Abstract
Global rise in the prevalence of endemic chronic kidney disease of unknown etiology (CKDu) possess major health issues. The prevalence of CKDu is also rising in the Indian population. Besides environmental factors, genetic factors play an important role in the predisposition to CKDu. In the present study, we have analyzed the association of single nucleotide polymorphisms (SNPs) in three genes with the susceptibility to CKDu. This was a case–control study with a total of 180 adult subjects (CKD = 60, CKDu = 60, Healthy = 60) from central India. We performed KASP genotyping assay to determine the allele frequency of SNP genotypes. We used the odds ratio (OR) to assess the association of individual SNPs, rs34970857 of KCNA10, rs6066043 of SLC13A3, and rs2910164 of miR-146a with CKDu and CKD susceptibility. In the case of rs34970857 of the KCNA10 gene, we noted a significantly increased OR for CKDu versus healthy control (Dominant model; CKDu versus control, CT + CC versus TT, OR = 3.96, p = 0.004). In the recessive and homozygous model, we observed significantly increased OR for rs6066043 of SLC13A3 gene, CKDu versus healthy control {(Recessive model; CKDu versus control, GG versus AA + GA, OR = 2.41, p = 0.03; homozygous model, GG versus AA, OR = 3.54, p = 0.04)}. CC genotype of rs34970857 of the KCNA10 gene and the GG genotype of the SLC13A3 gene are significantly associated with the susceptibility of CKDu.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, chronic kidney disease (CKD) has emerged as one of the leading causes of morbidity and mortality (Jager et a1. 2019; Bikbov et al. 2020; Health and Estimates: Life expectancy and leading causes of death and disability [Internet]. 2022; Foreman et al. 2018). In 2017, the estimated number of CKD cases was 843.6 million worldwide, out of which most population belonged to lower or middle-income countries (Jager et al. 2019; Stanifer et al. 2016). Compared to developed countries, the burden of CKD is increasing rapidly in developing countries, including India. (Stanifer et al. 2016). According to Kidney Disease Improving Global Outcomes (KIDGO), CKD is categorized into five stages, stages – 1–5 (Cheung et al. 2021), and finally reaching end-stage kidney disease (ESKD) or kidney failure, and the patients require kidney replacement therapy (KRT) (O’Hare et al. 2007). According to a study, the age-adjusted incidence rate of kidney failure in India is 229 per million, and more than 100,000 new CKD patients require KRT annually (Modi and Jha 2006). The most common risk factors of CKD include hypertension, diabetes mellitus, glomerulonephritis, chronic tubulointerstitial nephritis, and cystic or hereditary disease (Stanifer et al. 2016). However, a category of CKD patients lack these risk factors, and the disease is termed as “Chronic Kidney Disease with Unknown Etiology” (CKDu) (Paranagama et al. 2018). Clinically, the absence of hypertension and edema characterizes CKDu, and the histological appearance of the disease reveals tubule-interstitial pathology, similar to toxic nephropathies (Redmon et al. 2021).
CKDu is multifactorial, and several risk factors, including low socioeconomic status, occupational hazards, excessive use of pain killers (non-steroidal anti-inflammatory drugs), tobacco consumption, frequent use of contaminated alcohols, dehydration, and infection of Hantavirus associated with the pathogenesis of CKDu (Jayasumana et al. 2017; John et al. 2021; Fitria et al. 2020; Mendley et al. 2019; Sunil-Chandra et al. 2020). Occupational hazards associated with CKDu include the use of heavy metals and agrochemicals and excessive exposure to extremely hot and humid conditions. Besides environmental factors, genetic predisposition like polymorphisms in APOL1 and FGF23 genes are associated with the risk of CKDu. Two genome-wide association studies (GWAS) that included over 130,000 patients found eight single nucleotide polymorphisms (SNPs) located in or near MPPED2, DDX1, SLC47A1, KCNA10, SLC13A3, CDK12, CASP9, and INO80 associated with reduced GFR (Arambegedara et al. 2022). Importantly, SNP in SLC47A1 was associated with decreased GFR in nondiabetic individuals (O’Seaghdha and Fox 2011; Wuttke and Köttgen 2016).
The occurrence of the CKDu within families i.e., familial clustering and regional heterogeneity, takes place worldwide, suggesting a role of genetic predisposition in the CKDu pathogenesis. Genetic differences in ethnicities are known to have a strong association with the prevalence and risk of CKDu. For example, Americans of African descent who carry the APOL1 polymorphic genotype have higher rates and faster progression of CKDu, especially when exposed to occupational factors and heat stress (Reidy et al. 2018). In Sri Lanka, a few SNPs associate with CKDu in some endemic communities (Nanayakkara et al. 2015; Nanayakkara et al. 2014); therefore, studies are warranted to explore the association of SNPs with a genetic predisposition to CKDu in the Indian population.
In a genome-wide association study (GWAS) from Sri Lanka, researchers found a highly significant association for an SNP in sodium-dependent dicarboxylate transporter member 3 (SLC13A3) gene (rs6066043) with CKDu (Nanayakkara et al. 2014). The same group performed the whole-exome sequencing of eight CKDu patients and eight controls, and direct sequencing of candidate loci of 301 CKDu and 276 controls. This study identified 11 SNPs in seven genes, including LAMB2 (laminin beta 2), SLC7A13, PRCP (Prolylcarboxypeptidase Angiotensinase C), KNG1 (Kininogen 1), BANK1 (B cell scaffold protein with ankyrin repeats), TJP1 (tight junction protein 1) and KCNA10 (voltage-gated K channel) associated with CKDu. Among them, an SNP (rs34970857; c.658G > A/p.V220M) in the KCNA10 gene was significantly associated with CKDu (Nanayakkara et al. 2015). The voltage-gated potassium channel, encoded by KCNA10, is expressed in the heart, aorta, and kidney and regulates the transportation of potassium across the membrane (Yao et al. 2002). Dysfunction of the voltage-gated potassium channel in different locations can cause hypertension or kidney disease (Simino et al. 2011). Sodium-dependent dicarboxylate transporter, encoded by the SLC13A3 gene, is expressed in kidney tubular cells and associated with the pathogenesis of hypertension (Simino et al. 2011). Only a single GWAS from India has evaluated the role of genetic predisposition in CKDu (Prasad et al. 2019), which showed that two SNPs, namely rs992037 in PRKN, and rs61774076 in KAZN are associated with CKDu (Prasad et al. 2019).
In the past few years, microRNA-related SNPs also have shown a significant correlation with multiple kidney functions. SNPs in miR-6741-3p, miR-518b, miR-146a, and miR-1295a play a significant role in the development of CKD, tubulointerstitial lesions, collapsing glomerulopathy, and acute kidney injury (AKI) (Safdar et al. 2021; Wang et al. 2011). To date, there is no unequivocal evidence of recognizable possible environmental, occupational, or genetic factors responsible for CKDu. Therefore, this study aimed to determine the association of three SNPs (KCNA10; rs34970857, SLC13A3; rs6066043, miR-146a; rs2910164) with CKDu susceptibility.
Materials and Methods
Subjects
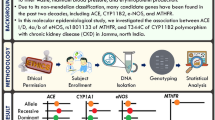
It was a case–control study design, where we recruited subjects from central India, both urban and rural. For a prevalence study, We enrolled CKD patients (N = 368) visiting the Nephrology outpatient department consecutively; based on the inclusion and exclusion criteria, the patients were categorized as CKD or CKDu (Fig. 1). We then randomly selected sixty patients with CKD (N = 60), and 60 patients with CKDu for genetic analysis. In addition, we enrolled 60 healthy controls in the study. We characterized CKD cases presented with glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 for three months or more, irrespective of the underlying cause; and the presence of albuminuria, defined as the albumin-to-creatinine ratio (ACR) > 30 mg/g in two of three spot urine specimens. We calculated the estimated GFR (eGFR) using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation (Michels et al. 2010). We used the following case definition to categorize CKD patients into CKDu. We further characterized CKDu patients as CKD patients presented without glomerulonephritis, pyelonephritis, renal calculi, polycystic kidneys, and nondiabetic {glycated hemoglobulin (HbA1c; < 6.5%)}. Furthermore, the blood pressure of CKDu patients with hypertension was below < 140/90 mm Hg for stages 1–3 (without receiving any anti-hypertensive treatment) or blood pressure below < 160/100 mm Hg in stages 4–5 (with one or two anti-hypertensive drugs) (Anupama et al. 2019). We also recruited unrelated healthy controls from the same region and ethnicity in the study. We ruled out CKD in the healthy controls by measuring eGFR, albumin-creatinine ratio, and proteinuria. Written informed consent from the participants was taken. The study was approved by the Institutional Human Ethics Committee-Postgraduate Research (IHEC-PGR), AIIMS Bhopal (IHEC-LOP/2019/MD0083).
DNA isolation
We collected venous blood (2 ml) in EDTA-containing vacutainer vials, extracted genomic DNA using the modified Phenol–chloroform method (Mustonen and Hemminki 1992), and stored it at − 40 °C. We then measured the DNA concentration by taking the absorbance at 260 nm using a nano-spectrophotometer (ThermoFisher). We ascertained the quality of DNA by calculating the A260/280 ratio.
SNP and Genotyping Assays
We selected three SNPs from the previously published GWAS, showing the association with CKDu or CKD (KCNA10; rs34970857, SLC13A3; rs6066043, miR-146a; rs2910164). We genotyped the selected SNPs with Kompetitive allele-specific PCR (KASP) genotyping assay (LGC Genomics, Hoddesdon, Herts, UK). KASP assay is a homogeneous, competitive allele-specific, and fluorescent-based SNP genotyping technique. The KASP Assay mixture is specific to the SNPs to be targeted and comprises two unlabelled, competitive, allele-specific forward primers, and one common universal reverse primer (Table 1). Each allele-specific primer consists of a unique tail sequence, complementary to universal FRET (fluorescent resonance energy transfer) cassettes, provided in the KASP master mix. One of the FRET cassettes is labeled with FAM (allele 1), and the other cassette is labeled with Hex (allele 2). During amplification, the relevant allele-specific primer amplifies DNA and generates complementary sequences of the tail region, allowing the completion between the tail sequence and FRET cassette, which results in the de-quenching of the FRET cassette, and the release of fluorescence. If the genotype is homozygous (allele 1 or allele 2) only one type of fluorescence will be generated. If the genotype is heterozygous (both allele 1 and allele 2), both fluorescent signals were emitted. This allows biallelic discrimination through competitive binding. We followed the manufacturer’s protocol (LGC Genomics) for standardization and validation of KASP chemistry. We took an equal number of controls and cases along with two non-template controls (NTC), and we confirmed the results by repeated genotyping.
Real-Time PCR Analysis
As per manufacturer instructions, we arbitrarily placed DNA samples from healthy controls, CKD, and CKDu patients on a 96-well PCR with 10 μL reaction mixture. Then, we added 10 ng of genomic DNA to each PCR reaction. We carried out the PCR reactions on a CFX-96 real-time thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA). We included two NTCs in each reaction plate to detect unwanted non-specific amplification and contamination. PCR mix contained the following components; KASP Master mix, assay mix, nuclease-free water, and a DNA sample. The PCR program was optimized and started with hot-start Taq activation at 94 ℃ for 15 min. We used a touchdown program with initial denaturation (94 ℃, 20 s), and primer annealing (61 ℃, 60 s) for ten cycles. Further, we performed 26 cycles of denaturation (94 ℃, 20 s), and amplification (55 ℃, 60 s). We decreased the annealing temperature 61 ℃ by 0.6 ℃ per cycle up to the extension temperature. Finally, we performed an endpoint plate read at 37 ℃ for 60 s with one cycle only. We included heterozygous or homozygous SNP genotypes, and we excluded the genotypes with imprecise results from the study. The genotype call rate ranged from 95–98%, and the genotype correlation between the duplicate samples was > 98.
Statistical Analysis
We performed statistical analysis using SPSS software (version 21; IBM, New York, USA). We presented categorical variables as n (%) of subjects and analyzed them using the χ2-test. We filtered the data based on non-deviation from the Hardy–Weinberg equilibrium. We performed the descriptive statistics and compared genotype distribution using the χ2-test. Finally, we used the OR and their corresponding 95% confidence intervals (CI) to assess the effect of each SNP on disease risk. P value < 0.05 was considered statistically significant.
Results
A total of 180 subjects were recruited in the study; baseline characteristics of subjects are shown in Table 2. CKD and healthy groups had approximately 60% males, whereas the CKDu group had 70% males. The mean age for CKD, CKDu, and healthy was 42.18, 46.88, and 32.80 years, respectively. Healthy controls recruited in the study were a little younger than the CKD and CKDu subjects; however, it should not affect the outcome of the genetic study. Median urine PCR was 3.00 and 1.17 for CKD and CKDu groups, respectively, while median urine ACR (mg/g) was 809.76, 320.39, and 12.76 for CKD, CKDu, and healthy groups, respectively. In the CKD group, around 90% of patients were in stage 5, while in the CKDu group, 19 patients were in stage 3, 27 belonged to stage 4, and 14 were in stage 5.
CT Genotype of KCNA10 was Associated with the Susceptibility of CKDu
In the KASP assay, the allelic polymorphism was categorized as homozygous for allele 1, homozygous for allele 2, and heterozygous (Fig. 2). The genotype call rate for SNPs assay was more than 90%. We analyzed the genotypic and allelic frequencies of three SNPs in KCNA10, SLC13A3 genes, and mir-146a. The allelic frequency of various genotypes is given in Fig. 3. To determine the association of specific genotypes with the risk of developing CKD or CKDu, we calculated the OR using five different genetic models, dominant, heterozygous, recessive, allele, and homozygous model.
The heterozygote CT was the major allele in KCNA10 (rs34970857) in all three groups, CKDu, CKD, and healthy subjects (Fig. 3). The frequency of the homozygous TT allele was 35.71%, which reduced to 18.86% and 12.28% in CKD and CKDu groups, respectively (Table 3). Accordingly, the frequency of the CT allele increased in patients with CKDu and CKD groups, compared to healthy controls (Fig. 3). The difference in the genotype distribution of TT was statistically significant. In the dominant and heterozygous genetic model, the CT allele was associated with an increased risk of CKDu (Table 4). We noted significantly increased OR for CKDu versus healthy control (Dominant model; CKDu versus control, CT + CC versus TT, OR 3.96 (CI = 1.51–10.37), p = 0.004; Heterozygous model, CT versus TT, OR = 4.00 (CI = 1.52–10.48), p = 0.004). Conversely, in the recessive model, the TT genotype decreased the risk of CKDu (Recessive model; TT versus CT + CC, OR = 0.252 (CI = 0.09–0.65) p = 0.004. In addition, we observed an increased OR for the CT genotype in CKD versus the healthy group; however, it did not reach statistical significance. Furthermore, we did not observe any statistically significant difference in the CT or TT allele frequency between CKD and CKDu groups (Table 4). Thus, the CT genotype was associated with susceptibility to CKDu, whereas the TT genotype was protective.
GG Genotype of SLC13A3 is Associated with the Susceptibility of CKD
The heterozygote GA was the major allele in SLC13A3 (rs6066043) in all three groups, CKDu, CKD, and healthy subjects (Fig. 3; Table 3). The frequency of the homozygous GG allele was 33.33%, which increased to 51.92% and 54.71% in CKD and CKDu groups, respectively. Accordingly, the frequency of the GA allele decreased in patients with CKDu and CKD, compared to healthy controls (Fig. 3). In the general population, AAF in the east Asian population is 55.49% homozygote GG; AAF was not available for south Asians (https://gnomad.broadinstitute.org/). The difference in the genotype distribution of GG among the groups was statistically significant. In the recessive and homozygous genetic model, the GG allele was associated with an increased risk of CKDu and CKD (Table 5). In the recessive and homozygous model, we noted significantly increased OR for CKDu versus healthy control {(Recessive model; CKDu versus control, GG versus AA + GA, OR 2.41 (CI = 1.10–5.28), p = 0.03; homozygous model, GG versus AA, OR = 3.54 (CI = 1.05–11.88), p = 0.04)}. Conversely, in the dominant and homozygous model, the AA genotype decreased the risk of CKDu {(Dominant model; AA + GA versus GG, OR = 0.41 (CI = 0.18–0.90) p = 0.03 Homozygous model; AA versus GG, OR = 0.28 (CI = 0.08–0.94) p = 0.04}. Similarly, we observed a significantly increased OR for the GG genotype in CKD versus healthy group {(Dominant model; CKD versus control, GG + GA versus AA, OR 4.17 (CI = 1.09–15.96), p = 0.04; homozygous model, GG versus AA, OR = 5.5 (CI = 1.34–22.50), p = 0.015); Allelic model, G versus A, OR = 2.09 (CI = 1.17–3.72), p = 0.014)}. Furthermore, we did not observe a statistically significant difference in the GG or GA allele frequency between CKD and CKDu patients (Table 5). Thus, the GG genotype was associated with increased susceptibility to CKDu and CKD, whereas the AA genotype was protective.
miR-146a SNP (rs2910164) was Not Associated with the Susceptibility of CKD
The heterozygote GC was the major allele in miR-146a (rs2910164) in all three groups, CKDu, CKD, and healthy subjects. The frequency of the homozygous GG allele was 43.87%, which increased to 49.12% in CKD groups (Table 3). No significant difference in the genotype frequency of GG or AA allele in CKDu and CKD, compared to healthy controls (Table 6).
Discussion
The lack of a precise definition that encompasses all of the characteristics of CKDu makes it challenging to study. There are subtle yet salient differences between the presentations of CKDu patients in the different regions, therefore, in our study, we used the CKDu case definition recommended by the Indian Society of Nephrologists (Anupama et al. 2019). In India, the distinction between CKDu and CKDs is based mainly on the decrease in eGFR (< 60 ml/min), bland urinary sediment, relatively low proteinuria, the absence of diabetes, and mild or no hypertension (Abraham et al. 2019). The resembling symptoms were first described in 1993 in a group of patients from Chennai, India, which stated that chronic interstitial nephritis was the leading cause of chronic kidney failure (Mani 1993). CKDu is endemic in various parts of the world. Over the years, many hypotheses have been proposed about the etiological and pathological aspects of CKDu. For example, environmental factors, heavy metals, fluoride, mycotoxins, herbicides, algal toxins, and pesticides are potential risk factors for CKDu (Wimalawansa 2014). Despite continuous efforts, the investigators could not identify the exact etiological factors of CKDu. The hot spots of CKDu identified in India are Andhra Pradesh, Telangana, Goa, Orissa, Maharashtra, Tamil Nadu, Chhattisgarh, and Punjab, but prevalence and risk factors for CKDu in Central India population have not been explored. With this hypothesis, we analyzed the frequency of three SNPs, which may predispose the central Indian population to CKDu.
In the present investigation, we studied three SNPs among three groups CKD, CKDu, and healthy subjects. For each SNP, we analyzed five different models: Dominant, Recessive, Heterozygous, Homozygous, and allelic. In rs34970857C > T SNP in the KCNA10 gene, we found a significant increase in CT genotypes in CKDu patients compared to healthy controls, and the C allele was associated with CKDu susceptibility. In contrast, the TT genotype and T allele were protective genetic factors for CKDu. In the case of SLC13A3, GG and AA genotypes were significantly associated with CKDu and CKD predisposition. The G allele in rs6066043 was associated with increased susceptibility towards CKD and CKDu, whereas the A allele was protective. Our findings are in accordance with a previous study from Sri Lanka, where rs34970857 SNP (c.658G > A/p.V220M) in the KCNA10 gene was identified as the most significant SNP, with an OR of 1.74 (Nanayakkara et al. 2015). Another study from the same group in Sri Lanka reported that genetic variation in SLC13A3 (rs6066043A > G) was significantly (p = 0.04, OR = 2.13) associated with the increased risk towards CKDu (Nanayakkara et al. 2015). However, that study did not include the CKD group, so it could not be concluded that rs6066043A > G was only associated with CKDu (Nanayakkara et al. 2015). In our study, rs6066043A > G was associated with susceptibility to both CKD and CKDu. It is plausible that rs6066043A > G may be associated with a common kidney injury pathway both in CKDu and CKD. Nonetheless, we did not observe any significant difference in the SNP frequency between CKD and CKDu. rs6066043A > G is located in the intergene region of SLC13A3 and P53RK; it is not clear how does rs6066043 G variant cause susceptibility to CKDu. A GWAS from India has shown that two SNPs, rs992037 in PRKN and rs61774076 in KAZN genes, are associated with CKDu (Prasad et al. 2019).
Two intronic SNPs (rs126917, rs2425885) in SLC13A3 are associated with type 2 diabetes-ESKD (Choi et al. 2016). SLC13A3 was identified as one of the 43 genes that can be employed as a protein biomarker to predict progressive renal fibrosis in mice. This finding suggested that SLC13A3 may also be a valuable molecular predictor for CKD in people (Ju et al. 2009). Therefore, genetic variation in the SLC13A3 gene may result in dysfunction, and abnormal kidney functions, which may lead to the development of CKDu. The present study found that the significant allele ‘G’ and GG genotype was predominant in the CKD population and significantly associated with CKD prevalence (p-value = 0.014; OR = 5.6772) compared to CKDu (p-value = 0.13; OR = 1.9169). Consistently, Nanayakkara et al. 2015 have done whole genome sequencing and reported the significant association of rs9656982 (T > C) SLC7A13 with susceptibility to CKDu (Nanayakkara et al. 2015). KCNA10 and SLC13A3 both works simultaneously to regulate blood pressure but do not affect each other expression. Similarly, miR-146a has not been explored to have any interaction with the selected gene regulation. Thus the SNPs analyzed in the current study may play an important role in kidney function but may work independently.
In our study, we also selected mir-146a C > G (rs2910164) SNP, but it was not significantly associated with a genetic predisposition to CKDu or CKD. However, in previous findings, the miR-146a expression dysregulation has been associated with kidney inflammation and deterioration of kidney function (Wang et al. 2011; Ichii et al. 2012). Wang et al. 2011 evaluated the levels of miR-146a and miR-155 in intra-kidney, and urine in 43 patients with IgA nephropathy (Wang et al. 2011) and found the levels of miR-146a and miR-155 of IgAN were higher than controls in intra-kidney and urinary samples (Wang et al. 2011). IgAN patients showed elevated intra-kidney and urinary levels of miR-146a and miR-155, and the degree of upregulation correlates with histological and clinical disease severity (Wang et al. 2011). Upregulation of miR-146a/b has been observed in B6.MRLc1 CKD mice, spontaneously develop kidney inflammation with age (Ichii et al. 2012). miR499A rs3746444 A/G genetic variant confounds genetic susceptibility with the development of type 2 diabetes-associated end-stage kidney disease (ESKD) for heterozygous (OR = 2.49, CI = 1.41–3.89), and homozygous (OR = 2.41, CI = 1.61–6.68) with 95% confidence interval in Saudi Arabia population. Sargazi et al. 2022, studied the widespread presence of three functional miR-146a gene polymorphisms (rs57095329 A/G, rs2910164 C/G, and rs6864584 T/C) in nondiabetic and diabetic patients with CKD (Sargazi et al. 2022). Authors found that rs57095329 A/G and rs2910164 C/G polymorphisms remarkably increased the risk of CKD under different genetic models as well as diabetic and nondiabetic patients (Sargazi et al. 2022). Conversely, the rs6864584 T/C genetic variant was co-related with a decreased risk of CKD in nondiabetic patients (Sargazi et al. 2022). However, in our study, we did not find any significant association with CKD/CKDu susceptibility. As CKDu is multifactorial, therefore the selected population might be associated with other risk factors or genetic variations other than mir-146a C > G (rs2910164) SNP. Another miRNA, miR-499 elevation, was demonstrated in ESKD patients with acute myocardial infarction (AMI) compared to other ESKD patients without AMI and the control group (> 100- fold, > 350-fold respectively, p = 0.001) (Abdel-Salam et al. 2019). Probably, mir-146a C > G (rs2910164) is not involved in the pathogenesis of CKDu.
Our study provided evidence for the involvement of genetic variants in CKDu predisposition. There was some difference in the baseline characteristics of subjects in the three groups, i.e., CKD, CKDu, and healthy; however, it should not affect genetic variations among the population. Current advanced technologies like high-resolution mass spectrometry and next-generation sequencing can identify the numerous SNPs. Therefore, investigating the combined effects of the SNPs with currently available diagnostic strategies of CKDu would be the best approach.
Conclusion
Our evaluations of the genetic variance with the susceptibility towards CKDu, and CKD patients have supported the evidence of a multifactorial origin of disease instead of a single gene. SNPs in KCNA10 and SLC13A3 were associated with CKDu and CKD predisposition with a high odd ratio; however, rs2910164 of miR-146a was not found significant. Therefore, detecting SNPs and evaluating their functional significance would reveal their role in the pathogenesis of CKDu and CKD. CKDu is not a single-gene disease, rather, it is a multifactorial disease. Therefore, it might be inferred that the pathogenesis happens in those who have a genetic predisposition to the disease when they are exposed to one or more environmental toxins.
Data Availability
The datasets used and/or analyzed under the current study are available on reasonable and authentic request from the corresponding.
References
Abdel-Salam DM, Alrowaili HI, Albedaiwi HK, Alessa AI, Alfayyadh HA (2019) Prevalence of Internet addiction and its associated factors among female students at Jouf University. Saudi Arabia J Egypt Public Health Assoc 94(1):12
Abraham G, Agarwal SK, Gowrishankar S, Vijayan M (2019) Chronic Kidney Disease of Unknown Etiology: Hotspots in India and Other Asian Countries. Semin Nephrol 39(3):272–277
Anupama Y, Sankarasubbaiyan S, Taduri G (2019) Chronic kidney disease of unknown etiology: Case definition for India – a perspective. Indian J Nephrol. https://doi.org/10.4103/ijn.IJN_327_18
Arambegedara D, Jayasinghe S, Udagama P (2022) Multi-pronged research on endemic chronic kidney disease of unknown etiology in Sri Lanka: a systematic review. Environ Sci Pollut Res 29(4):4893–4910
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 395(10225):709–733
Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH et al (2021) KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 99(3):S1-87
Choi S, Bae S, Park T (2016) Risk Prediction Using Genome-Wide Association Studies on Type 2 Diabetes. Genomics Inform 14(4):138–148
Fitria L, Prihartono NA, Ramdhan DH, Wahyono TYM, Kongtip P, Woskie S (2020) Environmental and Occupational Risk Factors Associated with Chronic Kidney Disease of Unknown Etiology in West Javanese Rice Farmers, Indonesia. Int J Environ Res Public Health 17(12):4521
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M et al (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet 392(10159):2052–2090
Health and Estimates: Life expectancy and leading causes of death and disability [Internet]. 2022Global Health Estimates: Life expectancy and leading causes of death and disability [Internet]. [cited 2022 May 9]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates
Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y (2012) Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney Int 81(3):280–292
Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C (2019) A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 34(11):1803–1805
Jayasumana C, Orantes C, Herrera R, Almaguer M, Lopez L, Silva LC et al (2017) Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational and environmental determinants. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 32(2):234–241
John O, Gummudi B, Jha A, Gopalakrishnan N, Kalra OP, Kaur P et al (2021) Chronic Kidney Disease of Unknown Etiology in India: What Do We Know and Where We Need to Go. Kidney Int Rep 6(11):2743–2751
Ju W, Eichinger F, Bitzer M, Oh J, McWeeney S, Berthier CC et al (2009) Renal gene and protein expression signatures for prediction of kidney disease progression. Am J Pathol 174(6):2073–2085
Mani MK (1993) Chronic renal failure in India. Nephrol Dial Transplant 8(8):684–689
Mendley SR, Levin A, Correa-Rotter R, Joubert BR, Whelan EA, Curwin B et al (2019) Chronic kidney diseases in agricultural communities: report from a workshop. Kidney Int 96(5):1071–1076
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT (2010) Performance of the Cockcroft-Gault, MDRD, and New CKD-EPI Formulas in Relation to GFR, Age, and Body Size. Clin J Am Soc Nephrol 5(6):1003–1009
Modi GK, Jha V (2006) The incidence of end-stage renal disease in India: a population-based study. Kidney Int 70(12):2131–2133
Mustonen R, Hemminki K (1992) 7-Methylguanine levels in DNA of smokers’ and non-smokers’ total white blood cells, granulocytes and lymphocytes. Carcinogenesis 13(11):1951–1955
Nanayakkara S, Senevirathna STMLD, Abeysekera T, Chandrajith R, Ratnatunga N, Gunarathne EDL et al (2014) An integrative study of the genetic, social and environmental determinants of chronic kidney disease characterized by tubulointerstitial damages in the North Central Region of Sri Lanka. J Occup Health. 56(1):28–38
Nanayakkara S, Senevirathna STMLD, Parahitiyawa NB, Abeysekera T, Chandrajith R, Ratnatunga N et al (2015) Whole-exome sequencing reveals genetic variants associated with chronic kidney disease characterized by tubulointerstitial damages in North Central Region. Sri Lanka. Environ Health Prev Med. 20(5):354–359
O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS et al (2007) Age affects outcomes in chronic kidney disease. J Am Soc Nephrol JASN 18(10):2758–2765
O’Seaghdha CM, Fox CS (2011) Genome-wide association studies of chronic kidney disease: what have we learned? Nat Rev Nephrol 8(2):89–99
Paranagama DGA, Bhuiyan MA, Jayasuriya N (2018) Factors associated with Chronic Kidney Disease of unknown aetiology (CKDu) in North Central Province of Sri Lanka: a comparative analysis of drinking water samples. Appl Water Sci 8(6):151
Prasad N, Prakash S, Khan AW, Bhadauria D, Gupta A (2019) Sun-201 genome wide analysis study to evaluate potential genetic risks and immunological pathways associated with chronic kidney disease of unknown etiology. ISN World Congr Nephrol WCN Abstr 4(7 Supplement):S242
Redmon JH, Levine KE, Lebov J, Harrington J, Kondash AJ (2021) A comparative review: Chronic Kidney Disease of unknown etiology (CKDu) research conducted in Latin America versus Asia. Environ Res 192:110270
Reidy KJ, Hjorten R, Parekh RS (2018) Genetic risk of APOL1 and kidney disease in children and young adults of African ancestry. Curr Opin Pediatr 30(2):252–259
Safdar M, Khan MS, Karim AY, Omar SA, Smail SW, Saeed M et al (2021) SNPs at 3’UTR of APOL1 and miR-6741-3p target sites associated with kidney diseases more susceptible to SARS-COV-2 infection: in silco and in vitro studies. Mamm Genome off J Int Mamm Genome Soc 32(5):389–400
Sargazi S, Mollashahi B, Sargazi S, Heidari Nia M, Saravani R, Mirinejad S et al (2022) Prevalence of miR146a Gene Polymorphisms in Diabetic and Non-diabetic Patients with Chronic Kidney Disease. Iran J Sci Technol Trans Sci 46(1):21–31
Simino J, Shi G, Arnett D, Broeckel U, Hunt SC, Rao D (2011) Variants on Chromosome 6p22.3 Associated with Blood Pressure in the HyperGEN Study: Follow-up of FBPP Quantitative Trait Loci. Am J Hypertens. 24(11):1227–33
Stanifer JW, Muiru A, Jafar TH, Patel UD (2016) Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 31(6):868–874
Sunil-Chandra NP, Jayaweera JAAS, Kumbukgolla W, Jayasundara MVML (2020) Association of Hantavirus Infections and Leptospirosis With the Occurrence of Chronic Kidney Disease of Uncertain Etiology in the North Central Province of Sri Lanka: A Prospective Study With Patients and Healthy Persons. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.556737
Wang G, Kwan BCH, Lai FMM, Chow KM, Li PKT, Szeto CC (2011) Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers 30(4):171–179
Wimalawansa SJ (2014) Escalating chronic kidney diseases of multi-factorial origin in Sri Lanka: causes, solutions, and recommendations. Environ Health Prev Med 19(6):375–394
Wuttke M, Köttgen A (2016) Insights into kidney diseases from genome-wide association studies. Nat Rev Nephrol 12(9):549–562
Yao X, Tian S, Chan HY, Biemesderfer D, Desir GV (2002) Expression of KCNA10, a voltage-gated K channel, in glomerular endothelium and at the apical membrane of the renal proximal tubule. J Am Soc Nephrol JASN 13(12):2831–2839
Acknowledgements
Not applicable.
Funding
This work was supported by the Department of Health and Research, New Delhi to ST (R. 12013/11/2021-HR/E-office: 8111586), and Indian Council of Medical Research grant (5/4/7-14/19/NCD-II) to MA.
Author information
Authors and Affiliations
Contributions
RK: Investigation, Data curation, Writing; ST: Methodology, Investigation; Writing- Original draft preparation; MA: Enrollment of subjects, Investigation; Analysis, Writing- reviewing, Funding acquisition; AA: Investigation; Data curation; SKG: Analysis, Writing- Reviewing, Supervision, AK: Conceptualization, Methodology, Analysis, Validation, Writing- Reviewing and Editing, Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Institutional Human Ethics Committee-Postgraduate Research (IHEC-PGR), AIIMS Bhopal dated 23.11.2019 (IHEC-LOP/2019/MD0083).
Consent to Participate
Informed consent was taken from all the participants (cases and healthy controls). While enrolling participants, a patient information sheet was handed over to all the participants before enrollment in the study.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, R., Tiwari, S., Atlani, M. et al. Association of Single Nucleotide Polymorphisms in KCNA10 and SLC13A3 Genes with the Susceptibility to Chronic Kidney Disease of Unknown Etiology in Central Indian Patients. Biochem Genet 61, 1548–1566 (2023). https://doi.org/10.1007/s10528-023-10335-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-023-10335-7