Abstract
Ascorbate peroxidase regulates toxic levels of hydrogen peroxide and catalyzes the reduction of H2O2 to water. A cDNA encoding cytosolic ascorbate peroxidase (Ahcapx) was isolated from the salt tolerant (ST) cell lines of Arachis hypogaea, that showed higher transcript level and 4.5 times higher total APX enzyme activity in the ST lines as compared to salt sensitive lines (SS). The transgenic tobacco plants overexpressing the Ahcapx gene were salinity and drought tolerant as compared to wild type (WT) plants. The transgenic plants had higher chlorophyll content and displayed enhanced germination rate under the abiotic stresses. In addition they also exhibited better photosynthetic efficiency, lower membrane damage and relatively higher values of ascorbate peroxidase activity under stress conditions. These results functionally validate a potential role of Ahcapx, as an important scavenger of reactive oxygen species useful in conferring stress tolerance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth and productivity are constantly challenged by various environmental adversities like drought, freezing stress, light, high temperature and salinity stress because of the increased production of toxic reactive oxygen species (ROS). ROS includes singlet oxygen (1O2), superoxide radical (O−2), hydrogen peroxide (H2O2) and hydroxyl radical (OH−) and their accumulation can cause cell damage and eventually cell death by damaging important biomolecules like lipids, proteins, DNA and RNA [2, 34]. The antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) scavenge ROS to protect plant cells. In addition, several non-enzymatic antioxidants such as carotenoids (e.g. zeaxanthin), tocopherols, ascorbic acid and glutathione also play a crucial role in balancing the cellular redox potential [34].
APXs are class I heme-peroxidases that catalyze the conversion of H2O2 to H2O using ascorbate as a specific electron donor [5]. The activity of antioxidant enzymes has been found to be higher in varieties that are more tolerant to environmental wear and tear [14, 16]. There are reports of higher abiotic stress tolerance in crops like Gossypium hirsutum [15] and Oryza sativa [10] carrying an efficient antioxidant system. Salt tolerant behavior in citrus and tomato has also been attributed to the increased activity of antioxidant enzymes like CAT, SOD and APX [16, 35]. Therefore, a correlation seems to exist between the efficient antioxidant enzymes and stress tolerance. It was supported by reports showing an increase in several antioxidant enzymes at both transcriptional and translational level during salt stress [8]. Overexpression of different isoforms of ascorbate peroxidase led to enhanced tolerance to various abiotic stresses in transgenic tobacco [29, 30, 43].
Arachis hypogaea or groundnut is an important cash crop and its edible oil and high protein content also makes it a valuable food crop. About 70 % of the global groundnut production is in semi-arid land where the problem of salinity is more severe due to irrigation. In our lab we had successfully generated and established salt tolerant lines of A. hypogaea [25]. In the present study, we report the isolation of cDNA encoding cytosolic ascorbate peroxidase (Ahcapx), one of the major H2O2 scavenging enzymes, from the salt tolerant line of Arachis hypogea which was able to grow on 200 mM NaCl and showed higher Ahcapx expression and total APX enzyme activity. Finally the isolated cDNA was functionally validated using transgenic approach and transgenic tobacco plants over expressing Ahcapx showed enhanced tolerance to salinity and drought stress.
Materials and methods
Plant material, callus induction and development of ST cell lines
The seeds of Arachis hypogaea var. JL-24 were obtained from Groundnut Research Institute, Junagarh, Gujarat. Callus was initiated and ST lines were generated [25].
Screening cDNA library
Arachis hypogaea cDNA library was screened with the cDNA probe of APX from Pennisetum glaucum. The positive clones were sequenced and primers were designed for RT-PCR from the sequences obtained using Primer3 Input (Version 0.4.0).
Cloning of Ahcapx cDNA from ST lines of A. hypogea using RT PCR
Total RNA was isolated from the salt tolerant lines of A. hypogaea using Tripure (Roche, Manheim Germany) following the manufacturer’s instructions and used for RT-PCR. The cDNA (1 μl) synthesized was taken as template for PCR using the APX specific primers. The purified DNA was ligated in pGEM-T vector and transformed in DH5α cells. The presence of the insert was confirmed by colony PCR using the gene specific primers and the positive clones were sequenced using M13 universal primers. The sequence alignment was performed using ClustalW program.
Northern analysis of Ahcapx transcript
RNA isolated from different tissues was used for Northern hybridization according to Sambrook et al. [40]. Radiolabelling of Ahcapx cDNA was done by using the Megaprime DNA labelling kit (Amersham Biosciences). After DNA transfer and cross-linking, the membrane was first treated in a prehybridization buffer containing 1 mM EDTA, 7 % SDS and 0.5 M sodium phosphate buffer for 1 h and then denatured radiolabeled probe was added in to it. The probe was allowed to hybridize for 16 h. After which the membrane was washed twice with 3X, 1X and 0.5X SSC containing 0.1 % SDS, exposed to imaging plate in a cassette and images were scanned using a phosphoimager (Fujifilm, FLA 5000). Northern analysis was also carried out to assess the transcript level under cold and osmotic stresses. For osmotic stress treatment, the SS cell lines were placed in 8 % mannitol containing liquid MSO medium and harvested at 0, 24 and 48 h. Cold stress was given to the SS or control cell lines by placing them at 4 °C. They were harvested at 0, 24 and 48 h.
Comparison of total APX activity in SS and ST lines
The APX enzyme activity was assessed according to Nakano and Asada [37]. The reduction in ascorbate concentration was monitored by reading the change in absorbance at 290 nm continuously for 180 s. (Extinction Coefficient of ascorbate was taken as 2.8 mM−1 cm).
Agrobacterium-mediated transformation of tobacco by pCAM-Ahcapx construct
The Ahcapx cDNA cloned in pGEMT vector was released by digestion with EcoRI and cloned at the EcoRI site in the pRT101 vector. The entire cassette of CaMV35S promoter, Ahcapx and terminator was released from pRT101 by HindIII digestion and cloned at the multiple cloning site of pCAMBIA 1301 vector to obtain pCAM-Ahcapx (Fig. 2a). This construct was then mobilized into Agrobacterium tumefaciens and used for Nicotiana tabacum transformation [23].
Molecular analyses of transgenic plants
The genomic DNA was extracted from the leaves of transformed and WT plants and PCR amplification was performed using gene specific primers (AhcapxF: 5′ AAG AAC GTC AAG CCA TG 3′ and AhcapxR—5′ AAC TTC TTG CAT TCA TCA CT 3′). The Southern blot analysis was carried out to confirm the integration of Ahcapx in PCR positive tobacco transgenic plants [40]. Expression of transgene was checked by RT-PCR using actin as internal control. The RT-PCR products were visualized by electrophoresis on 1.2 % agarose gel.
Leaf disc assays
Leaf discs were prepared from healthy and fully expanded leaves of transgenic and WT plants and floated in 10 ml solution of various concentration of sodium chloride and mannitol. Chlorophyll content in the leaf discs were measured after 72 h following the method by Arnon et al. [3]. In all these experiments, the leaf discs floated in water served as the control.
Germination assay
The salt and drought stress tolerance of WT and transgenic plants was also tested at the seed germination stages. Seeds of WT and transgenic lines L2, L4 and L5 were germinated on MS medium, supplemented with either NaCl (200 and 400 mM) or mannitol (200 and 400 mM) to assess salt and drought tolerance respectively. These seeds were also placed on MS medium to serve as control. The germination was scored after 10 days of growth under 16 h light and 8 h of dark period.
Preparation of crude enzyme extracts
Crude enzyme was extracted from leaves of WT and transgenic tobacco plants according to the method of Rao et al. [39]. One gram of fresh leaves was homogenized at 4 °C in 2 ml of medium containing 5 mM EDTA and 2 mM AsA in 50 mM K-phosphate buffer (pH 7.8). The homogenate was centrifuged at 15,000 g (Sigma 3 K30) for 15 min at 4 °C. The supernatant was used for determination of protein content and APX and superoxide dismutase (SOD) activities.
APX and SOD enzyme assay
Ascorbate peroxidase activity was assayed according to Asada and Nakano [37] as mentioned earlier. Superoxide dismutase assay was carried out according to Beauchamp and Fridovich [7]. The activity was assayed by SOD’s ability to inhibit photochemical reduction of Nitroblue Tetrazolium (NBT) at 560 nm. The assay was carried out in 3.2 ml reaction volume having 50 mM Na-P buffer, 0.66 mM, EDTA•Na2,10 mM L-methionine, 33 μM NBT, 0.0033 mM riboflavin and 200 μl of sample. The reaction mixture was kept in dark bottles by covering the reaction with aluminium foil. To 3 ml of reaction mixture, 200 μl of enzyme extract was added while the control/blank had 200 μl of extraction buffer only. The reaction tubes were illuminated with luminescent lamps for 10 min. The extinction at 560 nm was read against the blank. One enzyme unit of SOD is defined as that amount of protein (in mg) causing a 50 % inhibition of the photoreduction. The SOD activity was calculated by the following equation.
MDA and H2O2 content
The malonyldialdehyde (MDA) content assay was done according to Heath and Packer [21]. Leaf tissue (0.5 g) was ground in 2.5 ml of 0.1 % TCA buffer and then centrifuged at 10,000 g at 4 °C for 30 min. To 1 ml supernatant, 4 ml of 20%TCA with 0.5 % TBA solution was added and heated at 95 °C for 30 min followed by quick cooling in an ice bath. After 15 min it was again centrifuged at 10,000 g at 4 °C for 30 min OD of the supernatant was taken at 600 nm, 532 nm and 450 nm. MDA content was calculated using the following equation.
H2O2 assay was done according to Velikova et al. [45]. 0.5 g of leaf tissue was ground and mixed with 5 ml of 0.1 % (w/v) TCA. The homogenate was then transferred to the eppendorf tube and centrifuged at 12,000 g for 15 min at room temperature. To 5 ml of supernatant 0.5 ml of potassium phosphate buffer (pH 7.0, 1 M) and 1 ml of potassium iodide (1 M) was added. The mixture was mixed by vortexing and its absorbance taken at 390 nm using a UV-visual spectrophotometer. Concentration was determined from the equation derived from standard graph plotted with known dilution.
Leaf staining with DAB and NBT to assess H2O2 and superoxide
Detached leaves were infiltrated with 8–10 mL diaminobenzidin (DAB) solution (1 mg DAB mL−1, Sigma pH 3.8) in water and nitroblue tetrazolium (NBT) to assess the level of H2O2 and superoxide respectively, according to the methods by Giacomelli et al. [13] and Scarpeci et al. [41] with a little modification. The leaves were left into the solution overnight in the dark. The solution containing the leaves was then removed and leaves boiled in absolute ethanol for 10 min to remove chlorophyll. The leaves were then dipped in 20 % glycerol and photographed.
Measurement of Fv/Fm ratio
Photosynthetic activity was measured as photochemical yield (Fv/Fm), which represents the maximum quantum yield of photosystem II, by recording the chlorophyll fluorescence using a portable chlorophyll fluorescence meter (Handey-PEA, Hansatech, UK) after 30 min of dark adaptation of the leaves. Measurements were taken at (25 °C) using saturating light pulse of white light (8,000 μmol/m2s−1 for 0.8 s).
Stress tolerance of the plants
The T1 generation seeds were surface sterilized and germinated on MS medium containing hygromycin (30 mg l−1). The 6 days old seedlings were transferred to plastic pots containing agropeat and vermiculite (3:1 v/v) at 25 °C, 60 % relative humidity and and 16 h light/8 h dark photoperiod with light supplied at an intensity of 150 μmol/m2 s−1 in growth chamber for 1 month prior to stress treatment with well water supply. For salt stress treatment, the plants were irrigated with 200 mM of NaCl every third day for 15 days. For drought treatments the plants were not watered for 15 successive days.
Statistical analysis
All experiments were performed at least three times independently. Results were assessed by Student’s t test. Significance was defined as P < 0.05.
Results
Cloning and computational analysis of Ahcapx cDNA
Based on the nucleotide sequence of the ascorbate peroxidase gene obtained after screening of the cDNA library, primers were designed to isolate ascorbate peroxidase from salt tolerant (ST) lines of Arachis hypogaea using RT-PCR. The amplified cDNA was cloned in pGEMT and sequence and the sequence was submitted to the Genebank with an accession no. EF165068. The computational analysis showed that Ahcapx cDNA (753 bp) encodes a cytosolic protein of 250 amino acids of molecular weight of 27.05 kDa with an acidic pI of 5.52. The deduced amino acid sequence demonstrated maximum identity (94 %) with the ascorbate peroxidase from Vigna unguiculata and 93 and 92 % with Glycine max and Medicago truncatula, respectively. Sequence alignment revealed the presence of highly conserved active site (Arg 38 to His 42 (R-L-A-W-H)) of the enzyme. The AhCAPX protein also contains the conserved distal (His 42) and proximal histidine (His 163) that have been reported to be involved in activity of the enzyme [12] (Fig. 1a). The heme binding site (DIVALSGGHTL) and the N-terminal sequence (GKSYPTV) was highly conserved in Vigna, Glycine, Pisum and spinach. The presence of phosphorylation sites as assessed by NetPhos 2.0 showed that the entire protein has 9 putative phosphorylation sites with 4 sites at Ser (152, 162, 173 and 196), 2 at Thr (46 and 59) and 3 at Tyr (93, 95 and 235) suggesting the possible posttranslational modification of this protein.
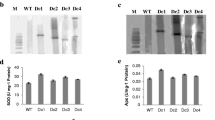
a Multiple sequence alignment of AhCAPX with the previously reported APX from other plants. ClustalW program was used for multiple sequence alignment. Gaps are represented as ‘-’. ‘*’, ‘:’ and ‘.’ indicate identical amino acid residues, conserved substitutions and semi-conserved substitutions in all sequences used in the alignment respectively. Closed and open boxes show the active site and heme binding site of the APX respectively. b The Ahcapx transcript level in SS and ST lines. c Ahcapx transcript level in SS line under cold and mannitol stress Total RNA was probed with radiolabelled Ahcapx probe and equal loading is shown by methylene blue stained rRNA on nylon membrane
Higher transcript level and total APX activity of Ahcapx in ST cell lines
In an attempt to unravel the role of cytosolic ascorbate peroxidase in salt tolerance of ST lines the expression pattern of Ahcapx gene was checked. Higher transcript level in ST cell lines was corroborated with the 4.5 times higher APX enzyme activity (data not shown) in the ST cell lines as compared with the SS cell lines (Fig. 1b).
Upregulation of Ahcapx gene in response to cold and drought stress
The transcript level of the Ahcapx in the SS cell line after 24 and 48 h of cold (4 °C) and drought stress (8 % mannitol) was studied. At 4 °C, an increase of 2.5 fold was observed after 24 h which was further increased to 4 fold after 48 h of cold treatment. Similarly Ahcapx transcript was found to increase by 1.5 and 4.5 fold after 24 and 48 h of mannitol treatment respectively (Fig. 1c).
Generation of transgenic tobacco plants overexpressing Ahcapx gene
The function of ascorbate peroxidase cDNA isolated from Arachis hypogaea was validated using transgenic approach. The Ahcapx cDNA cloned in pCAMBIA1301 was used for tobacco transformation. The incorporation and copy number of the transgene was confirmed by PCR and DNA gel blot analysis respectively. PCR analysis detected an expected band of 973 bp corresponding to the Ahcapx gene (in lane marked + ve) in all putative transgenic plants (lane 1 to 9) while it was absent in the case of WT plant (Fig. 2b). Lines L1 to L5 were subjected to Southern blot showed single copy in lines L2, L4, and L5 while two copies were observed in lines L1, and L3 (Fig. 2c). RT-PCR was carried out to study the expression of the transgene (Fig. 2d) which revealed successful expression of Ahcapx in lines L1, L2, L3, L4 and L5.
Molecular and biochemical analysis of transgenic Nicotiana tabacum plantlets. a Schematic diagram of the Ahcapx construct cloned in pCAMBA1301 used for tobacco transformation b PCR analysis of DNA using the Ahcapx gene specific primers. A band of 973 bp that corresponds to the Ahcapx was amplified only in all the putative transgenic plants (lane 1 to 9) but not in WT. M denotes 1 kb marker and + ve denotes positive control. c The DNA was digested with NcoI for Southern blot analysis, marked above. The blot was probed with radiolabeled Ahcapx. Single copy was observed in lines L2, L4, and L5 while two copies were observed in lines L1 and L3 d RT-PCR analysis also detected the Ahcapx transcript in transgenic lines (L1, L2, L3, L4 and L5)
Delayed aging, higher chlorophyll content and better germination rate demonstrated by transgenic tobacco under stress
The leaf discs excised from 65 days old transgenic lines L2, L4 and L5, as well as from WT plant were incubated in sodium chloride (200, 400 & 600 mM) and in mannitol (200, 400 & 600 mM). The leaf discs of the transgenic lines showed delayed senescence as compared to WT plant after 72 h of incubation (Fig. 3a and c). The estimation of chlorophyll content in the leaf disc further supported our visual observation. At 200, , and 600 mM of NaCl there was 40, 79 and 93 % reduction in chlorophyll content respectively in the leaf discs of WT plant as compared to transgenic lines which showed only 17, 52 and 70 % reduction in L2, 18, 53 and 79 % reduction in L4 and 18, 54 and 80 % reduction in L5 respectively (Fig. 3b). Similarly at 200, 400 and 600 mM of mannitol there was 37, 67 and 91 % reduction in chlorophyll content respectively in the leaf discs of WT plant as compared to transgenic plant which showed only 16, 45 and 67 % reduction in L2, 16, 45 and 71 % reduction in L4 and 17, 47 and 74 % in L5 respectively (Fig. 3d).
Retardation of stress induced senescence of leaf discs in transgenic N. tabacum . The leaf discs of WT and transgenic lines (L2, L4 and L5) were floated on 200, 400, and 600 mM NaCl and mannitol solution. a Visual representation of WT and transgenic lines L2 and L4 shown under a NaCl and c mannitol. Chlorophyll content as estimated in WT and transgenic lines (L2, L4 and L5) on the third day under b NaCl and d mannitol
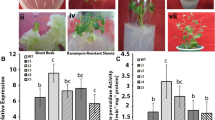
We also carried out germination assay to compare the effect of salt and drought stress on transgenic plants as well as WT plants. Seeds of WT plants and transgenic line L2, L4 and L5 (T1 generation) were germinated on MS medium containing 200 and 400 mM NaCl or similar concentrations of mannitol. At 200 mM mannitol, higher percentage of germination was observed in the transgenic lines L2, L4 and L5 (84 to 88 %) as compared to WT seeds (54 %) after 10 days. The transgenic seedlings were healthy and green in comparison to the WT seedlings. Even at 400 mM mannitol the transgenic lines showed more germination (80 to 83 %) in contrast to control (30 %) (Fig. 4a and b). The transgenic line also showed higher germination rates (>70 %) than WT seeds (30 %) in MS media supplemented with 200 mM NaCl (Fig. 4c and d) after 10 days of plating. However, no seed germination could be observed at 400 mM NaCl in both transgenic and control seeds (data not shown).
Seed germination of WT and transgenic lines (L2, L4 and L5) on MS medium and MS medium containing 200 and 400 of mannitol and 200 mM NaCl. Visual representation of WT and transgenic lines (L2 and L4) during seed germination shown under b mannitol and c NaCl. The percentage of germination under b mannitol and d NaCl, is given as a bar diagram in WT and transgenic lines L2, L4 and L5
Enhanced SOD and APX activities under stress
The ascorbate peroxidase activity under well watered condition was slightly higher in the transgenic plants as compared to the control plants. When treated with 200 mM NaCl solution, APX activity increased in WT plants as well in transgenic lines showing more evident increase in transgenic plants (Fig. 5a). The APX activity in the WT plants increased by 1.2 fold of the original value, whereas the APX activity in L2, L4 and L5 transgenic lines increased by 2.3, 1.7 and 1.6 fold, respectively. When exposed to drought conditions (irrigation withheld for 15 days) the APX activity of the transgenic plants was higher than that of WT plants. The APX activity of WT plants increased by 1.09 fold as compared to plants under well watered condition, whereas the APX activity in L2, L4 and L5 increased to 2.5 fold, 1.7 fold and 1.5 respectively. Under similar condition of NaCl and drought stress the SOD activity was also monitored. The activity was almost similar in WT plants and transgenic lines under well watered conditions. But when exposed to NaCl and drought stress, SOD activity was also observed to increased in all plants with 2.3 fold, 2 fold and 1.7 fold increase under NaCl stress and 2.1 and 2 and 1.6 fold under drought stress in transgenic lines L2, L4 and L5 respectively (Fig. 5b).
Changes in the activities of antioxidant enzymes a APX and b SOD in WT and transgenic lines (L2, L4 and L5) without and under salt and drought stress (water withdrawn for 15 successive days). c Level of H2O2 and d MDA content in WT and transgenic lines (L2, L4 and L5) without and after the aforementioned stresses respectively
Lower H2O2 and MDA content
To test whether there is a change in H2O2 content with increase in APX activity and its affect on lipid peroxidation and MDA content, H2O2 and MDA content was measured under stress. The increase in H2O2 content was more in WT plants (71 and 83 % under salt and drought stress respectively) as compared to that in transgenic lines L2, L4 and L5 under salt (41, 44 and 45 % respectively) and drought (34, 40 and 39 % respectively) stresses (Fig. 5c). The increase in MDA level was also more in WT plants under salt and drought stress (120, and 110 % respectively) as compared to the transgenic lines L2 (58.6 and 50 % respectively), L4 (59 and 53 % respectively) and L5 (64 and 58 % respectively) (Fig. 5d). Furthermore we also analysed the intracellular levels of H2O2 and superoxide’s in leaves of transgenic lines and WT under salt and drought stress by diaminobenzidine (DAB) and Nitroblue tetrazolium (NBT) staining respectively and the results showed higher H2O2 and O−2 content in WT as compared to the transgenic lines L2, L4 and L5 under both the stresses (Fig. 6a and b).
Level of a H2O2 and b superoxide in WT and transgenic lines (L2, L4 and L5), as detected by DAB and NBT staining respectively, before and after the aforementioned stresses. c Fv/Fm ratio in WT and transgenic plants (L2, L4 and L5) without and after salt and drought stresses (water withdrawn for 15 successive days), showing photosystem II photosynthetic efficiency. Fv is the total amount of variable fluorescence and Fm is the maximum fluorescence yield. d Morphology of WT and transgenic plant after drought (upper panel) and salt stress (lower panel) induced by mannitol and NaCl respectively
Transgenic plants showed better photosynthetic efficiency and less membrane damage under salt and drought stress
The photosynthetic efficiency (Fv/Fm) of transgenic plants compared to WT plants under stress was determined by measuring the quantum yield. It was observed that the photosynthetic efficiency of transgenic plant was slightly higher than WT plants when without stress. Reduction in Fv/Fm was observed in WT as well as in transgenic lines on application of stress. After 15 days of salt and drought stress, the reduction in Fv/Fm was 33 and 38 % in WT plant respectively, while it was 10 and 13 % in line L2, 14 and 16 % in line L4, and 15 and 17 % in line L5 respectively (Fig. 6c).
Stress tolerance of plants
The transgenic plants were also assessed for drought and salinity stress. For testing the drought tolerance of transgenic vs. WT plants, 1 month old plants were subjected to drought stress by withholding the water supply for 15 days (see “Materials and Methods”). While the WT plants showed severe wilting, the transgenic plant showed less wilting (Fig. 6d, upper panel). To test whether Ahcapx expression could enhance salt tolerance, 1 month old plants were treated with 200 mM NaCl every third day for 15 days and the plant survival monitored. The WT plants were severely affected under these conditions as evidenced by their stunted growth and chlorophyll loss. However, only a few leaves of transgenic plants bleached and their stems were still erect (Fig. 6d, lower panel).
Discussion
The harmful effects of salt stress are prevented by a complex mechanism of tolerance which involves many genes [17]. A major consequence of by salt stress is the release of reactive oxygen species, which damage the vital cellular organelles and hinder the normal physiological processes in plants, leading to growth retardation and eventually death of the plant [20]. Ascorbate peroxidase is one of the key antioxidant enzymes that helps in the detoxification of ROS, released during stressful conditions in plants. The APX catalyses the conversion of H2O2, a potential ROS to H2O, and protects the organism from its deleterious effects [4]. In the present investigation, a cytosolic ascorbate peroxidase was isolated from the salt tolerant (ST) cell lines of Arachis hypogaea (Ahcapx). The in silico analysis of the gene revealed that its shows close homology with the other species of legume family. Among post-translational modifications , protein phosphorylation is the most commonly occurring phenomenon [24, 44]. Reversible phosphorylation of Serine, Threonine and Tyrosine residues in a protein has been reported during plants’s regulatory mechanism under biotic and abiotic stresses [26, 27, 48]. Furthermore, in maize, specific kinases have been shown to be involved in upregulation of activities of different antioxidant enzymes by phosphorylation under water stress by Xu et al. [47]. In a similar way, occurrence of putative phosphorylation sites suggests that this enzyme might also undergo phosphorylation under various stresses. Higher transcript level of Ahcapx in ST cell lines suggested that it is salt inducible. The higher tolerance of ST lines in 200 mM NaCl containing medium correlated with the higher transcript level as well as higher APX enzyme activity. It can be fairly hypothesized from these results that H2O2 detoxification is operative in the cell lines of Arachis hypogaea by virtue of APX enzymes and might facilitate the cell lines to thrive in salt stress. Reports from other groups also showed increase in APX activity in salt induced oxidative stress resulting in greater tolerance in plants [15, 22]. The transcript was also upregulated under other abiotic stresses like cold and drought stress when applied to these cell lines. Earlier reports suggest that on exposure to low temperature more superoxide radical dismutates to form H2O2 [38]. Since ascorbate peroxidase is the key H2O2 scavenging enzyme found in cytosol and different organelles, a higher level of APX expression is expected and has been shown upon the aforementioned stresses [49]. Salt stress directly imposes osmotic stress leading to generation of reactive oxygen species [19]. Extracellular application of mannitol, reduces water potential outside the cell. This situation mimics drought condition and leads to osmotic stress [50].
To further elucidate the potential of Ahcapx, it was overexpressed in tobacco and the tolerance of transgenic tobacco plants was evaluated. The transgenic plants were more tolerant to salt and drought stress. The overexpression of cytosolic APX has been shown to confer higher tolerance to transgenic plants in prior studies also [6, 11, 31, 46]. The leaf discs of the transgenic plants also exhibited delayed senescence and higher chlorophyll content as compared to leaf discs of WT plant which suggests that APX, which is involved in scavenging the excess reactive oxygen species (H2O2) produced under stress conditions may lower the ROS mediated membrane damage, thus protecting the leaf discs from bleaching. This observation supports the earlier reports where the transgenic plants over-expressing antioxidant genes such as APX in chloroplasts exhibits less chlorophyll degradation [1, 28, 36].
In a study, a cytosolic APX gene derived from pea when overexpressed in tomato showed decreased electrolyte leakage, better germination and tolerance to chilling and salt stress in the transformants [46]. In this study, the overexpression of Ahapx in tobacco also led to the increased enzyme activity of APX and SOD as they are known to work in coordination, in regulating ROS in plants, thus helping the plants to perform better under stress. In a study carried out by Gupta et al. 1993 [17], the over expression of CuZn SOD in tobacco not just increased SOD enzyme activity but also enhanced APX activity, which further supports their coordinated action [18]. The increased enzyme activity and enhanced tolerance signifies the overexpression of Ahcapx gene in plants can lead to transgenics capable of performing well under array of abiotic stress.
In the present study, similar to the earlier reports, WT plants carrying only endogenous gene also showed increased enzyme activity under stress [9, 10, 16, 32, 42]. Since the transgenic plants have cumulative activity of the transgene (Ahcapx) and the endogenous APX, these plants have higher enzyme activity and hence better tolerance under stress. The adverse effect of stress directly affects the photosynthetic activity and reduces the overall plant growth. The transgenic plants were also able to maintain higher Fv/Fm ratio under stress in comparison to WT plants, thus indicating better photosynthetic efficiency [33]. Reactive oxygen leads to membrane lipid peroxidation and malonyldialdehyde (MDA) accumulation under oxidative stress. However on the contrary, Ahcapx overexpressing plants showed less membrane damage and lower MDA accumulation as compared to WT plants under salt and drought stress. In conclusion the higher APX and SOD activity in the transgenic plants lowered the H2O2 content, thus minimizing the membrane damage and MDA content. This result is in concurrence with the earlier work [42], where it has been shown rice and tobacco overexpressing ascorbate peroxidase has lower level of MDA and H2O2 content under stress conditions, which further support our findings.
Thus the overexpression of salt inducible Ahcapx in tobacco led to the development of transgenic plants able to withstand abiotic stresses and exhibit better photosynthetic efficiency. Thus, this gene is vital for upcoming crop development programs.
References
Allen RD, Webb RP, Schake SA. Use of transgenic plants to study antioxidant defenses. Free Radic Biol Med. 1997;23:473–9.
Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99.
Arnon DI. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949;24:1–15.
Asada K. Ascorbate peroxidase – a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:121–410.
Asada Y, Miyake J. Photobiological hydrogen production. J Biosci Bioeng. 1999;88:1–6.
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A. Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant. 2004;121:231–8.
Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87.
Bueno P, Piqueras A, Kurepa J, Savoure A, Verbruggen N, Van Montagu M. Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci. 1998;138:27–34.
Caverzan A, Passaia G, Barcellos RS, Werner RC, Lazzarotto F, Margis-pinheiro M. Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol. 2012;35:1011–9.
Dionisio-Sese M, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9.
Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599–613.
Fishel LA, Farnum MF, Mauro JM, Miller MA, Kraut J, Liu YJ. Compound I radical in site-directed mutants of cytochrome c peroxidase as probed by electron paramagnetic resonance and electron-nuclear double resonance. Biochemistry. 1991;30:1986–96.
Giacomelli L, Masi A, Ripoll DR, Lee MJ, van Wijk KJ. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol Biol. 2007;65:627–44.
Gomez JM, Hernandez JA, Jimenez A, del Rio LA, Sevilla F. Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Radic Res. 1999;31:S11–8.
Gossett DR, Millhollon EP, Lucas MC, Banks SW, Marney MM. The effects of NaCl on antioxidant enzyme activities in callus tissue of salt-tolerant and salt-sensitive cotton cultivars (Gossypium hirsutum L.). Plant Cell Rep. 1994;13:498–503.
Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta. 1997;203:460–9.
Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Gen. 2014;2014, ID701596.
Gupta AS, Webb RP, Holaday AS, Allen RD. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol. 1993;103:1067–73.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Clarendon; 1985.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. London: Oxford University Press; 2000.
Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98.
Hernandez JJA, Mullineaux P, Sevilla F. Tolerance of pea plants (Pisum sativum) to long term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ. 2000;23:853–62.
Horsch RB, Fraley RT, Rogers SG, Sanders PR, Lloyd A. A simple and general method for transferring genes into plants. Science. 1985;227:1229–31.
Hunter T. The age of crosstalk: phosphorylation ubiquitination, and beyond. Mol Cell. 2007;28:730–8.
Jain M, Mathur G, Koul S, Sarin NB. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep. 2001;20:463–8.
Jiang Y, Yang B, Harris NS, Deyholos MK. Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J Exp Bot. 2007;58:3591–607.
Kersten B, Agarwal GK, Durek P, Neigenfind J, Schulze W, Walther D. Plant phosphoproteomics: an update. Proteomics. 2009;9:964–88.
Kwon SY, Jeong YZ, Lee HS, Kim JS, Cho KY, Allen RD. Enhanced tolerance of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant Cell Environ. 2002;25:873–82.
Lee Y, Ahmad R, Lee H, Kwak S, Shafqat MN, Kwon SY. Improved tolerance of Cu/Zn superoxide dismutase and ascorbate peroxidase expressing transgenic tobacco seeds and seedlings against multiple abiotic stresses international. J Agric Biol. 2013;15:171–85.
Liu ZB, Cai H, Han J, Zhou J. L. A novel thylakoid ascorbate peroxidase from Jatrophacurcas enhances salt tolerance in transgenic tobacco. Int J Mol Sci. 2014;15:171–85.
Lu Z, Liu D, Liu S. Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2007;26:1909–17.
Mahajan S, Tuteja N. Cold, salinity and drought stresses:an overview. Arch Biochem Biophys. 2005;444:139–58.
Maxwell K, Johnson GN. Chlorophyll fluorescence- a practical guide. J Exp Bot. 2000;51:659–68.
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8.
Mittova V, Guy M, Tal M, Volokita M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot. 2004;55:1105–13.
Murgia I, Tarantino D, Vannini C, Bracale M, Carravieri S, Soave C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004;38:940–53.
Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–80.
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–4.
Rao MV, Paliyath G, Ormrod DP, Murr DO, Watkins CB. Influence of salisylic acid on H2O2 production, oxidative stress and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–49.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989.
Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol. 2008;66:361–78.
Sharma P, Dubey RS. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings expossed to toxic concentrations of aluminium. Plant Cell Rep. 2007;26:2027–38.
Singh N, Mishra A, Jha B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar Biotechnol (NY). 2014;16:321–32.
Temporini C, Calleri E, Massolini G, Caccialanza G. Integrated analytical strategies for the study of the phosphorylation and glycosylation in proteins. Mass Spectrom Rev. 2008;27:207–36.
Velikova VYI. Edreva A oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66.
Wang Y, Wisniewski ME, Meilan R, Webb R, Fuchigami L, Boyer C. Overexpression of cytosolic ascorbate peroxidase in tomato (Lycopersicon esculentum) confers tolerance to chilling and salt stress. J Am Soc Hortic Sci. 2005;130:167–73.
Xu S, Ding H, Su F, Zhang A, Jiang M. Involvement of protein phosphorylation in water stress-induced antioxidant defense in maize leaves. J Integr Plant Biol. 2009;51:654–62.
Zamharir MG, Mardi M, Alavi SM, Hasanzadeh N, Nekouei MK, Zamanizadeh HR. Identification of genes differentially expressed during interaction of Mexican lime tree infected with Candidatus phytoplasma aurantifolia. BMC Microbiol. 2011;11:1.
Zhang Z, Zhang Q, Wu J, Zheng X, Zheng S, Sun X. Gene knockout study reveals that cytosolic ascorbate peroxidase 2(OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS One. 2013;8(2), e57472.
Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73.
Acknowledgments
UPOE Project from JNU, UGC, CSIR, DST-FIST and DST-PURSE is acknowledged for the funding. We thank Dr MK Reddy for the kind gift of Arachis hypogaea library and APX probe. Groundnut Research Institute, Junagarh, is acknowledged for providing the seeds (Var JL-24) for carrying out the investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Divya Chavhan Shrivastava and Arun Vincent Kisku contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shrivastava, D.C., Kisku, A.V., Saxena, M. et al. Stress inducible cytosolic ascorbate peroxidase (Ahcapx) from Arachis hypogaea cell lines confers salinity and drought stress tolerance in transgenic tobacco. Nucleus 58, 3–13 (2015). https://doi.org/10.1007/s13237-015-0134-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-015-0134-3