACE is located on the long arm of chromosome number 17 (17q23.3) and consists of 26 exons and 25 introns with a length of 26 kb (GeneCards) (Fig. 2). ACE I/D polymorphism is characterized by the insertion (I-allele) or deletion (D-allele) of 287 bp Alu repeat sequence within the intron 16. The highest ACE activity has been found in DD homozygous genotype and the lowest in II homozygous genotype whereas ID heterozygous have intermediate activity [13, 38]. DD- genotype of ACE increases the protein expression which in turn is responsible for a high level of angiotensin II.
In the present study the frequency of the risk allele (D) was slightly higher in CKD cases (0.47) and controls (0.43) (Table 4). Likewise, a higher frequency of wild I-allele was observed in the controls than in patients (0.57 vs. 0.53) which was found in line with previous studies [30, 41]. The allelic frequencies reported in the present study were also comparable to the findings of other researchers worldwide [5, 36, 49]. ACE locus is well known for its heterogeneity, as both D and I-allele are responsible for the susceptibility of disease in different populations.
The present study did not find any association of ACE I/D polymorphism with CKD in the study population. In respect of support to our study, different research suggests a non-significant association with chronic kidney diseases [7, 13, 17, 28, 50, 56]. However, several studies have depicted a strong association of ACE I/D polymorphism with CKD phenotypes such as ESRD (End Stage Renal Diseases) patients in contrast to the results of the present study [1, 3, 15, 20, 31, 33, 36, 51, 53, 59, 66]. There have been also reported the gender-wise effect of ACE where it is found that female is prone to CKD in different population [10, 38].
Aldosterone reductase is a rate-limiting enzyme for the synthesis of aldosterone from deoxycorticosterone where it catalyzes the conversion of 11-beta-hydroxycorticosterone into corticosterone then to 18-hydroxycorticosterone and finally 18-hydroxycorticosterone into aldosterone [47]. Aldosterone reductase is encoded by the CYP11B2 (8q24.3) (GeneCards) (Fig. 2) and mainly expressed in the zona glomerulosa of the adrenal cortex of the kidney and its expression is primarily regulated by angiotensin II and potassium. Concerning the common T344C variant located at a putative binding site for the steroidogenic transcription factor (SF-1) [45] and are associated with elevated serum aldosterone level which is a potent cause of various diseases such as high blood pressure, ischemic stroke, renal function and ESRD (End-Stage Renal Disease) [44]. It has been reported that T-allele has five times less affinity than C-allele. The higher affinity of the C-allele leads to increased aldosterone synthase production and hence aldosterone level in the serum [65].
In the present study, the -344 C allele was found to confer the risk of CKD [OR1.33 (1.03–1.72), p = 0.03], and also CC genotype was significantly associated with the risk of CKD susceptibility. Consistent with our study, different independent research and meta-analyses have shown the association of CYP11B2 T-344C polymorphism with CKD7 [43]. It has been also shown that TT genotype was significantly associated with increased risk in the Asian population [64, 67]. But in contrast, some studies reported the lack of association of T-344C polymorphism with CKD. Vasudevan and the group did not find any association between CYP11B2 polymorphism for ESRD in Malaysian subjects [62]. Lee and colleagues have suggested that the CYP11B2 polymorphism might not be a genetic marker for cardiovascular morbidity with CKD in Korean ESRD patients [32]. Another investigation reported that there was no association between the CYP11B2 genotype and the progression of renal failure among ESRD patients [34]. Few studies have also failed to report the relationship of T-344C polymorphism with other diseases viz. HTN and T2DM [22, 44, 52] are considered risk factors for CKD.
Another important regulator of vascular tone is eNOS (endothelial Nitric Oxide Synthase), which is important for catalyzing the synthesis of nitric oxide (NO) from L-arginine. eNOS (7q35-36) featured with 26 exons of the length of 21 kb is majorly expressed in the glomerular afferent and efferent arterioles (GeneCards) (Fig. 2). NO act as a potent vasodilator and regulates endothelial function by exerting vaso-protective effects by scavenging superoxide radicals and suppressing platelet aggregation, leukocyte adhesion, and smooth muscle cell proliferation [21, 48]. But, the presence of eNOS polymorphism such as the VNTR (Variable Number of Tandem Repeats) 4a/b polymorphism reduces the eNOS activity thereby reducing the NO level and therefore altering the blood nitrite and nitrate concentration and enzyme formation [4, 35]. The reduction in NO release may predispose individuals to vascular abnormalities and endothelial dysfunction which leads to the progression of chronic renal failure, and ESRD [48].
eNOS was found to be significantly associated with the CKD utilizing different models (Table 5). In support, different research groups have supported the fact of association and showed a significant rise in the likelihood of the disorder [9, 11, 19]. It has been also shown that the eNOS 4a allele was a risk factor for ESRD in children with CKD [18, 44]. This might help to understand the gene is vital in the progression of diseases which has been also shown to a 6.3 folds risk in the progression of diabetic nephropathy [2, 39]. By polling different studies and increasing, the power of the study, meta-analysis has shown that eNOS 4a/b polymorphism is a potent candidate for CKD in Asian but not in non-Asian [25, 68]. However, some other studies observed that VNTR intron 4a/b polymorphism shows no association with renal disease comorbidity with T2DM in Caucasian-Brazilians [48], diabetic nephropathy in Iraq population [4], Egyptian population [55], and Japanese population [54].
MTHFR (1p36.3) a gene with 11 exons and a length of 1980 bp encodes for Methylenetetrahydrofolate reductase, which plays a major role in the folate and homocysteine pathway where it catalyzes the conversion of 5,10-methylenetetrahydrofolate (5,10-MTHF) to 5-methyltetrahydrofolate (5-MTHF) (GeneCards). 5-5-MTHF is a circulating form of folate and it also acts as a peroxynitrite scavenger it also affects endothelial function by diminishing the generation of vascular superoxide and enhancement in nitric oxide levels [6]. Missense mutation from C to T in exon 4 at nucleotide 677 causes the substitution of alanine (A) to valine (V) at codon 222 within the catalytic region of the MTHFR protein, resulting in the production of thermolabile form of the enzyme [52] which decreases the enzyme activity. Decreases in the activity of MTHFR lead to a decreased pool of methyl-THF and are associated with hyper-homo-cysteinemia, particularly in the folate-deficient states [57].
In the present study, the allelic model suggests that the risk allele ‘T’ was adding approximately sixfold risk [OR 5.98, (p = 0.002)] towards CKD development. Other than an allelic model, the heterozygous co-dominant model with OR 5.10, 95% CI [1.71–15.22], (p = 0.001) observed a significant association of MTHFR C677T gene polymorphism with CKD. Concerning our study, different meta-analyses suggested that the MTHFR 677 T allele was more likely to increase the risk of DN in Asian, West Asian, and Chinese populations but not in East Asian or Japanese populations, and also among Africans or Caucasians, North Chinese than in South Chinese population [12, 23, 63]. Dong and group reported that the “677 T” was significantly responsible for the decreased kidney function in hypertensive Chinese males [16]. Significant association of TT genotype of C677T with CKD and the researchers also provided 1.39 folds risk associated with TT-genotype [26, 27, 37]. It has been also shown that the rare variant may increase the mortality risk in patients with ESRD [27]. It has been also shown that a higher risk of ESRD was observed for “677 TT” genotype in diabetic patients [14], and the suggested significant association of C677T polymorphism with CKD progression in DN [46]. The chance of risk of CVD (Cardio Vascular Diseases) in ESRD patients significantly increases due to the presence of 677 T.
Apart from such positive association studies, some studies indicate the non-association of C677T. In the Mexican population, there was no significant association was observed [24]. It has been found that C677T could have a protective role on renal function among dialysis patients in end-stage renal failure [60]. A protective role on renal function in non-diabetic patients without hyper-homocysteinemia and an adequate alimentary regimen of MTHFR C677T polymorphism were also reported [61]. Non-significant association of MTHFR C667T polymorphism with cardiovascular risk in end-stage renal disease and mortality [8, 42].
Enclosing the section, conflicting results have been reported by the different research groups and this might be due to differences in ethnicity and geographical background. Also, the disparity might be due to the different sample sizes, and techniques used for the genotyping.
Conclusion
Chronic kidney diseases are described by the decreased ability of the kidney to perform its usual functions, which include removing waste products from the blood, controlling blood pressure, and producing erythropoietin (CKD). In conclusion, this molecular epidemiology study shows the variation in CYP11B2, eNOS, and MTHFR (T344C, 4a/b allele, and rs1801133 respectively) significantly increases the risk of CKD in the region of Jammu of the north Indian population.
Data and material availability
The data that has been generated in the present study, has been included in the manuscript.
Abbreviations
- CKD:
-
Chronic kidney diseases
- NKF:
-
National kidney foundation
- eGFR:
-
Estimated glomerular filtration rate
- GBD-2019:
-
Global burden disorder- 2019
- ACE :
-
Angiotensin-converting enzyme
- e-NOS :
-
Endothelial nitric oxide synthase
- MTHFR :
-
Methylenetetrahydrofolate reductase
- CYP11B2 :
-
Cytochrome P450 family 11 subfamily B member 2
- VNTR:
-
Variable number of tandem repeats
- ASCOMS:
-
Acharaya Shri Chander college of medical sciences
- UT:
-
Union territory
- EDTA:
-
Ethylenediaminetetraacetic acid solution
- GAS:
-
Genetic association study
- IEC:
-
Institutional ethical committee
- GUI:
-
Graphical user interface
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- WHR:
-
Waist hip ratio
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- PR:
-
Pulse rate
- FBS:
-
Fasting blood sugar
- LDL-C:
-
Low density lipoprotein-cholesterol
- HDL-C:
-
High-density lipoprotein-cholesterol
- HWE:
-
Hardy–Weinberg equilibrium
- ESRD:
-
End stage renal diseases
- SF-1:
-
Steroidogenic transcription factor
- HTN:
-
Hypertension
- T2DM:
-
Type 2 diabetes
- 5,10-MTHF:
-
5,10-Methylenetetrahydrofolate
- 5-MTHF:
-
5-Methyltetrahydrofolate
- CVD:
-
Cardio vascular diseases
References
AbdeL-Aziz AF, EL-Saeed AF, EL-Dahshan K, AL-Sayed Ebead B. Association of angiotensin-I-converting enzyme (ACE) insertion/deletion gene polymorphism with end stage renal disease in Egyptian patients. Br J Med Med Res. 2014;4(8):1763–71.
Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Sud K, Bhansali A, Khullar M. Endothelial nitric oxide synthase gene haplotypes and diabetic nephropathy among Asian Indians. Mol Cell Biochem. 2008;314:9–17.
Ali S, Mehr MT, Bilal M, Zubair M, Khan AS, Mehmood N. Angiotensin 1 converting enzyme encoding gene polymorphism in renal patients. Pakistan J Med Health Sci. 2022;16(08):890–890.
Al-Janabi LM, Algenabi AHA, Mohammed AJ. Endothelial nitric oxide synthase (eNOS) 4b/a gene polymorphism and the risk of diabetic nephropathy in type 2 diabetes. Tai-Qar Med J. 2016;11(1):65–75.
Anbazhagan K, Sampathkumar K, Ramakrishnan M, Gomathi P, Gomathi S, Selvam GS. Analysis of polymorphism in renin angiotensin system and other related genes in south Indian chronic kidney disease patients. Clin Chim Acta. 2009;406:108–12.
Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114(11):1193–201. https://doi.org/10.1161/CIRCULATIONAHA.106.612325.
Arfa I, Abid A, Nouria S, Elloumii Zghal H, Malouche D, Mannai L, Zorgati MM, Ben AN, Zouari B, Ben Ammar S, Ben Rayana MC, Hmida S, Blousa-Chabchoub S, Abdelhak S. Lack of association between the angiotensis converting enzyme gene (I/D) polymorphism and diabetic nephrology in Tunisian type 2 Diabetic patients. J Renin Angio Aldo. 2008;S-9:32–6.
Aucella F, Margaglione M, Grandone E, Vigilante M, Gatta G, Forcella M, Ktena M, Min AD, Salatino G, Procaccini DA, Stallone C, Gesualdo L. The C677T methylenetetrahydrofolate reductase gene mutation does not influence cardiovascular risk in the dialysis population: results of a multicentre prospective study. Nephrol Dial Transplant. 2005;20:382–6.
Bellini MH, Figueira MN, Piccoli MF, Marumo JT, Cendoroglo MS, Neto MC, Dalboni MA, Batista MC, Goes MA, Schor N. Association of endothelial nitric oxide synthase gene intron 4 polymorphism with end-stage renal disease. Nephrology. 2007;12:289–93.
Bhagat M, Raina JK, Sharma M, Sharma R, Panjaliya RK, Bali SK, Tripathi NK. Association analysis of ACE I/D genotype with gynoid chronic kidney disease patients of Jammu region (J&K). Int J Recent Sci Res. 2017;8(12):22115–7.
Buraczynska M, Ksiazek P, Zaluska W, Nowicka T, Ksiazek A. Endothelial nitric oxide synthase gene intron 4 polymorphism in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;19:2302–6.
Chen H, Wei F, Wang L, Wang Z, Meng J, Jia L, Sun G, Zhang R, Li B, Yu H, Pang H, Bi X, Dong H, Jiang A, Wang L. MTHFR gene C677T polymorphism and type 2 diabetic nephropathy in Asian populations: a meta-analysis. Int J Clin Exp Med. 2015;8(3):3662–70.
Choudhry N, Nagra SA, Shafi T, Mujtaba G, Abiodullah M, Rashid N. Lack of association of insertion/deletion polymorphism in angiotensin converting enzyme gene with nephropathy in type 2 diabetic patients in Punjabi population of Pakistan. Afr J Biotech. 2012;11(6):1484–9.
Cimponeriu DG, Vladica M, Apostol PP, Panaite C, Craciun AM, Ungureanu D, Moldovan C, Serafinceanu C, Gavrila L, Cheta DM. The MTHFR C677T and eNOS ID polymorphisms increase the risk for ESRD in Romanian diabetic and nondiabetic patients. Diabetes. 2007;1(56):A92.
Deepashree GA, Ramprasad E, Jayakumar M, Paul SF, Gnanasambandan R. ACE ID gene polymorphism contributes to chronic kidney disease progression but not NOS3 gene among type 2 diabetes with nephropathy patients. Endocr Metab Sci. 2021;4:100100.
Dong Q, Tang G, He M, Cai Y, Cai Y, Xing H, Sun L, Li J, Zhang Y, Fan F, Wang B, Sun N, Liu L, Xu X, Hou F, Shen H, Xu X, Huo Y. Methylenetetrahydrofolate reductase C677T polymorphism is associated with estimated glomerular filtration rate in hypertensive Chinese males. BMC Med Genet. 2012;13:74.
Elhawary NA, Bogari N, Rashad M, Tayeb MT. Null genetic risk of ACE gene polymorphisms with nephropathy in type 1 diabetes among Egyptian population. Egyptian J Med Human Genet. 2011;12:187–92.
Elshamaa MF, Sabry SM, Bazaraa HF, Koura HF, Elghoroury EM, Kantoush NA, Thabet EH, Abd-El Haleem DA. Genetic polymorphism of ACE and the angiotensin II type1 receptor genes in children with chronic kidney disease. J Inflamm. 2011;8:20.
Elumalai R, Periasamy S, Ramanathan G, Lakkakula BNKS. Role of endothelial nitric oxide synthase VNTR (intron 4 a/b) polymorphism on the progression of renal disease in autosomal dominant polycystic kidney disease. J Renal Inj Prev. 2014;3(3):69–73.
Fawwaz S, Balbaa M, Fakhoury H, Borjac J, Fakhoury R. Association between Angiotensin-converting Enzyme Insertion/Deletion Gene Polymorphism and End-stage Renal Disease in Lebanese Patients with Diabetic Nephropathy. Saudi J Kidney Dis Transpl. 2017;28(2):325–9.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837d. https://doi.org/10.1093/eurheartj/ehr304.
Freitas SR, Cabello PH, Moura-Neto RS, Dolinsky LC, Boia MN. Combined analysis of genetic and environmental factors on essential hypertension in a brazilian rural population in the Amazon region. Arq Bras Cardiol. 2007;88(4):447–51.
Gao XH, Zhang GY, Wang Y, Zhang HY. Correlations of MTHFR 677C.T polymorphism with cardiovascular disease in patients with end-stage renal disease: a meta-analysis. PLoS ONE. 2014;9(7):e102323. https://doi.org/10.1371/journal.pone.0102323.
Gutierrez-Amavizca BE, Orozco-Castellanos R, Ortíz-Orozco R, Padilla-Gutierrez P, Valle Y, Gutierrez-Gutierrez N, Garcia-Garcia G, Gallegos-Arreola M, Figuera LE. Contribution of GSTM1, GSTT1, and MTHFR polymorphisms to end-stage renal disease of unknown etiology in Mexicans. Indian J Nephrol. 2013;23(6):438–43.
He Y, Fan Z, Zhang J, Zhang Q, Zheng M, Li Y, Zhang D, Gu S, Yang H. Polymorphism of eNOS gene association with diabetic nephropathy: a meta-analysis. Mutagenesis. 2011;26(2):339–49.
Hishida A, Okada R, Guang Y, Naito M, Wakai K, Hosono S, Nakamura K, Turin TC, Suzuki S, Niimura H, Mikami H, Otonari J, Kuriyama N, Katsuura S, Kubo M, Tanaka H, Hamaiima N. MTHFR, MTR and MTRR polymorphisms and risk of chronic kidney disease in Japanese: cross-sectional data from the J-MICC study. Int Urol Nephrol. 2013;45(6):1613–20.
Jamison RL, Shih MC, Humphries DE, GuarinoDP KJS, Goldfarb DS, Warren SR, Gaziano JM, Lavori P. Effect of the MTHFR C677T and A1298C polymorphisms on survival in patients with advanced CKD and ESRD: a prospective study. Am J Kidney Dis. 2009;53(5):779–89.
Jayapalan JJ, Muniandy S, Pheng CS. Null association between ACE gene I/D polymorphism and diabetic nephropathy among multiethnic Malaysian subjects. Trop J Pharm Res. 2010;9(5):431–9.
Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl. 2013;3(4):368–71. https://doi.org/10.1038/kisup.2013.79.
Kundal BR, Jasrotia R, Raina JK, Bhardawaj R, Panjaliya RK, Kumar P. Angiotensin converting enzyme (ACE) I/D gene polymorphism in susceptibility of migraine. Indian J Appl Res. 2016;6(6):57–9.
Lakkakula BV, Khare RL, Verma HK, Pattnaik S. Genetic association of ACE gene I/D polymorphism with the risk of diabetic kidney disease; a meta-analysis. J Nephropathol. 2019;8(4):e44–e44.
Lee JE, Bae SY, Kim JY, Pyo HJ, Kwon YJ. Aldosterone Synthase Gene (CYP11B2) Polymorphism in Korean End-Stage Renal Disease Patients on Hemodialysis. Electrolyte Blood Press. 2009;7:67–72.
Lin C, Yang HY, Wu CC, Lee HS, Lin YH, Lu KC, Chu CM, Lin FH, Kao SY, Su SL. Angiotensin-converting enzyme insertion/deletionpolymorphism contributes high risk for chronic kidney disease in asian male with hypertension–a meta-regression analysis of 98 observational studies. PLoS ONE. 2014;9(1):1–16.
Lovati E, Richard A, Frey BM, Frey FJ, Ferrari P. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int. 2001;60:46–54.
Mehmetoglu I, Yilmaz G, Kurban S, Acar H, Duzenli MA. Investigation of eNOS gene intron 4 A/B VNTR and intron 23 polymorphisms in patients with essential hypertension. Turk J Med Sci. 2010;40(3):479–84.
Movvaa S, Alluric RV, Komandurc S, Vattamc K, Eppac K, Mukkavalid KK, Mubigondab S, Sahariab S, Shastryd JC, Hasana Q. Relationship of angiotensin-converting enzyme gene polymorphism with nephropathy associated with type 2 diabetes mellitus in Asian Indians. J Diabetes Complicat. 2007;21:237–41.
Mtiraoui N, Ezzidi I, Chaieb M, Marmouche H, Aouni Z, Chaieb A, Mahjoub T, Vaxillaire M, Almawi WY. MTHFR C677T and A1298C gene polymorphisms and hyperhomocysteinemia as risk factors of diabetic nephropathy in type 2 diabetes patients. Diabetes Res Clin Pract. 2007;75(1):99–106.
Nagamani S, Perumal MS, Perumal RLS, Kesavan C, Muthusamy K. ACE DD genotype associated with females chronic kidney diseases patient of Tamilnadu population. Egyptian J Med Genet. 2015;16:29–33.
Neugebauer S, Baba T, Wantanabe T. Association of the nitric oxide synthase gene polymorphism with an increased risk for progression to diabetic nephropathy in type 2 diabetes. Diabetes. 2000;49(3):500–3.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–80. https://doi.org/10.1053/j.ackd.2014.03.003.
Panjaliya RK, Sethi S, Sharma M, Sharma R, Kumar P, Gupta S. Association of insertion/deletion polymorphism of Alu angiotensin converting enzyme insertion/deletion genotype with type 2 diabetes mellitus and hypertension in J&K population: a case control study. Glob Sci Res J. 2013;1(1):016–20.
Poduri A, Mukherjee D, Sud K, Kohli HS, Sakhuia V, Khullar M. MTHFR A1298C polymorphism is associated with cardiovascular risk in end stage renal disease in North Indians. Mol Cell Biochem. 2008;308(1–2):43–50.
Prasad P, Tiwari AK, Kumar KMP, Ammini AC, Gupta A, Gupta R, Sharma AK, AK, Rao AR, Nagendra R, Chandra TS, Tiwari SC, Rastogi P, Gupta BL and Thelma BK,. Chronic renal insufficiency among Asian Indians with type 2 diabetes: I. Role of RAAS gene polymorphisms. BMC Med Genet. 2006;7:42.
Purkait P, Raychodhury P, Bandhyopadhya S, Naidu JM, Sarkar BN. Analysis of aldosterone synthase gene promoter (- 344 C >T) polymorphism in Indian diabetic nephropathy patients. J Diabetes Metab. 2013;4(5):1–5.
Rajan S, Ramu P, Umamaheswaran, Adithan C. Association of aldosterone synthase (CYP11B2 C-344T) gene polymorphism & susceptibility to essential hypertension in a south Indian Tamil population. Indian J Med Res. 2010;132:379–438.
Ramanathan G, Harichandana B, Kannan S, Elumalai R, Paul S. Association between end-stage diabetic nephropathy and MTHFR (C677T and A1298C) gene polymorphisms. Nephrology. 2017. https://doi.org/10.1111/nep.13208.
Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4(8):225. https://doi.org/10.1186/gb-2003-4-8-225.
Santos KG, Crispim D, Canani LH, Ferrugem PT, Gross JL, Roisenberg I. Association of eNOS gene polymorphisms with renal disease in Caucasians with type 2 diabetes. Diabetes Res Clin Pract. 2011;91:353–62.
Sauca O, Cojocaru D. Angiotensin-convering enzyme insertion/deletion polymorphism in type i diabetic nephropathy. Genet Asp Diabetic Nephrop. 2011; 15–19.
Schiavello T, Burke V, Bogdanava N, Jasik P, Melson S, Boudville N, Robertson K, Angelicheva D, Dworniczak B, Lemmens M, Horst J, Todorav V, Dimitrak D, Sulowicz W, Krasniak A, StomporT BL, Hallmayer J, Kalaydjieve L, Thomas M. Angiotensin- converting enzyme activity and the ACE Alu polymorphism in autosomal dominant polycystic kidney disease. Nephrol Dial Transp. 2001;16:2323–7.
Shanmuganathan R, Kumaresan R, Giri P. Prevalence of angiotensin converting enzyme (ACE) gene insertion/deletion polymorphism in South Indian population with hypertension and chronic kidney disease. J Postgrad Med. 2015;61(4):230–4.
Sharma M, Raina JK, Bhagat M, Panjaliya RK, And SS, Kumar P. Study of association of CYP11B2 C-344T gene polymorphism with hypertension and type 2 diabetes mellitus in the populace of J&K state. Int J Mol Biol. 2016;7(2):124–8.
Shen W, Jiang XX, Li YW, He Q. I/D polymorphism of ACE and risk of diabetes-related end-stage renal disease: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2019;23(4):1652–60.
Shimizu T, Onuma T, Kawamori R, Makita Y, Tomino Y. Endothelial nitric oxide synthase gene and the development of diabetic nephropathy. Diabetes Res Clin Pract. 2002;58:179–85.
Shoukry A, Shalaby SM, Abdelazim S, Abdelazim M, Ramadan A, Ismail MI, Fouad M. Endothelial nitric oxide synthase gene polymorphisms and the risk of diabetic nephropathy in type 2 diabetes mellitus. Genet Test Mol Biomarkers. 2012;16:574–9.
Sikdar M, Purkait P, Raychoudhury P, Bhattacharya SN, Naidu JM, Sarkar BN. ACE gene insertion/deletion polymorphism and type-2 diabetic nephropathy in eastern Indian population. Human Biol Rev. 2013;2(1):66–76.
Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, Halašová E, Lehotský J. the molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. 2016;17(10):1733. https://doi.org/10.3390/ijms17101733.
Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35(2):329–vii. https://doi.org/10.1016/j.pop.2008.01.008.
Tripathi G, Dharmani P, Khan F, Sharma RK, Pandirikkal V, Agrawal S. High prevalence of ACE DD genotype among north Indian end stage renal disease patients. BMC Nephrol. 2006;7:15.
Trovato FM, Catalano D, Ragusa A, Martines GF, Pirri C, Buccheri MA, Di Nora C, Trovato GM. Relationship of MTHFR gene polymorphisms with renal and cardiac disease. World J Nephrol. 2015;4(1):127–37. https://doi.org/10.5527/wjn.v4.i1.127.
Trovato GM, Catalano D, Ragusa A, Martines GF, Tonzuso A, Pirri C, Buccheri MA, Nora CD, Trovato FM. Renal insufficiency in non-diabetic subjects: relationship of MTHFR C677t gene polymorphism and left ventricular hypertrophy. Ren Fail. 2013;35(5):615–23.
Vasudevan R, Ali ABT, Mansoor MS, Zulkifli NF, Ismail P. Analysis of T34C genetic polymorphism of CYPIIB2 gene in Malaysian end stage renal disease subjects. Res J Biol Sci. 2011;6(5):213–8.
Xiong X, Lin XK, Xiao X, Qin DP, Zhou DY, Hu JG, Liu Y, Zhong XS. Association between MTHFR C677T polymorphism and diabetic nephropathy in the Chinese population: An updated meta-analysis and review. Nephrology (Carlton). 2016;21(1):5–12. https://doi.org/10.1111/nep.12541.
Xu H, Wang X, Liu M, Shao X, He X. Association of aldosterone synthase (CYP11B2) -344 T/C polymorphism with diabetic nephropathy: A meta-analysis. J Renin-Angiotensin-Aldosterone Syst: JRAAS. 2016;17(1):1470320316633896.
Yilmaz M, Sari I, Bagci B, Gumus E, Ozdemir O. Aldosterone synthase CYP11B2 gene promoter polymorphism in a Turkish population with chronic kidney disease. Iran J Kidney Dis. 2015;9:209–14.
Yousef HM, Abo-Elmagd YE, Yousif MM, Emam WAE. Association of angiotensin converting enzyme gene polymorphism and diabetic nephropathy in type 2 diabetes mellitus in sharkia governorate. ZUMJ. 2014;20(3):463–9.
Zhang L, Chen X, Qin H, Jiang L, Qin Y. Association between CYP11B2-344T/C gene polymorphism and end-stage renal disease susceptibility: a meta-analysis. Int J Clin Exp Med. 2017;10(6):8728–34.
Zintzaras E, Papathanasiou AA, Stefanidis I. Endothelial nitric oxide synthase gene polymorphisms and diabetic nephropathy: A Huge review and meta-analysis. Genet Med. 2009;11(10):695–706.
Acknowledgements
The authors gratefully acknowledged the patients and their family’s cooperation in giving their permission to participate in the study. Also the authors are highly thankful to Department of Nephrology, Government medical college, Jammu. The Institute of Human Genetics, University of Jammu, which provided the lab space, is also gratefully acknowledged by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PK contributed to the study design, MB, JKR, MS and AS drafted the manuscript, edited the pictures and tables, MB, JKR, and MS analyse the data, MB, KM, IS collected the data, RKP and PK edited the manuscript, PK finalizes the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Every subject was made aware of the nature and scope of the study and their consent was taken before the blood sample collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Samik Bhattacharya; Reviewers: Mohd Younis, Javaid Sheikh.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhagat, M., Raina, J.K., Sharma, M. et al. Genetic association study of ACE I/D, 4a/b of eNOS, rs1801133 of MTHFR, and T344C of CYP11B2 with chronic kidney disease (CKD) in the Jammu region of North Indian population. Nucleus 67, 371–384 (2024). https://doi.org/10.1007/s13237-023-00433-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-023-00433-7